Abstract
In eukaryotic cells, the endoplasmic reticulum (ER) is an organelle that is responsible for protein folding and assembly, lipid and sterol biosynthesis, and free calcium storage. In the past decade, intensive research effort has been focused on intracellular stress signaling pathways from the ER that lead to transcriptional and translational reprogramming of stressed cells. These signaling pathways, which are collectively termed Unfolded Protein Response (UPR), are critical for the cell to make survival or death decision under ER stress conditions. In recent years, research in the cancer field has revealed that ER stress and the UPR are highly induced in various tumors and are closely associated with cancer cell survival and resistance to anti-cancer treatments. Identifying the UPR components that are activated or suppressed in malignancy and exploring cancer therapeutic potentials by targeting the UPR are hot research spots. In this review, we summarize the recent progress in understating UPR signaling in cancer and its related therapeutic potential.
Keywords: Endoplasmic reticulum, ER stress, unfolded protein response, cancer, malignancy, cancer therapy
Introduction
Inside a eukaryotic cell, there are numerous organelles that play essential roles in cell survival and functions. Besides the nucleus, the largest organelle is endoplasmic reticulum (ER), an extensive membranous labyrinth network of tubules, vesicles and sac that surrounds the nucleus and expands to the cytosol [1]. The ER has been primarily recognized as a compartment for protein folding and assembly, a pool of free calcium, and a site for lipid and sterol biosynthesis [2, 3]. Approximately one-third of newly synthesized proteins are translocated into the ER where they fold and assemble with the help of a series of molecular chaperones and folding catalysts. Inside the ER, co- and post-translational modifications, including disulfide bond formation and N-linked glycosylation, play important roles in the folding and oligomeric assembly of proteins. The ER provides a high-fidelity quality control system to ensure that only correctly folded proteins can be transported out of the ER while unfolded or misfolded proteins are retained in the ER and eventually degraded [2]. As a protein-folding compartment, the ER is exquisitely sensitive to alterations in homeostasis. A number of biochemical, physiologic to pathologic stimuli, such as those that cause ER calcium depletion, altered glycosylation, nutrient deprivation, oxidative stress, DNA damage, or energy perturbation/ fluctuations, can interrupt the protein folding process and subsequently cause accumulation of unfolded or misfolded proteins in the ER - a condition referred to “ER stress” [3-8]. To ensure the fidelity of protein folding and to handle the accumulation of unfolded or misfolded proteins, the ER has evolved a group of signal transduction pathways, the unfolded protein response (UPR) signaling pathways, to alter transcriptional and translational programs [3, 7]. The basic UPR pathways in mammalian cells consist of three main signaling cascades initiated by three primary ER-localized protein stress sensors: IRE1α (inositol-requiring 1 alpha), PERK (double-strand RNA-activated protein kinase-like ER kinase), and ATF6 (activating transcription factor 6).
IRE1α is a protein kinase and endoribonuclease [9, 10], PERK is a protein kinase that is known to phosphorylate alpha-subunit of eukaryotic translation initiation factor (elF2α) [11, 12], and ATF6 is a basic leucine zipper (bZIP) transcription factor of CREB/ATF family [13]. The primary role of the UPR is to prevent the cell from ER stress by reducing the amount of proteins translocated into the ER lumen, increasing retro-translocation and degradation of ER-localized proteins, and augmenting the protein-folding capacity of the ER (Figure 1). However, if the attempt to recover from ER stress fails, the UPR will induce cell death programs to eliminate the stressed cells [14].
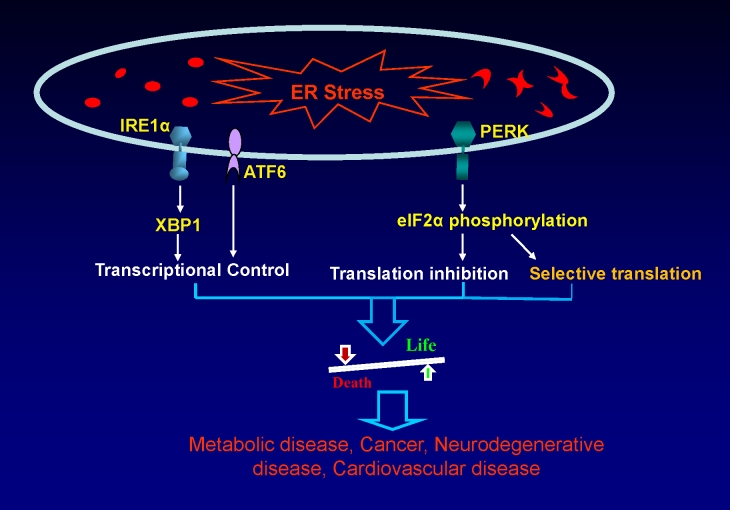
Figure 1.
Role of UPR signaling in health and disease. Under ER stress, three ER stress sensors IRE1α, PERK and ATF6, are activated to alter transcriptional and translational programs to protect the cell from stress caused by the accumulation of unfolded or misfolded proteins. The UPR is critical for the cell to make survival or death decisions under ER stress conditions by altering translational and transcriptional programs. Regulation through UPR signaling is crucial for the development of a variety of diseases, including metabolic disease, cancer, neurodegenerative disease, and cardiovascular disease.
During tumorgenesis, the high proliferation rate of cancer cells requires increased activities of ER protein folding, assembly, and transport, a condition that can induce physiological ER stress [15]. Moreover, as the tumor grows, cancer cells experience increasing nutrient starvation and hypoxia, which are strong inducers for the accumulation of unfolded or misfolded proteins in the ER and the activation of the UPR pathways [15, 16]. Indeed, accumulating evidence has demonstrated that the UPR is an important mechanism required for cancer cells to maintain malignancy and therapy resistance. Additionally, the possibility of targeting the UPR signaling as a novel therapeutic strategy has greatly inspired the cancer research community and pharmaceutical industry.
The UPR pathways
When cells encounter ER stress, an immediate response will be the activation of ER stress sensor PERK through its homo-dimerization and auto-phosphorylation [17]. Activated PERK phosphorylates translation initiation factor elF2α, leading to protein translational attenuation in general. PERK-mediated translation attenuation provides a survival signal, as this can reduce the ER workload by preventing newly-synthesized proteins from entering into the ER which is saturated by unfolded or misfolded proteins. This is evidenced by the fact that the inactivation of PERK-mediated UPR pathway reduces cells’ ability to survive ER stress [18, 19]. However, while general protein translation is inhibited, PERK-mediated elF2α phosphorylation can lead to preferential translation of specific mRNAs that contain multiple upstream open reading frames in their 5'-untranslated regions (ORFs). These upstream ORFs are bypassed only when elF2α is phosphorylated, thus allowing translation of the mRNA [20]. One of those mRNAs is known to encode the transcription factor 4 (ATF4). Under ER stress, phosphorylated elF2α selectively initiates translation of atf4 mRNA [21]. ATF4 subsequently activates expression of genes involved in cell metabolism, anti-oxidative response, and ER stress-associated apoptosis [18, 22].
Along with PERK-mediated translational repression, IRE1α- and ATF6-mediated UPR pathways are also activated to increase protein folding capacity and ER-associated protein degradation. Under ER stress, IRE1α is activated through its homo-dimerization and auto-phosphorylation. Activated IRE1α can function as an endoribonuclease to initiate removal of a 26 nucleotide intron from the mRNA encoding X -box binding protein 1 (XBP1) [17]. This unconventional mRNA splicing generates a translation frameshift that enables the spliced Xbp1 mRNA to encode a functional potent bZIP transcription factor. The spliced XBP1 can activate expression of a group of ER chaperones and enzymes to help protein folding, maturation, secretion, as well as degradation of misfolded proteins [23]. In addition to its endoribonuclease activity, phosphorylated IRE1α can also serve as a scaffold protein that recruits tumour-necrosis factor (TNF)-receptor-associated factor 2 (TRAF2), leading to activation of JUN N-terminal kinase (JNK)-mediated signaling pathways [24]. Notably, the pro-apoptotic B-cell lymphoma 2 (BCL-2) family members BAX and BAK can directly bind to the cytosolic domain of IRE1α and augment its kinase and endoribonuclease activities [25, 26]. The interaction of BCL-2 family members with IRE1α may provide a molecular link between ER stress and apoptosis pathways. On activation of the UPR, ATF6 is also released from the ER membrane, and transits to the Golgi compartment where it is processed by proteases to produce an activated bZIP transcription factor that activates expression of UPR target genes [13]. Similar to spliced XBP1, cleaved ATF6 also activates expression of a group of genes involved in protein folding, secretion, and degradation in the ER [23, 27]. However, recent evidence suggests that ATF6, but not XBP1, is dispensable for the differentiation, function, or survival of specialized cell types where the UPR signaling is required [28, 29].
If the stressed cells fail to adapt to and recover from ER stress through the UPR-mediated survival programs, the UPR will initiate apoptotic pathways to remove the stressed cells. The well-defined pathway involved in the transition from ER stress to apoptosis is mediated by a transcription factor called GADD153/CHOP that is downstream of the PERK/elF2α UPR pathway [14, 30-32]. Under prolonged ER stress, activated PERK phosphorylates elF2α, which can selectively induce translation of the mRNA encoding ATF4. ATF4 induces a pro-apoptotic factor GADD153/CHOP to mediate ER stress-induced apoptosis. This is probably a case in some viral infections in which the organism utilizes ER stress-induced apoptosis to eliminate the infected, stressed cells in order to limit viral replication [33, 34]. Additionally, as part of the UPR program, ER-associated Protein Degradation (ERAD) is responsible for the degradation of aberrant or misfolded proteins in the ER and, in addition to this “quality control” function, also accounts for the degradation of several metabolically regulated, active ER proteins [35, 36]. During the process of ERAD, molecular chaperones and associated factors recognize and target substrates for retrotranslocation to the cytoplasm, where they are polyubiquitinated and degraded by 26S proteasome. ERAD is essential for maintaining ER homeostasis, and disruption of ERAD is closely associated with ER stress-induced apoptosis [37].
The UPR in malignancy
Cancer cells possess rapid glucose metabolism and fast growth rate, which leads to poor vascu-larisation of tumor mass, low oxygen supply, nutrient deprivation, and pH changes [16, 38]. On the other hand, cancer cells can express mutant proteins that cannot be correctly folded, and experience insufficient ER energy supply, alteration of the redox environment, and viral infection [39]. All of these can cause ER stress and activation of the UPR. Increasing evidence suggests that the UPR provides survival signaling pathways required for tumor growth. Indeed, increased expression of the UPR components, including the UPR trans-activators XBP1 and ATF6, ER stress-associated pro-apoptotic factor CHOP, as well as ER chaperones GRP78/BIP, GRP94, and GRP170, have been detected in breast cancer, hepatocellular carcinomas, gastric tumors, and esophageal adenocarcinomas [40]. Cancer cells may adapt to ER stress and evade stress-induced apoptotic pathways by differentially activating the UPR branches [41-43]. Here, we discuss recent advances in understating the roles of different UPR components in malignancy.
ER chaperone GRP78/BiP
GRP78/BiP (glucose-regulated protein of 78 kDa) is an abundant ER chaperone that uses ATP/ADP cycling to regulate the protein folding process [44, 45]. It has been proposed that the initial activation of three ER stress sensors, including IRE1α, PERK and ATF6, depends on the dissociation of GRP78 in response to ER stress [7]. Recent studies suggested that GRP78 plays critical cytoprotective roles in oncogenesis [38, 44]. Increased expression of GRP78 has been observed in a variety of cancers [46-48]. GRP78 over-expression was shown to provide important survival signals for cancer cells during oncogenesis and confers drug resistance in both proliferating and dormant cancer cells [15].
The evidence that GRP78 is required for cancer cell survival came from the observation that suppression of GRP78 in fibrosarcoma cells inhibited their ability to form tumors in vivo [49]. GRP78 has also been implicated in promoting tumor cell proliferation. Over-expression of GRP78 correlated with increased proliferation rates of a range of glioma cells, while the knock-down of GRP78 resulted in decreased proliferation rates of glioma cells [41]. Dong et al showed that Grp78 heterozygosity prolonged the latency period and significantly impeded tumor growth in a genetic mouse model of breast cancer where GRP78 expression level was reduced by half [50]. Their results suggested that GRP78 regulates cancer progression through three mechanisms, including enhancement of tumor cell proliferation, protection against apoptosis, and promotion of tumor angiogenesis. Recently, in a large series of breast cancer cases, expression of GRP78 and XBP-1 was observed in 76% and 90% of the breast cancers [47]. The results suggested that the UPR is activated in the majority of breast cancers and probably confers resistance to chemotherapy.
Additionally, a link between the high GRP78 expression level and poor clinical outcome of cancer therapy has been observed. For example, high levels of GRP78 expression correlate with increasing tumor grade in hepatocellular carcinoma, poor clinical outcome in breast cancer, high rates of recurrence and mortality in prostate cancer, and high rates of nodal metastasis and reduced survival in gastric cancer[48, 51-53].
UPR signaling through IRE1α/XBP1
The UPR signaling through ER stress sensor IRE1α and trans-activator XBP1 controls the upregulation of a broad spectrum of UPR-related genes involved in protein folding, transport, and ERAD [23]. In addition to classical UPR -related genes, the IRE1α/XBP1 arm of the UPR also regulates expression of the genes involved in cell differentiation, inflammation, lipogenesis, and apoptotic pathways [54]. A number of recent studies suggested that the IRE1/XBP1 arm of the UPR is essential for malignancy maintenance under oncogenic stress. Transformed mouse embryonic fibroblasts or human fibrosarcoma tumor cells (HT1080) that lack XBP1 displayed the inability to grow as tumor xenografts in SCID mice [55, 56]. Instead, XBP1-deficient cells showed increased apoptosis and decreased clonogenic survival under ER stress or hypoxia condition. Furthermore, expression of the dominant-negative form of IRE1α or inhibition of XBP1 by RNAi reduced blood vessel formation during tumorgenesis in an intradermal angiogenesis model or a human tumor xenograft model [57]. However, expression of spliced XBP-1 restored angiogenesis in IRE1α dominant -negative expressing cells, suggesting that the UPR signaling through IRE1α/XBP1 is crucial for angiogenesis in the early stage of tumor development. Interestingly, the un-spliced form of Xbp1 mRNA was shown to encode a rapid-turnover protein that can function as a dominant negative factor to inhibit spliced XBP1 activities [58, 59]. While high expression levels of spliced XBP1 were associated with increased tumor survival, high levels of the unspliced form of XBP1 caused increased apoptosis of tumor cells [60].
UPR signaling through PERK/elF2α
During tumorgenesis, cancer cells need to tolerate a subset of oncogenesis-associated cellular stresses including DNA damage, hypoxia, proteotoxic, mitotic, and oxidative stress [16]. In order to adapt to and overcome the stress, tumor cells remodel the transcriptional and translational programs by activating pro-survival signaling pathways. The UPR signaling through PERK/elF2α has been demonstrated to confer a survival advantage for tumor cells under hypoxic stress [61]. Hypoxic stress can activate PERK, leading to phosphorylation of elF2α in tumor cells [61, 62]. Transformed mouse embryonic fibroblasts from the PERK-deficient animals and HT29 colorectal carcinoma cells expressing dominant-negative PERK exhibited lower survival rates under hypoxic conditions, compared to wild type cells [62]. These cells formed smaller tumors and displayed higher levels of apoptotic activity in hypoxic areas than the wild-type control cells [63]. Additionally, tumors derived from PERK-deficient mouse embryonic fibroblasts exhibited limited ability to stimulate angiogenesis [64]. Furthermore, the critical role of PERK/elF2α-mediated UPR signaling in hypoxia survival is supported by a study with mouse embryonic fibroblasts expressing a “knock-in” mutant of elF2α (S51A) that cannot be phosphorylated by PERK [63]. These cells displayed an increased susceptibility to hypoxia with virtually no survival under prolonged hypoxia conditions.
The therapeutic potential of targeting the UPR components
The importance of the UPR in malignancy maintenance has inspired great interest in exploring therapeutic potentials by targeting the UPR components. Tumor cells grow under oncogenic stress caused by hypoxia, nutrient deprivation, DNA damage, metabolic and oxidative stress and therefore rely on an activated UPR for survival [15, 47]. However, most normal cells are not subjected to stress, and the UPR pathways remain inactive state in these cells. This discrepancy between tumor cells and normal cells may offer an advantage for the agents that target the UPR to achieve the specificity in cancer therapy. In the following, we provide some representative evidence for cancer therapeutic applications by targeting UPR components.
GRP78/BiP as a cancer therapeutic target and biomarker
Expression of GRP78 protein correlated with both the rate of patient survival and the depth of tumor invasion. In human cancers, elevated GRP78 level generally indicates the higher pathologic grade, recurrence, and poor patient survival in breast, liver, prostate, colon, and gastric cancers, although lung cancer is an exception [15]. Additionally, GRP78 expression was positively correlated with increasing tumor thickness and with increasing dermal tumor mitotic index [65]. These observations have inspired the idea of targeting GRP78 for cancer therapy. Indeed, recent studies supported that knock-down of GRP78 can suppress cancer cell growth and improve the sensitivity of cancer cells to the treatments. Knockdown of GRP78 by siRNA could slow down the growth of glioma cells and increase their sensitivity to chemotherapeutic agents, including temozolomide, 5-fluorouracil and CPT-11 [41]. The cytotoxic effect of GRP78 knockdown has been confirmed in many cancer cell lines [66, 67], although one study suggested that the pro-survival role of GRP78 in tumorgenesis is possibly cell-line specific [68]. Researchers have been actively screening for a specific GRP78 inhibitor as an anticancer agent [69-71].
GRP78 is an abundant molecular chaperone that localizes to the ER lumen. However, recent evidence suggested that a sub-fraction of GRP78 localized to the surface of specific cell types, including malignant cells [72]. Preferential expression of GRP78 on the surface of tumor cells but not in normal organs suggests that surface GRP78 can serve as a biomarker for cancer-specific therapy. Indeed, some of recent studies supported that ER chaperones GRP78 and GRP94 are effective biomarkers for indicating aggressive behavior and poor prognosis in cancer [51, 53, 73, 74], although there is evidence that GRP78 as a cancer biomarker might be tumor-type specific [15, 52, 75].
Proteasome inhibitors
The ubiquitin-proteasome pathway is one of central players in the regulation of several diverse cellular processes. Proteasome inhibitors can block the action of proteasomes, inhibit the degradation of proteins critically involved in regulation of cell proliferation and survival, and eventually lead to growth inhibition and apoptosis. Proteasome inhibitors have been intensively studied in the treatment of cancers. Bortezomib (Velcade; PS-341) is a highly selective and reversible proteasome inhibitor that has been approved for clinical use against multiple myeloma and is in clinical trials as a single agent or in combination with chemotherapeutics against other solid tumor malignancies [76, 77]. The in vitro studies have confirmed the cytotoxic effects of bortezomib on a broad range of cancer cell types, including prostate, lung, breast, colon, and non-Hodgkin's lymphoma [78-81]. It can induce additive or synergistic cytotoxic activities against cancer cells when combined with other antineoplastic agents [81-83]. Although the mechanisms involved in its anti-cancer activity are still being elucidated, bortezomib was recently shown to cause the accumulation of misfolded proteins in the ER and ER stress-associated apoptosis by inhibiting 26S proteasome activity and subsequent ERAD machinery [58, 84-86]. Moreover, bortezomib was shown to suppress the IRE1α/XBP1 arm of the UPR by inhibiting IRE1α endoribonuclease/ kinase activity and by stabilizing the unspliced form of XBP1, a dominant negative for the functional XBP1 protein [58, 59]. In addition to bortezomib, therapeutic potentials of other proteasome inhibitors were also investigated. For example, BU-32 (NSC D750499-S), a highly selective proteasome inhibitor, was effective in suppressing in vitro and in vivo breast cancer cells, on which bortezomib has limited effect [87].
ERAD inhibitor
Under ER stress, ERAD removes aberrant or misfolded proteins from the ER through protein retrotranslocation and ubiquitin-proteasome degradation systems [36, 88]. Defects in ERAD cause the accumulation of misfolded proteins in the ER and thus trigger ER stress-induced apoptosis [37]. In the process of ERAD, a cytosolic ATPase named p97 plays key roles in extracting misfolded proteins that are polyubiquitinated and transporting them to the proteasome for degradation. Recently, Eeyarestatin I (Eerl), a chemical inhibitor that can block ERAD, has been shown to have preferential cytotoxic activity against cancer cells [89, 90]. Eerl can target p97 complex to inhibit deubiquitination of p97-associated ERAD substrates, which is required for the degradation process [90]. Like bortezomib, Eerl induces an integrated stress response in the ER as well as apoptosis via the Bcl-2 homology3 (BH3)-only pro-apoptotic protein NOXA. Eerl activates the CREB/ATF transcription factors ATF3 and ATF4, which form a complex capable of binding to the NOXA promoter and activate NOXA expression [89]. Interestingly, Eerl was found to be able to block ubiquitination of histone H2A to relieve its inhibition on NOXA transcription [89]. These studies suggested that the ERAD inhibitor Eerl may represent a novel class of anticancer drugs that integrate ER stress response with epigenetic mechanisms to induce cell death.
Other therapeutic potential associated with ER stress
Several other distinct agents have been reported to have anti-cancer potentials by modulating ER stress response. Versipelostatin, a novel macrocyclic compound, showed highly selective cytotoxicity to glucose-deprived tumor cells and in vivo tumors by inhibiting GRP78 induction and expression of the UPR trans-activators XBP1 andATF4 [69, 91]. An engineered fusion protein, epidermal growth factor-SubA (EGF-SubA), was reported to be highly toxic to growing and confluent epidermal growth factor receptor-expressing cancer cells, and its cytotoxicity is thought to be mediated by rapid cleavage of GRP78 [70]. Systemic delivery of EGF-SubA resulted in a significant inhibition of human breast and prostate tumor xenografts in mouse models. Additionally, Salazar et al reported that δ-tetrahydrocannabinol, the main active component of marijuana, induces human glioma cancer cell death through stimulation of ER stress-associated autophagy [92]. δ-tetrahydrocannabinol can induce ceramide accumulation and the ER stress response that triggers autophagy through inhibition of the Akt/mammalian target of rapamycin complex 1 axis. The δ-tetrahydrocannabinol-induced autophagic death of human and mouse cancer cells suggested that cannabinoid administration may be an effective strategy for cancer therapy.
Concluding remarks
Significant progress has been made in elucidating the mechanism and role of the ER stress response in oncogenesis and cancer therapy resistance. The related findings have raised an exciting possibility of targeting the UPR components as an effective strategy for cancer therapy and overcoming drug resistance. For future research, it is important to delineate the distinct roles of the UPR branches that may provide survival or death signal in tumorgenesis or cancer therapy. The related information will be essential for pharmaceutical design toward controlling cancer through modulating UPR signaling. Research in this topic will significantly advance our understanding of cancer biology and be informative to its therapeutic application against cancer.
Acknowledgments
The research in the Zhang laboratory is partially supported by the American Heart Association (AHA) Scientist Development Award (0635423Z), the AHA Grant-in-Aid (09GRNT2280479), the Department of Defense Idea Grant (BC095179P1), and the Karmanos Cancer Institute pilot grant. The research in the Yang laboratory is partially supported by the Department of Defense Breast Cancer Program grants (BC083945 and BC083954) and the Karmanos Cancer Institute pilot grant.
References
- 1.Porter KR, Claude A, Fullam EF. A Study of Tissue Culture Cells by Electron Microscopy : Methods and Preliminary Observations. J Exp Med. 1945;81:233–246. doi: 10.1084/jem.81.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 4.McMillian DR, Gething MJ, Sambrook J. The cellular response to unfolded proteins: Intercom-partmental Signaling. Current Opinions in Biotechnology. 1994;5:540–545. doi: 10.1016/0958-1669(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 5.Sidrauski C, Chapman R, Walter P. The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends. Cell Biol. 1998;8:245–249. doi: 10.1016/s0962-8924(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 6.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 8.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 10.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticu-lum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol.Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic -reticulum- resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 13.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymol. 2008;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroder M, Kaufman RJ. The Mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 18.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 19.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman RJ. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci. 2004;29:152–158. doi: 10.1016/j.tibs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 22.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by trans-membrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 25.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 26.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 27.Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CH0P pathway and is involved in cell death. Embo J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 33.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 34.Jordan R, Wang L, Graczyk TM, Block TM, Romano PR. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J Virol. 2002;76:9588–9599. doi: 10.1128/JVI.76.19.9588-9599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 36.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and ge-nomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 37.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic re-ticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–246. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao JR, Chang KC, Chen CW, Wu SY, Su IJ, Hsu MC, Jin YT, Tsai ST, Takada K, Chang Y. Endoplasmic reticulum stress triggers XBP-1-mediated up-regulation of an EBV oncoprotein in nasopharyngeal carcinoma. Cancer Res. 2009;69:4461–4467. doi: 10.1158/0008-5472.CAN-09-0277. [DOI] [PubMed] [Google Scholar]
- 40.Boelens J, Lust S, Offner F, Bracke ME, Vanhoecke BW. Review. The endoplasmic reticulum: a target for new anticancer drugs. 2007;21:215–226. In Vivo. [PubMed] [Google Scholar]
- 41.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 42.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res. 2008;21:358–367. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 45.Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Yoshimastu T, Morita M, Yasumoto K. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Scriven P, Coulson S, Haines R, Balasubramanian S, Cross S, Wyld L. Activation and clinical significance of the unfolded protein response in breast cancer. Br J Cancer. 2009;101:1692–1698. doi: 10.1038/sj.bjc.6605365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara 0, Yamamoto N, Yamamoto M. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepato-carcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 49.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/ C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in trans-gene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 51.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, Wei D, Yao J, Fang S, Xie K. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 53.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, Lee AS, Pinski J. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Cancer Res. 2005;3:669–677. doi: 10.1158/1541-7786.MCR-05-0181. [DOI] [PubMed] [Google Scholar]
- 57.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, Le QT, Koong AC. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tirosh B, Iwakoshi NN, Glimcher LH, Ploegh HL. Rapid turnover of unspliced Xbp-1 as a factor that modulates the unfolded protein response. J Biol Chem. 2006;281:5852–5860. doi: 10.1074/jbc.M509061200. [DOI] [PubMed] [Google Scholar]
- 60.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, Rudland PS, Sibson DR. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 61.Fels DR, Koumenis C. The PERK/elF2alpha/ ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 62.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor elF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, Bell JC. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, Thompson JF, Hersey P. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 66.Tsutsumi S, Namba T, Tanaka Kl, Arai Y, Ishihara T, Aburaya M, Mima S, Hoshino T, Mizushima T. Celecoxib upregulates endoplasmic reticulum chaperones that inhibit celecoxib-induced apoptosis in human gastric cells. Oncogene. 2006;25:1018–1029. doi: 10.1038/sj.onc.1209139. [DOI] [PubMed] [Google Scholar]
- 67.Zu K, Bihani T, Lin A, Park YM, Mori K, Ip C. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene. 2006;25:546–554. doi: 10.1038/sj.onc.1209071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki T, Lu J, Zahed M, Kita K, Suzuki N. Reduction of GRP78 expression with siRNA activates unfolded protein response leading to apoptosis in HeLa cells. Arch Biochem Biophys. 2007;468:1–14. doi: 10.1016/j.abb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Saito S, Furuno A, Sakurai J, Sakamoto A, Park HR, Shin-Ya K, Tsuruo T, Tomida A. Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Res. 2009;69:4225–4234. doi: 10.1158/0008-5472.CAN-08-2689. [DOI] [PubMed] [Google Scholar]
- 70.Backer JM, Krivoshein AV, Hamby CV, Pizzonia J, Gilbert KS, Ray YS, Brand H, Paton AW, Paton JC, Backer MV. Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia. 2009;11:1165–1173. doi: 10.1593/neo.09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 72.Papalas JA, Vollmer RT, Gonzalez-Gronow M, Pizzo SV, Burchette J, Youens KE, Johnson KB, Selim MA. Patterns of GRP78 and MTJ1 expression in primary cutaneous malignant melanoma. Mod Pathol 2009. 2009;152 doi: 10.1038/modpathol.2009.152. doi: 10.1038/modpathol. (in press) [DOI] [PubMed] [Google Scholar]
- 73.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, Lee AS, Cote RJ. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–5993. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 75.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immu-notherapy. Cancer Biol Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 76.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- 77.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 78.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 79.Ling YH, Liebes L, Jiang JD, Holland JF, Elliott PJ, Adams J, Muggia FM, Perez-Soler R. Mechanisms of proteasome inhibitor PS-341-induced G (2)-M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin Cancer Res. 2003;9:1145–1154. [PubMed] [Google Scholar]
- 80.Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171:88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 81.Cusack JC, Jr., Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS., Jr. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 82.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 83.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Schlossman R, Munshi NC, Hideshima T, Anderson KC. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 84.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, Koong AC, Koumenis C. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 2008;68:9323–9330. doi: 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nawrocki ST, Carew JS, Dunner K, Jr., Boise LH, Chiao PJ, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res. 2005;65:11510–11519. doi: 10.1158/0008-5472.CAN-05-2394. [DOI] [PubMed] [Google Scholar]
- 87.Agyin JK, Santhamma B, Nair HB, Roy SS, Tekmal RR. BU-32: a novel proteasome inhibitor for breast cancer. Breast Cancer Res. 2009;11:R74. doi: 10.1186/bcr2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCracken AA, Brodsky JL. Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD) Bioessays. 2003;25:868–877. doi: 10.1002/bies.10320. [DOI] [PubMed] [Google Scholar]
- 89.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Q, Li L, Ye Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J Biol Chem. 2008;283:7445–7454. doi: 10.1074/jbc.M708347200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin-Ya K. Novel antitumor and neuroprotective substances discovered by characteristic screenings based on specific molecular targets. Biosci Biotechnol Biochem. 2005;69:867–872. doi: 10.1271/bbb.69.867. [DOI] [PubMed] [Google Scholar]
- 92.Salazar M, Carracedo A, Salanueva IJ, Hernan-dez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, Gon-zalez-Feria L, lovanna JL, Guzman M, Boya P, Velasco G. Cannabinoid action induces auto-phagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]