Abstract
Naïve and recall CD4+ T cell responses were probed with recombinant influenza A viruses incorporating the ovalbumin (OVA) OT-II peptide. The extent of OT-II specific CD4+ T cell expansion was greater following primary exposure, with secondary challenge achieving no significant increase in numbers despite higher precursor frequencies. Adoptive transfer experiments with OT-II TCR-transgenic T cells established that the predominant memory set is CD62Lhi, while the CD62Llo precursors make little contribution to the recall response. Unlike the situation described by others where the transfer of very large numbers of in vitro activated CD4 effectors can modify the disease process, providing CD62Lhi or CD62Llo OT-II-specific T cells at physiological levels neither enhanced virus clearance nor altered clinical progression. Some confounding effects of the transgenic model were observed, with decreasing primary expansion efficiency correlating with greater numbers of transferred cells. This was associated with increased levels of mRNA for the pro-apoptotic molecule Bim in cells recovered following high dose transfer. However, even with very low numbers of transferred cells, memory T cells did not expand significantly following secondary challenge. A similar result was recorded in mice primed and boosted to respond to an endogenous IAb-restricted epitope derived from the influenza virus hemagglutinin glycoprotein. Depletion of CD8+T cells during secondary challenge generated an increased accumulation of OT-II-specific T cells, but only at the site of infection. Taken together, significant expansion was not a feature of these secondary influenza-specific CD4 T cell responses virus and the recall of memory did not enhance recovery.
Keywords: T cells, Memory, Viral
Introduction
Antigen-specific CD4+ T cells are the central regulators of adaptive immunity, providing help via cell surface receptor contact and the secretion of cytokines to: activate dendritic cells, promote antibody maturation and class-switching as well as provide key factors for the survival and expansion of CD8+ T cells (1, 2). The part played by CD4+ T cells in resolving infection has been analyzed using CD4+ T cell-depleted or MHC class II−/− (MHC II−/−) animals. In a variety of infections, clearance of the pathogen is delayed, the extent of viral pathology is increased and memory B cell and CD8+ T cell responses are impaired (3–5). For example, MHC II−/− mice mount relatively normal CD8+ cytotoxic T lymphocyte (CTL) responses following primary lymphocytic choriomeningitis virus (LCMV) infection. However secondary challenge is characterized by greatly diminished CD8+ T cell expansion, an effect attributed to the aberrant expression of TRAIL on CD8+ memory CTLs generated without CD4+ T help (6). Other studies with Listeria monocytogenes indicate that immune CD4+ T cells secrete an as yet unidentified survival factor that promotes CD8+ T cell memory (7, 8). In both sets of experiments the end result is failure of the CD8+ recall response. The situation for the influenza A viruses is less dramatic: while there is evidence of a partial defect in the absence of a concurrent CD4+ T cell immunity, the CD8+ CTLs still expand and retain the immunodominance profiles characteristic of wildtype (wt) mice (9, 10).
Individual CD4+ T cell expansions from the naïve repertoire generally look to be smaller than the concurrent CD8+ CTL responses, with at least some of the effect being attributed to the greater diversity of MHC II-restricted epitopes (11–14). This, combined with the lack of widely available staining (tetramer or dimer) reagents has resulted in the quantitative analysis of CD4+ T cell responses being relatively under-addressed. In one study following primary infection with a recombinant influenza A virus containing the OVA323–339 peptide (OT-IIp), adoptively transferred TCR-transgenic (Tg) cells replicated and trafficked to the lung and airways (11). The response was generally lower in magnitude than that characteristic for CD8+ CTLs, but was comparable to the endogenous CD4+ T cell response.
Other adoptive transfer studies utilized large numbers of in vitro expanded TCR-Tg CD4+ T cells specific for an A/Puerto Rico/34 (PR8) influenza A virus hemagglutinin epitope (15–19). These experiments indicated that such effectors operate to promote the direct clearance of virus via cytolytic mechanisms. Analysis with this model also showed that memory CD4+ T cells are generated from effector precursors as early as three days following initial stimulation (18), a finding consistent with experiments from our group characterizing the in vivo induction of CD8+ T cell memory (20). Similar studies using Tg or polyclonal in vitro activated CD4+ T cell populations transferred in vivo demonstrated significant differences in the recall potential and effector function of CD62Lhi and CD62Llo subsets (21). More recent experiments have suggested that these differences may be influenced by the amount and quality of co-stimulation following secondary virus challenge (22).
The present analyses utilize wt mice and the OT-II (22) TCR-Tg system (OT-IIT CD4+ T cells) to dissect the characteristics of primary and memory CD4+ T cell responses in prime/boost experiments with recombinant H1N1 (PR8) and H3N2 (HKx31) viruses incorporating the OT-II peptide (H1ova and H3ova) in their respective HA (H1 and H3) proteins (23). The analysis focuses on questions relating to central and effector memory phenotype (based on CD62L and CD44) the extent of clonal expansion, and the capacity to mediate cellular, rather than antibody-mediated, protective immunity that we and others (2, 20) have addressed in great detail for virus-specific CD8+ T cells. By all the criteria analyzed, these two arms of cell-mediated immunity look to be rather different. Secondary expansion of the OT-IIT cells appears severely limited reflecting, perhaps, that the establishment and persistence of memory is in some way compromised by aspects of the TCR-Tg model (24, 25). Indeed, we found that high dose transfers resulted in the upregulation of message for the pro-apoptotic molecule Bim. Since transferring excess TCR-Tg cells can be counterproductive, we tested low number transfers more typical of physiological conditions. This, however, fails to generate a memory base for substantial secondary expansion. Overall, the findings using this TCR-Tg model are consistent with what is seen for endogenous responses to influenza A, suggesting that memory CD4+ T cells play little part in secondary effector responses in this model.
Materials and Methods
Viruses, Mice, and Sampling
Reverse genetics protocols were used to insert the OVA323–339 sequence (ISQAVHAAHAEINEAGR) after the glycines at residue 173 of the H1 (PR8, H1N1) and 174 of the H3 (the A/Aichi HA of HKx31) HA glycoproteins (23). No residues were removed. The resultant H1ova and H3ova viruses were rescued in 10d-old embryonated chicken eggs after the engineered plasmids were transfected into cocultures of 293 T cells and MDCK cells. A known epitope (26) in the H3 HA (I-AbHA192SLYVQASGRVTVSTRR) protein of x31 (SLY1) was inserted into the H1 HA by the same method (H1sly). Female C57Bl/6J (B6) mice were purchased from The Jackson Laboratory, while the OT-II/Thy1.1+ TCR-Tg mice were bred at St. Jude Children’s Research Hospital. All mice were held under specific pathogen-free conditions. Priming with the H1ova or H1sly viruses was by i.p. injection with 108 EID50 of the H1 viruses. Intraperitoneal priming results in aborted replication allowing very high doses to be given, generating robust and consistent cellular memory. Further, this protocol avoids the accumulation of cells in the lungs allowing the examination only of recruited memory responses following challenge and not cells remaining from a primary infection (either innate or adaptive). Intranasal (i.n.) challenge with the H3 viruses was performed following anesthesia by i.p. injection of 2,2,2-tribromoethanol (Avertin), i.n. delivery of 106 EID50 of the H3wt or H3ova viruses as described in the figure legends. The mice were anesthetized again at the time of sampling and exsanguinated by sectioning the axillary artery. Inflammatory cell populations were recovered from the infected respiratory tract by bronchoalveolar lavage (BAL), followed by removal of the mediastinal lymph nodes (MLNs) and spleen to prepare single-cell suspensions for lymphocyte analysis.
Identifying OT-II CD4+ T Cells
The OT-II-specific CD4+ T cell response was analyzed by flow cytometry utilizing a Becton Dickinson FACSCalibur and FloJo software, or a MoFlo sorter for cell separation. Spleen and BAL lymphocytes were stained with Thy1.1 phycoerythrin (PE)(OX-7), CD4 PE-Cy5 (L3T4), and in some cases, Vα2 FITC (B20.1). In some experiments, cells were also stained for CD44 and CD62L. Cells were negatively gated for CD8 and MHCII. The TCR-Tg populations used in cell transfer studies were first labeled with the carboxyfluorescein diacetate succinimidyl ester (CFSE).
Real time RT-PCR analysis
The primers for Bim, Nor1 and GAPDH were described previously (27). RNA from OT-IIT cells sorted at intervals after infection was extracted using Trizol reagent and amplified by a separate reverse transcription step, followed by amplification using ABI SYBR Green MasterMix according to the manufacturer’s instructions. Every sample was analyzed in triplicate for each product on an ABI 7500 instrument with the ddCT Relative Quantitation protocol.
Cell transfers
Cell transfers were done in 0.2 mL PBS via intravenous injection in the lateral tail vein. The numbers of cells transferred are given for each experiment in the figure legends.
CD4+ T Cell ELISPOT
An established ELISPOT assay was used to quantify OT-II- or SLY1-specific IFN-γ-producing CD4+ T cells in spleen after stimulation with SLY1192–207, OVA323–339 peptide or no peptide, and the number of IFN-γ producers was measured as spots per 106 cells after 48 h at 37°C.
Virus titration
Lung homogenates were titered by plaque assay on Madin-Darby canine kidney (MDCK) cells. Near confluent 25-cm2 monolayers of MDCK cells were infected with 1 ml aliquots of diluted lung homogenate for 1 h at 37°C. Cells were washed with PBS, 3 ml of MEM containing 1 mg/ml L-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (Worthington Biochemical). After adding agarose (0.9%), the cultures were incubated at 37°C with 5% CO2 for 72 h. Plaques were visualized with crystal violet.
Statistical analysis
Statistical analyses were performed using InStat (Graphpad) software. In most cases, ANOVA (Kruskal-Wallis) followed by Tukey's post-test or Student's t-test were used to compare results.
Results
Primary and secondary OT-IIT expansion
Questions relating to both naïve and memory CD4+ T cell precursor frequencies are readily probed using adoptively transferred, Thy-1-different OT-IIT populations. The extent of proliferation following primary challenge was analyzed by giving 6×105 naïve OT-IIT cells to wt (Thy1.2+) B6 mice, which were then infected i.n. with variant H3N2 influenza A viruses on the following day. The spleen and BAL populations were harvested and the extent of expansion was assessed for the Thy1.1+ OT-IIT cells by comparing the values resulting from exposure to the H3wt or H3ova virus. Consistent with findings for similar TCR-Tg models, stimulation with the OT-II epitope greatly increased the number of OT-II T cells, representing a 16x expansion over the value found following infection with the wt virus (Figure 1A). In the BAL, the OT-IIT cells dominated the CD4+ T cell component, constituting >50% of those in the airways (Primary, Figure 1B).
Figure 1.
Primary and secondary expansion of transgenic OT-IIT cells. (A) 6×105 OT-IIT cells were transferred i.v. into wt B6 mice, which were challenged i.n. soon after with H3wt (white) or H3ova (black) and the spleens harvested after a further 10d. The values show the relative expansion of the OT-IIT cells (comparing H3ova vs. H3wt infected). Cell number was determined by FACS analysis of Thy1.1+CD4+ cells. (B) Shows the % total CD4+ (left) and CD4+OT-IIT (right) cells in the BAL after primary or secondary infection. (C) 6×105 OT-IIT cells were given i.v. to wt B6 mice, which were infected i.p. with H1wt or H1ova and rested for 50d, when these “memory” mice were harvested for analysis. Identically primed mice were challenged i.n. with either H3wt or H3ova and the spleen 8d later. (D) Representative FACS plots of OT-IIT cells (right panels) in the BAL following primary (upper) or secondary (lower) challenge. Control BAL samples (left panels) from B6 mice infected with wt viruses are shown for comparison. Each panel is representative of two independent experiment with 5 mice per group. The * indicates p<0.05 by ANOVA (Kruskal-Wallis, Tukey's post-test).
To test for the establishment of memory, naïve B6 mice were given 6×105 naïve OT-IIT cells, then infected i.p. with a high dose of the H1ova or H1wt viruses. After 50d the mice primed with the H1wt virus had few detectable OT-IIT cells, while individual spleens from the H1ova group contained substantial numbers (d30, Figure 1C). Groups of these H1ova or H1wt immune mice were then challenged i.n. with the homologous H3ova or H3wt virus, and spleen and BAL populations were harvested 8d later (d8, Figure 1C). While there was some indication (not statistically significant) that OT-IIT memory precursors had increased approximately 3-fold (to 3×105 cells/spleen) in numbers, this was much lower than the expansions that occur normally for CD8+ T cells (see ref (10) and PA and PB1 in Figure 5, below). Furthermore, the OT-IIT set only constituted approximately 10% of CD4+ T cell numbers in the secondary BAL compartment, a considerable drop in relative prevalence from that found following primary challenge (Figure 1D).
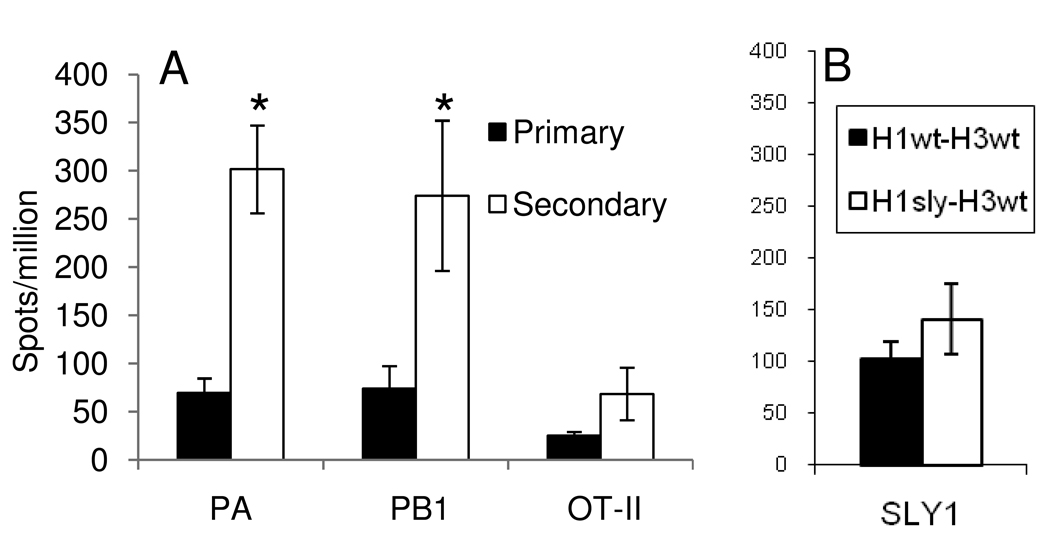
Figure 5.
Limited clonal expansion by endogenous OT-IIe specific cells in wt mice. (a) The wt B6 mice were infected i.n. with H3ova (primary) or primed i.p. with H1ova, rested for at least 30d, then challenged i.n. with H3ova (secondary). Spleens were harvested on day 10 (primary) or day 8 (secondary) and assayed by ELISPOT as described in the Methods. PA and PB1 refer to the DbPA224, and KbPB1703 CD8+ T cell responses, respectively. (b) The wt B6 mice were primed i.p. with H1wt or H1sly, rested for at least 30d, then challenged i.n. with H3wt (which contains the SLY1e). Spleens were harvested on day 8 and assayed by ELISPOT as described in the Methods. Data are representative of at least two independent experiments. The * indicates p<0.05 by ANOVA (Kruskal-Wallis, Tukey's post-test).
Phenotypes of precursor and expanded CD4+ memory T cells and lack of protection
The results presented in Figure 1 indicate that OT-II-specific CD4+ memory T cells show less capacity for further expansion than the comparable virus or OT-I specific CD8+ sets (20). Does this difference correlate in any way with the relative prominence of “effector” (CD62Llo) versus “central” (CD62Lhi) memory precursors (28, 29)? Following adoptive transfer, influenza virus-specific CD44hiCD8+ CD62Lhi and CD62Llo memory T cells both show evidence of a substantial capacity for further expansion and effector function (20). Also, is there evidence that physiological numbers of either “effector’ or “central” CD4+ memory T cells can protect?
The phenotypic and functional characteristics of immune OT-IIT cells were thus analyzed using the experimental plan illustrated at the top of Figure 2. Naive (6×105) OT-IIT cells were transferred to B6 mice, which were then infected i.n with the H3ova virus and “rested’ for at least 30d. Spleen populations were then sorted to isolate the Thy1.1+CD44hi, CD62Lhi or CD62lo subsets, and 1×105 OT-IIT CD44hiCD62Lhi or CD44hiCD62Llo memory T cells were transferred into individual recipients (Figure 2B). Along with controls that were either injected with diluent (PBS) or given an equivalent number of naïve OT-IIT cells, all mice were infected i.n with either a potentially lethal (high path, Figure 2 CD) or readily survivable (low path, Figure 2 EF) dose of the H1ova or H3ova virus, respectively.
Figure 2.
CD62L phenotype correlates with proliferative capacity but not protection. The experimental protocol is illustrated in (A). 1×105 CD44hiCD62Lhi and CD44hiCD62Llo OT-IIT cells were sorted from the spleens of H1ova i.n. primed mice 30d after infection and transferred into naïve recipients. Other mice were given in equivalent numbers of naïve OT-IIT cells. Representative plots of sorted cells are displayed in (B), with the CD4+Thy1.1+CD8−MHC Class II− cells in gated in from the left panel displayed in the right panel stained for CD44 and CD62L (sorting on hi and lo cells). All groups were then challenged i.n. with either a potentially lethal (CD) or sublethal (EF) dose of H1ova (104 EID50) or H3ova (106 EID50) , respectively . The OT-IIT cell counts in the spleen (C,E) for each cell population show greater expansion for the naïve and antigen-experience CD62Lhi vs CD62Llo phenotype. (D,F) Relative weight loss (initial weight normalized to 1) after infection of the different groups of mice. Each panel is representative of at least two independent experiments with 4–5 mice per group. The * indicates p<0.05 by ANOVA (Kruskal-Wallis, Tukey's post-test), compared to naïve transfer.
Following either high (Figure 2C) or low (Figure 2E) path virus challenge, the extent of OT-IIT cell expansion on d8 was found to be no greater for CD44hiCD62Lhi memory T cells than for an equivalent number of naïve precursors, while the smallest counts were found in those given the primed CD44hiCD62Llo set (~1/4 of the naive and CD62Lhi sets). In several experiments, there was no significant difference between the number of cells recovered from the BAL in either the CD44hiCD62Lhi- or CD44hiCD62Llo -recipient animals (data not shown). The numbers recovered for all subsets were lower than from the spleen and the trend (2/3 experiments) was towards higher recovery from the CD44hiCD62Lhi-recipient animals, despite the “tissue tropism” expected of CD44hiCD62Llo cells. Furthermore, neither the immune CD44hiCD62Lhi nor the CD44hiCD62Llo memory T cells conferred any greater protection against virus-induced weight loss (Figure 2 DF), virus growth, or mortality (Table I) than naïve, or no, OT-IIT cells. In addition, all the transferred OT-II T cell subsets gave rise to CD44hiCD62Llo and CD44hiCD62Lhi OT-IIT progeny on d8 (data not shown), with those from the memory CD44hiCD62Llo precursors being least likely to express their starting phenotype after further antigen challenge. Again, though the evidence for greater proliferative capacity of the CD44hiCD62Lhi versus CD44hiCD62Llo T cells is what might be expected from the broader literature in this field, the findings are generally different from the situation found previously for influenza A virus-specific CD8+ T cells (30).
Table I.
Lung titers and mortality in mice receiving CD4 memory or naïve populations following lethal H1ova influenza challengea
| PBS (no cells) | Naïve cells | CD62Lhi | CD62Llo | |
|---|---|---|---|---|
| Day 8 Titer (log10 PFU) |
4.71±0.048 | 4.03±0.15 | 4.21±0.13 | 4.56±0.24 |
| Mortality by day 8 or 30% weight loss |
5/15 (33%) | 4/14 (29%) | 5/14 (36%) | 6/14 (43%) |
Mice were infected with a lethal dose (1 MLD50) of H1ova (104 EID50). Lung titers were determined by MDCK plaque assay. Titers are representative of at least two independent experiments, with 3–5 mice per group. Mortality was determined across three independent experiments. Mice were humanely euthanized after 30% weight loss. No statistical differences were found among the groups by Cox Proportional Hazards test.
Cell number effects on recall efficiency
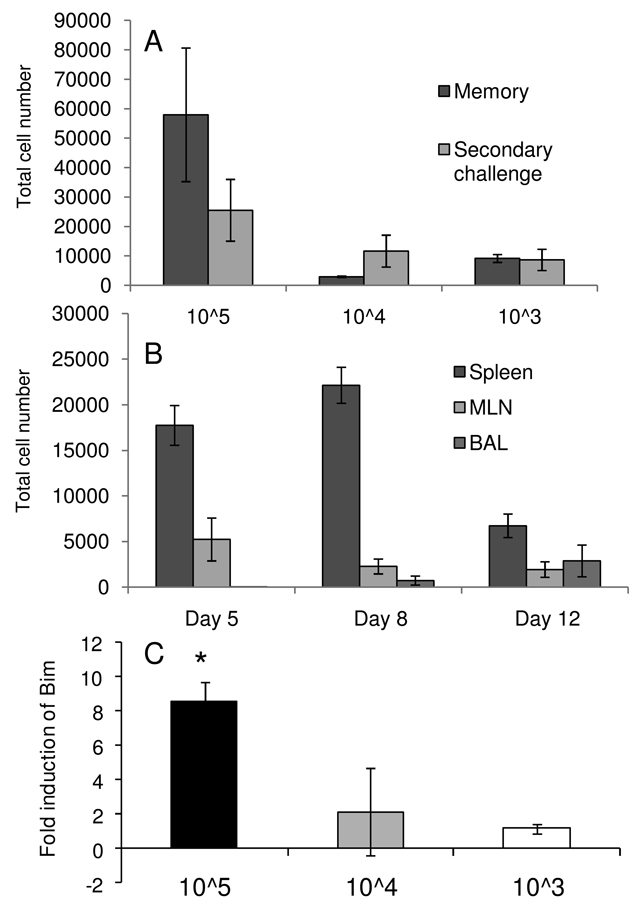
Transferring too many TCR-Tg cells can be counterproductive, as giving lower cell doses can result in greater proliferation and total cell accumulation (27, 31). Similarly, resting CD4+ T cell numbers can decline when large numbers of clonotypic T cells are transferred, while more heterogeneous populations may be maintained (25). To test whether we would see more efficient expansion at smaller cell doses, we gave recipient mice 103, 104, or 105 naïve OT-IIT cells. Mice were infected i.p. with the H1ova virus. Some were sampled 30d later to determine the numbers of OT-IIT memory T cells, while others were challenged i.n. with H3ova on d30 and OT-IIT cells were counted for BAL, MLN and spleen samples taken 8d later. None of the mice showed any evidence of substantial CD4+ T cell expansion from the memory compartment, irrespective of the initial, naive TCR-Tg numbers (Figure 3A). The results were no different from those found for the high dose transfers analyzed in Figure 1. We repeated the analysis of the 103 transfer at additional time points to see if increased expansion might occur at earlier or later time points than previously analyzed (Figure 3B). Non-statistically significant increases were observed between day 5 and the peak of the response in the spleen on day 8, with contraction being obvious by 12. While numbers at day 5 were slightly higher in the MLN, there was little variation across all three time points for any organ.
Figure 3.
Increasing transfer numbers decreases per cell proliferative capacity. (A) 105, 104, or 103 naïve OT-IIT cells were transferred into naïve mice. Mice were primed with H1ova, rested for thirty days and either analyzed for splenic OT-IIT memory responses (memory) or challenged with H3ova (secondary) and analyzed eight days later for splenic OT-IIT expansion. (B) Time course of OT-IIT expansion following secondary infection. 103 naive OT-IIT cells were transferred into naive mice, which were subsequently primed, rested and challenged as in (A). OT-IIT cell were enumerated in the spleen, BAL and MLN on the indicated days after infection. (C) Bim expression is increased in memory OT-IIT cells from high dose transfer. Relative quantitation (ddCT) of Bim levels in d30 memory splenic OT-IIT cells compared to splenic cells isolated on d5 following primary infection, with data normalized to internal controls. Data represent averages of at least three mice per group and are representative of two independent experiments. The * indicates p<0.05 by ANOVA (Kruskal-Wallis, Tukey's post-test).
Despite the poor secondary expansion observed in all three transfer situations, the hierarchy in memory generation efficiency was 103 > 104 > 105, taking into account the expected expansions if these 10x-different cell populations had behaved equivalently (Figure 3A). Based on previous reports (27), we investigated whether key regulators of apoptosis (Bim and Nor1) might be selectively modifying memory T cell homeostasis for the higher dose transfers. Using real time RT-PCR we compared the level of induction of these factors between a primary response on d5 and d30 memory. No difference was observed in Nor1 mRNA levels for the three groups (data not shown), however, Bim levels were significantly elevated in the TCR-Tg memory set recovered from the 105 transfer (Figure 3C). This likely explains some of the inefficiency of the high dose transgenic transfers. Overall though, even at endogenous levels of precursor frequency, no significant memory expansion was observed in secondary OT-IIT responses.
CD8+T cell depletion enhances OT-IIT memory
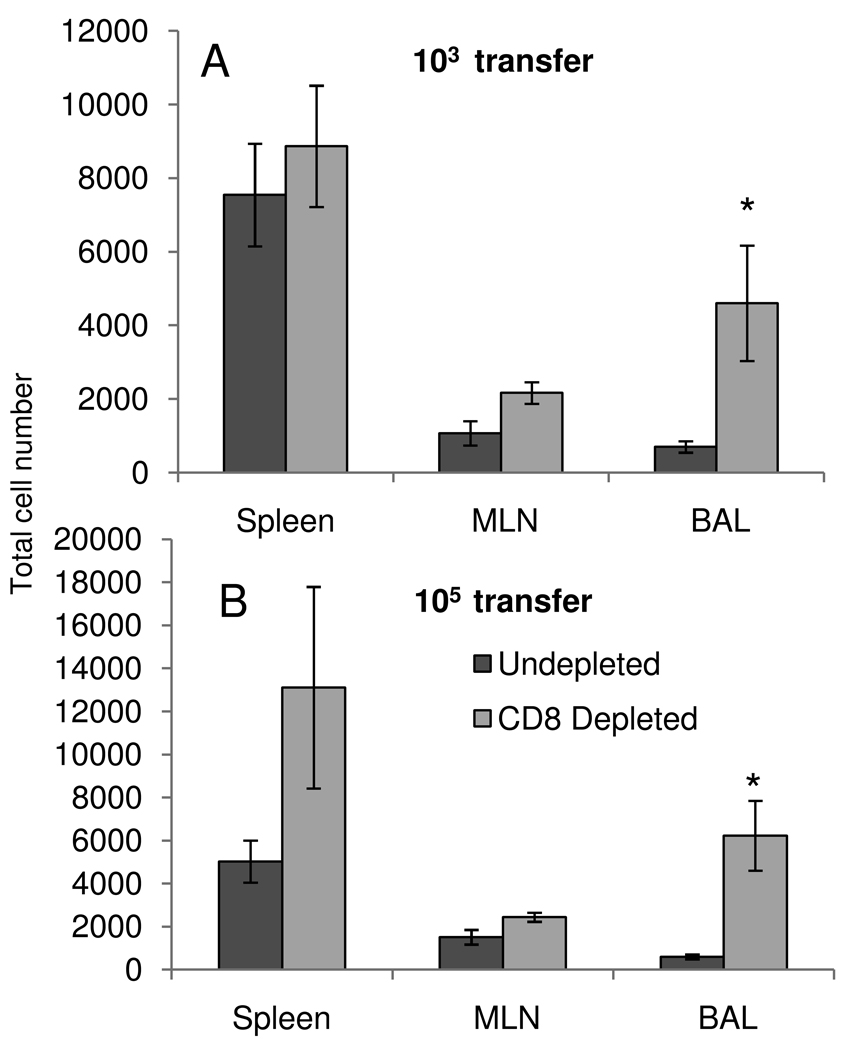
Secondary anti-influenza responses are characterized by effective viral control by cross-reactive, memory CD8+ T cells. Is this efficient cell-mediated clearance limiting the activation and expansion of memory CD4+ T cells? We tested this hypothesis by transferring either 103 or 105 OT-IIT cells into naive animals, priming with H1ova, and resting the animals for 30 days to generate OT-IIT memory cells. We then depleted CD8 T cells from these animals by intraperitoneal administration of an anti-CD8 monoclonal antibody (2.43), followed by infection with H3ova. The antibody was administered twice prior to infection and subsequently on alternating days until d8, when the mice were euthanized and BAL, spleen and MLN were analyzed for OT-IIT expansion (Figure 4). There was a ten-fold increase in the number of OT-IIT cells recovered from the BAL of the 105 transfer group (Figure 4B), while there was an 8-fold increase in the 103 transfer group (Figure 4A). Higher numbers of OT-IIT cells were found in all organs examined, but these differences were only statistically significant in the BAL.
Figure 4.
CD8 depletion enhances OT-IIT accumulation. 103 (A) or 105 (B) OT-IIT cells were transferred into naive mice, which were primed (H1ova), rested, and challenged (H3ova) as in Figure 3. Three days prior to secondary challenge, mice were inject interperitoneally with either an anti-CD8 monoclonal antibody(2.43) or vehicle control. Injections continued on alternating days thereafter. On day 8 after infection, OT-IIT cells were enumerated from the spleen, BAL and MLN. Data represent averages of 3–4 mice/group and two independent experiments. The * indicates p<0.05 by Student's t-test between control and depleted animals.
Endogenous CD4+T cell responses
To determine if the poor secondary expansions observed in our TCR-Tg model could be replicated for an endogenous epitope, we compared the magnitude of the recall, endogenous, “OT-IIe”-specific CD4+ T cell response to that for two subdominant, secondary CD8+ sets (Fig 5A). The CD8+DbPA224 and CD8+KbPB1703 T cell populations were essentially comparable in size following priming and boosting with the H1wt and H3wt or H1ova and H3 ova viruses though, as might be expected, only the latter combination led to the expansion of an OTIIe-specific set. The numbers of antigen-specific CD4+ T cells were, however, significantly lower than the counts for the two CD8+ T cell responses.
An endogenous MHC Class II-restricted epitope, SLY1, derived from the x31 HA, has been described previously (26). We engineered this sequence into the same site on the H1 HA and measured the magnitude of the response (Figure 5B) in mice primed with H1wt and challenged with H3wt (a primary response to SLY1), or mice primed with H1sly and challenged with H3wt (a secondary response to SLY1) The magnitude of this and OT-IIe-specific endogenous responses were comparable, regardless of the priming regimen. There was no statistically significant increase in response magnitude resulting from earlier exposure to this epitope.
Discussion
Analyzing the characteristics of CD4+ T cell expansion, phenotype and possible effector function following primary or secondary challenge with engineered influenza A viruses has thus shown intriguing differences from the more familiar CD8+ CTL response profiles (32, 33). While CD4+ OT-IIT cells respond robustly on primary infection with OT-IIp+ influenza A viruses (expanding 16×) the extent of further proliferation following secondary challenge is much less (approximately 3× and not significant). This is very different from the established profile for most influenza A virus specific CD8+ T cell responses (10, 34). Perhaps, though the OT-II system has been widely used (20), OT-IIe is either a very “poor” epitope or the OT-IIT TCR is sub-optimal. This would, however, also need to be true for the “native” H3SLY1 response. As a general point, there is still a great deal to learn about what constitutes “best fit” for peptide+MHC complexes and TCRs, and how that in turn translates into response profiles.
The possibility of intraclonal competition limits the wide applicability of TCR-Tg models and emphasizes the need to transfer physiological numbers of cells (25, 31). Phenotypic differences can be induced by varying the cell dose, suggesting that a compromised response profile (including functional impairment) can be a consequence of giving too many TCR-Tg cells (24). In this influenza virus model, transferring decreasing numbers of TCR-Tg cells did increase the efficiency of memory formation, though not to the dramatic extent described for the LCMV system where a 100× lower cell dose can expand to higher absolute counts (31). The moderate increase in expansion efficiency observed in our transfer experiments may be partially explained by enhanced message levels for the pro-apoptotic molecule Bim found in “memory” cells generated following high dose transfer. Combined with previous reports using LCMV, this suggests a universal apoptosis “enhancement” mechanism for minimizing clonal dominance in CD4+ T cell populations. Studies showing a role for self-MHCII complexes in homeostatic proliferation and survival suggest one mechanism by which high doses of TCR-Tg T cells may compete for a scarce resource (specific self-MHCII complexes), resulting in apoptosis (35, 36). Even so, despite this effect, secondary expansion was severely compromised for even low dose OT-IIT transfers, a finding replicated with the endogenous SLY1 epitope.
Within the OT-IIT memory CD4+ population, the “central memory” CD62LhiCD44hi subset showed a substantially greater capacity for further expansion than the CD62LloCD44lo “effector memory” subset. In fact, the sorted CD62Lhi OT-IIT cells behaved much like the more potent naïve set following challenge with a lethal virus. Furthermore, the CD44/CD62L partitioning of CD4+ OT-II T cell memory contrasts with comparable experiments using endogenous influenza A virus-specific CD8+ memory CTLs, which showed no difference in “transfer capacity” for the “central” and “effector” populations defined by the CD62L marker (20). In that study, the “optimal” CD8+ memory T cells were found to localize preferentially to the draining MLN which, when compared to the spleen, contained more potent CD62Lhi and CD62Llo memory CTL precursors at all time points tested. However, when we tested CD4+ OT-IIT memory populations from the MLN and the spleen, there appeared to be no significant advantage for the lymph node precursors (data not shown).
Direct intratracheal transfer of effector CD4+ T cell populations from Sendai- or influenza-infected lung showed some efficacy in reducing viral titers following challenge. This correlated with a rapid production of IFN-γ and was based on the transfer of 5×105 polyclonal effectors (37). It may be that the limited memory numbers generated in our system did not provide enough cytokine support to generate a protective effect. Overall, the numbers of CD4+ OT-IIe specific T cell memory generated in this system provided no protection against virus-induced pathology (as measured by weight loss and mortality) and caused no increase in the magnitude of concurrent CD8+ T cell responses (data not shown). This contrasts with findings from a Sendai virus pneumonia model that shows robust CD4+ T cell recall responses following in vivo virus challenge, and with an influenza-based experimental system where very large numbers of in vitro stimulated, TCR-Tg or polyclonal CD4+ memory T cells are transferred (15, 21, 38). In these instances, CD4+ effectors seemed to contribute some measure of protection and even mediate virus clearance. Other experiments (32) that did not use this in vitro culture step showed no evidence, however, that influenza-specific CD4+ T cells can control virus in the absence of antibody.
Might the relative CD4+ T help independence of influenza-specific CD8+ T cell responses, when compared with other viral systems, be a function of a relatively weak CD4+ T cell response? The robust CD8+T cell secondary response is capable of controlling infection and in its absence we did observe increased numbers of OT-IIT cells in the BAL. However, the numbers in other organs were only slightly elevated and this was not statistically significant. It does suggest some mechanism for competitive exclusion of OT-IIT cells being exerted by CD8+T cells, possibly by the removal of antigen.
Alternatively, others have concluded that long-lived CD4+ T cell memory is not a general feature of immunity, especially where CD4+ T help is relatively dispensable for potent effector recall responses (39, 40). These studies came to similar conclusions as our own, showing that memory CD4+ T cells proliferate poorly in comparison to naive cells in an immunization based model or in response to systemic LCMV infection. Increased CD4+T cell secondary responses thus are only a function of higher precursor frequency in the memory compartment, though LCMV infection during challenge did drive stronger memory CD4+ T cell proliferation than immunization. Our experiments have extended these findings to a localized infectious model system where, in contrast to LCMV, we have shown relatively robust CD8+ T cell recall responses in CD4-deficient animals (though the memory compartment itself does appear partially compromised) (9, 10, 41). Perhaps the strong priming environment provided by the inflammatory milieu in influenza virus infection allows comparatively poor CD4+ T cell memory and functionally independent CD8+ T cell responses. This hypothesis warrants further investigation.
Acknowledgments
The authors thank Rachael Keating and Katherine Kedzierska for discussion of experiments and the manuscript.
Abbreviations
- BAL
bronchoalveolar lavage
- CTL
cytotoxic T lymphocyte
- EID50
egg 50% infective dose
- HA or H
hemagglutinin
- H1ova and H3ova
H1N1 and H3N2 influenza A viruses engineered to express the OTII epitope
- H1wt and H3wt
wildtype H1N1 (PR8) and H3N2 (HKx31) viruses
- i.n.
intranasal(ly)
- MLN
mediastinal lymph node
- N
viral neuraminidase
- MDCK
Madin–Darby canine kidney
- OT-IIp
ISQAVHAAHAEINEAGR peptide
- SLY1p
SLYVQASGRVTVSTR peptide
- OT-IIe
H2IAb+OT-IIp epitope
- SLY1e
H2IAb+SLY1p
- OT-IIT
TCR transgenic CD4+ T cells specific for the OT-II epitope
- Tg
transgenic.
Footnotes
This study was supported by National Institutes of Health Grants AI70251 (to P.D.), AI95357 (to R.W.), and AI065097 (to P.T.), by funds from the Australian National Health and Medical Research Council, and by private donations to ALSAC at St. Jude Children’s Research Hospital.
Reference List
- 1.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen JP, Bartholdy C, Wodarz D, Thomsen AR. Depletion of CD4+ T cells precipitates immunopathology in immunodeficient mice infected with a noncytocidal virus. J Immunol. 2001;166:3384–3391. doi: 10.4049/jimmunol.166.5.3384. [DOI] [PubMed] [Google Scholar]
- 5.Su HC, Cousens LP, Fast LD, Slifka MK, Bungiro RD, Ahmed R, Biron CA. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J Immunol. 1998;160:5007–5017. [PubMed] [Google Scholar]
- 6.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 7.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas PG, Brown SA, Keating R, Yue W, Morris MY, So J, Webby RJ, Doherty PC. Hidden epitopes emerge in secondary influenza virus-specific CD8+ T cell responses. J Immunol. 2007;178:3091–3098. doi: 10.4049/jimmunol.178.5.3091. [DOI] [PubMed] [Google Scholar]
- 11.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Crowe SR, Miller SC, Brown DM, Adams PS, Dutton RW, Harmsen AG, Lund FE, Randall TD, Swain SL, Woodland DL. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine. 2006;24:457–467. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 13.Richards KA, Chaves FA, Krafcik FR, Topham DJ, Lazarski CA, Sant AJ. Direct ex vivo analyses of HLA-DR1 transgenic mice reveal an exceptionally broad pattern of immunodominance in the primary HLA-DR1-restricted CD4 T-cell response to influenza virus hemagglutinin. J Virol. 2007;81:7608–7619. doi: 10.1128/JVI.02834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell TJ, Brown DM, Hollenbaugh JA, Charbonneau T, Kemp RA, Swain SL, Dutton RW. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clin Immunol. 2004;113:89–100. doi: 10.1016/j.clim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 16.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 17.Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL. Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med. 2005;201:1101–1112. doi: 10.1084/jem.20041852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Farber DL. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc Natl Acad Sci U S A. 2002;99:11802–11807. doi: 10.1073/pnas.192263099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol. 2007;179:64–70. doi: 10.4049/jimmunol.179.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadzadeh M, Hussain SF, Farber DL. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J Immunol. 2001;166:926–935. doi: 10.4049/jimmunol.166.2.926. [DOI] [PubMed] [Google Scholar]
- 22.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 23.Thomas PG, Brown SA, Yue W, So J, Webby RJ, Doherty PC. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006;103:2764–2769. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 26.Arnold PY, Vignali KM, Miller TB, La Gruta NL, Cauley LS, Haynes L, Scott Adams P, Swain SL, Woodland DL, Vignali DA. Reliable generation and use of MHC class II:gamma2aFc multimers for the identification of antigen-specific CD4(+) T cells. J Immunol Methods. 2002;271:137–151. doi: 10.1016/s0022-1759(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 27.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 29.Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, Artis D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitmire J, Benning KN, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008;180:6777–6785. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topham DJ, Doherty PC. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 34.Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J Immunol. 2001;166:4627–4633. doi: 10.4049/jimmunol.166.7.4627. [DOI] [PubMed] [Google Scholar]
- 35.Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4(+) T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown SA, Hurwitz JL, Zirkel A, Surman S, Takimoto T, Alymova I, Coleclough C, Portner A, Doherty PC, Slobod KS. A recombinant Sendai virus is controlled by CD4+ effector T cells responding to a secreted human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 2007;81:12535–12542. doi: 10.1128/JVI.00197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 40.MacLeod MK, McKee A, Crawford F, White J, Kappler J, Marrack P. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci U S A. 2008;105:14521–14526. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]