Abstract
The prevalence of hypertension is increased in winter and in cold regions of the world. Cold temperatures make hypertension worse and trigger cardiovascular complications (stroke, myocardial infarction, heart failure, etc.). Chronic or intermittent exposure to cold causes hypertension and cardiac hypertrophy in animals. The purpose of this review is to provide the recent advances in the mechanistic investigation of cold-induced hypertension (CIH). Cold temperatures increase the activities of the sympathetic nervous system (SNS) and the renin-angiotensin system (RAS). The SNS initiates CIH via the RAS. Cold exposure suppresses the expression of eNOS and formation of NO, increases the production of endothelin-1 (ET-1), up-regulates ETA receptors, but down-regulates ETB receptors. The roles of these factors and their relations in CIH will be reviewed.
Keywords: cold-induced hypertension, cold-induced cardiac hypertrophy, cold, blood pressure, sympathetic nervous system, renin-angiotensin system, endothelin, mineralocorticoid receptor, eNOS, c-myc
2. INTRODUCTION
Of the four seasons, the cold winter has the highest mortality and morbidity from cardiovascular complications (1–10). The prevalence of hypertension is increased in cold regions or in winter (1,4–8,10–12). Cold winters increase the severity of hypertension and trigger myocardial infarction and stroke in hypertensive patients (1,2,4,8,13–17). Therefore, it is important to study how cold temperatures cause cardiovascular diseases. Studies from our laboratory have shown that chronic exposure of rats to cold (5°C) for 1–3 weeks is accompanied by a significant elevation of resting (systolic, diastolic and mean) blood pressure (BP), tachycardia and cardiac hypertrophy (19–23). These signs characterize the development of the syndrome of hypertension during chronic exposure to cold, namely cold-induced hypertension (CIH). Chronic exposure to cold also induces hypertension in dogs, rabbits, sheep, and young oxen (24,25). The elevated BP of rats after 7 weeks of exposure to cold did not return to pre-cold exposure level even after discontinuance of cold treatment for 4 weeks (26). Thus, an elevation of BP induced by a longer period of cold exposure might not be reversible after return to a thermo-neutral temperature. Intermittent exposure of rats to cold also induced hypertension, with a sigmoid relationship between the hours per day exposed to cold and systolic BP (27). It should be mentioned that elevation of BP is an important cardiovascular response to cold exposure, which provides enhanced circulatory function for the increased non-shivering thermogenesis and metabolic rate for the purpose to maintain body temperature. Indeed, rats are able to maintain their core temperatures constant during exposure to cold (5±2°C) (21,28,29). In this sense, elevation of BP is an adaptive response to cold exposure. However, constantly elevated BP could cause cardiovascular and renal damage (e.g., cardiac and renal hypertrophy) seen in cold-exposed animals (18,19,21). Therefore, CIH is detrimental to the long-term cardiovascular health of cold-exposed subjects. To date, the pathogenesis of cold-induced hypertension (CIH) is not fully understood. Recent studies indicate that the mechanism of CIH may involve: the sympathetic nervous system (SNS), the renin-angiotensin-aldosterone system (RAS), nitric oxide (NO), and endothelin-1. Cold-induced cardiac hypertrophy (CICH), however, may be due to up-regulation of cell growth-related transcription factors.
3. RECENT ADVANCES IN THE SNS AND THE RAS IN CIH
The levels of circulating and urinary epinephrine and norepinephrine (NE) are increased significantly in cold-exposed rats (19,22,23,30,31,32). Thus, the sympatho-adrenal system is activated by chronic cold exposure. Our studies showed that central imidazoline and angiotensin II (AngII) receptors and hypothalamic mineralocorticoid receptors may be involved in the cold-induced activation of the SNS (22,23,33). The activated SNS may initiate CIH because therapeutic inhibition of the SNS or bilateral denervation of the renal sympathetic nerve prevented the development of CIH (22,23,32). However, both in vitro vascular contractile responsiveness and the in vivo pressor response to α1-adrenergic agonists are decreased significantly in cold-exposed animals (34–37), suggesting that cold exposure down-regulates vascular α1-adrenoceptors. Therefore, α1-adrenoceptors are not critical for SNS mediation of CIH.
On the other hand, cold exposure increases plasma renin activity and AngII formation (32,38), suggesting that the RAS is activated by cold exposure. Blockade of the RAS at different levels could prevent or attenuate cold-induced elevation of BP (32,39,40). For examples, a reduction of renin secretion (32), blockade of AT1 receptors (39), or inhibition of angiotensin I converting enzyme (40) abolishes the cold-induced increase in BP. Thus, the RAS may play a critical role in the development of CIH. Knockout of angiotensinogen gene delays and attenuates cold-induced elevation of BP despite elevated plasma level of NE (41), suggesting that the SNS initiates CIH via activation of the RAS (22,23,32,41). Indeed, inhibition of the SNS abolishes or attenuates the cold-induced increase in plasma renin activity and the RAS (22,23,32). The roles of renin and AT1 receptors in CIH have been evaluated by Wang et al (42) and Sun et al (43). Antisense inhibition of renin secretion by adenoviral delivery of renin antisense gene abolishes the cold-induced increases in renin, AngII and aldosterone and prevents the development of CIH (42). This result suggests that renin, the first component of the RAS, plays a critical role in the development of CIH. This study also demonstrates that antisense inhibition of renin may be an ideal approach for the control of CIH and other high renin-related hypertension because it suppresses the entire RAS. Knockout of the AT1A receptor gene delays and attenuates cold-induced elevation of BP (43), indicating that this receptor subtype mediates the role of the RAS in CIH. The most recent study showed that RNAi inhibition of mineralocorticoid receptors (MR) prevented the development of CIH (44). Interestingly, inhibition of MR abolished the cold-induced increases in plasma NE, plasma renin activity, and plasma aldosterone (44), suggesting a suppression of the SNS and the RAS. The suppression of the SNS was probably due to the inhibition of central MRs. Indeed, AAV delivery of MR-shRNA decreased the cold-induced up-regulation of MR in the hypothalamus (44), suggesting that hypothalamic MR may also be involved in the cold-induced activation of the SNS.
4. NITRIC OXIDE AND CIH
Nitric oxide (NO) is an important vasodilator that is involved in the regulation of blood pressure and endothelial function. Recent studies indicated that the plasma and urine levels of nitrite and nitrate, an index of NO, were decreased in cold-exposed animals (41,43), indicating that cold exposure suppressed the production of NO. The cold-induced decrease in NO production disappeared in angiotensinogen gene knockout mice (41), suggesting that the cold-induced suppression of NO production may be mediated by the RAS. A recent study revealed that eNOS protein expression was inhibited in the heart and aorta in wild-type mice (43). Interestingly, cold exposure failed to decrease eNOS expression in AT1A receptor knockout mice (43). These results further suggest that the inhibitory effect of cold exposure on eNOS expression may be mediated by AT1A receptors. The AT1 receptors may mediate cold-induced inhibition of eNOS because activation of AT2 receptors stimulates the formation of NO (45,46). It should be mentioned that data obtained from gene knockout animals may be complicated by associated compensatory changes. Thus, the hypotheses that the RAS and AT1A receptors may be involved in cold-induced suppression of NO production and eNOS expression need to be further tested in normal animals using appropriate blockade of the RAS.
To determine the potential role of the NO system in the development of CIH, Wang et al evaluated the effect of viral delivery of human eNOS gene on CIH (47). This study demonstrated that human eNOS gene delivery increased NO production and attenuated cold-induced elevation of blood pressure. Therefore, the NO system may be involved in the initiation and development of CIH. Interestingly, systemic eNOS gene delivery decreased plasma NE in cold-exposed rats (47), which suggests that the increased NO production may suppress the activity of the SNS. Notably, the cold-induced reduction in NO production was accompanied by an increase in the SNS activity. These results suggested that NO may be the mediator of cold-induced activation of the SNS. It has been reported that a decrease in central NO increases the SNS activity (48,49). On the other hand, NO could inhibit the release of norepinephrine in the brain (50). Indeed, eNOS gene delivery increased NO production in rostral ventrolateral medulla (rVLM), the cardiovascular center, in cold-exposed rats (47). In addition, eNOS gene delivery decreased plasma renin activity, probably due to the suppression of the SNS activity (47). Therefore, the NO system may mediate the role of the SNS in CIH.
5. COLD EXPOSURE AND THE ENDOTHELIN SYSTEM
The endothelin (ET) system plays an important role in the regulation of vascular tone, blood pressure (BP), myocardial contractility, fluid balance, and hemodynamics (51,52). The ETs are a group of vasoconstrictor peptides derived from vascular endothelial cells (53,54). Three ETs have been identified, ET-1, -2, and -3, all consisting of 21 amino acids. ET-1, the predominant representative of the ET family, is the most potent natural mammalian vasoconstrictor agent yet discovered (30–40 times stronger than AngII) (54), and is essential for cardiovascular regulation (52,54,55). The action of ET-1 is mediated by ET receptors. There are two types of ET receptors, ETA and ETB. ETB receptors can be further classified as ETB1 and ETB2 subtypes according to their distribution and function. The vasoconstrictive effect of ET-1 is mainly mediated by endothelin receptor type A (ETA) which increases intracellular calcium concentration (56). ET-1 also possesses vasodilatory effect (57), an action mediated by ETB receptors in endothelial cells (ETB1). ETB receptors have been found in VSMC (ETB2) and can mediate vasoconstriction and proliferation in multiple blood vessels.
A recent study indicated that chronic cold exposure increased ET-1 levels in cardiovascular and renal tissues (58). The most dramatic increase in ET-1 occurred in mesenteric resistance arteries as early as one week after exposure to cold when BP begins to rise (58), suggesting that ET-1 may contribute to the initiation of CIH. However, chronic exposure to cold did not alter plasma levels of ET-1. This is in contrast with acute cold exposure (minutes or hours) which increases plasma ET-1 levels (59,60). It should be emphasized that ET-1 is produced in endothelial cells and predominantly secreted toward the adjacent VSMC, supporting the notion that ET-1 is an autocrine/paracrine agent rather than a circulating hormone. Therefore, tissue levels of ET-1 are more important than plasma levels of ET-1 in assessing the contribution of the ET system to CIH. Physiologically, an increase in BP inhibits vascular ET formation. Therefore, the cold-induced increase in ET-1 production, at least during the early stage of cold exposure, is not due to hypertension-associated endothelial damage because CIH is not fully established until 5 weeks after exposure to cold (61). Thus, the cold-induced increase in ET-1 production may be due to endocrine changes associated with cold exposure, e.g., the activated RAS.
A previous study indicated that chronic cold exposure also increased ET-1 production in the heart (58). Interestingly, cold exposure increased ETA receptor expression but decreased ETB receptor expression in the heart which resulted in an increase in the ratio of ETA/ETB (58). It has been reported that the activation of the ET system can contribute to cardiac hypertrophy (62,63). ET-1 added directly to the cultured cardiomyocytes increases the size of the cells and increases the actin production (64). ET-1, produced locally by cardiomyocytes, is an important mediator for myocardial hypertrophy induced by thyroid hormone (65). Although ET-1 levels in the heart were not elevated until 5 weeks after exposure to cold, the ratio of cardiac ETA/ETB was markedly increased as early as 1 week of exposure to cold (58). It has been reported that the ETA receptor-mediated action plays an important role in the pathogenesis of DOCA-salt-induced hypertension and cardiac hypertrophy (66). However, the ETB receptor-mediated action protects against vascular and end organ damage in this model of hypertension. An increase in the ratio of ETA/ETB points out the necessity to evaluate if the alteration of the cardiac ET system is involved in CICH.
Chen and Sun reported that the renal cortex was predominately occupied by ETA receptors while the renal medulla (in the tubules) has a high abundance of ETB receptors (58). ETA receptors were up-regulated in renal cortex while ETB receptors were down-regulated in renal medulla in cold-exposed rats (58). Cold exposure increased ET-1 levels predominately in kidney cortex (58). An increase in ET-1 levels in the renal cortex decreases renal blood flow (RBF) and glomerular filtration rate (GFR) (51,67), resulting in antidiuresis and antinatriuresis. This effect is mediated by cortical vasoconstrictor effects of ETA receptors which were increased by cold exposure. In the renal medulla, however, ET-1 inhibits Na+-K+-ATPase activity and blocks the stimulatory effects of vasopressin on water reabsorption and thereby inducing natriuresis and diuresis (51,68,69). This effect is mediated by ETB receptors through increasing NO release and intracellular cGMP levels (70). The medullary ETB receptor protein expression was decreased throughout exposure to cold. Thus, the differential regulation of the ET system in the cortex and medulla tends to increase reabsorption of Na+ and water, thereby causing fluid retention which is seen in cold-exposed rats (71). ET-1 has proliferative effects, so it will be interesting to test if ET-1 contributes to cold-induced renal hypertrophy.
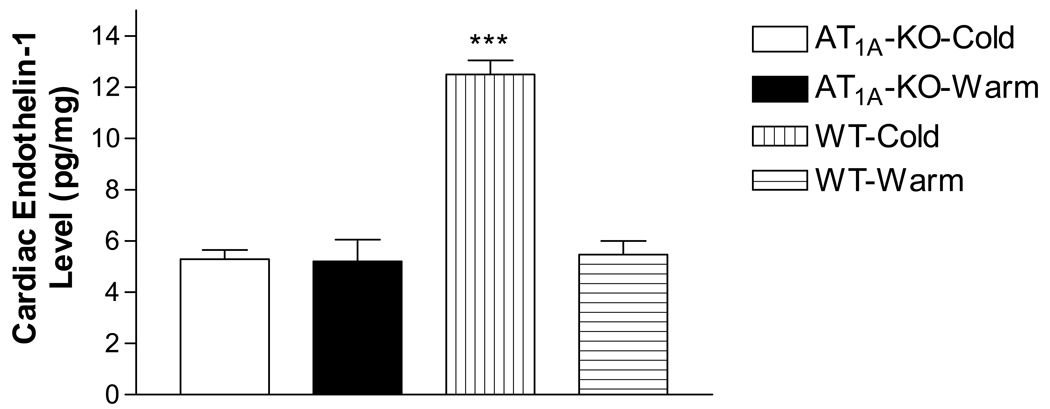
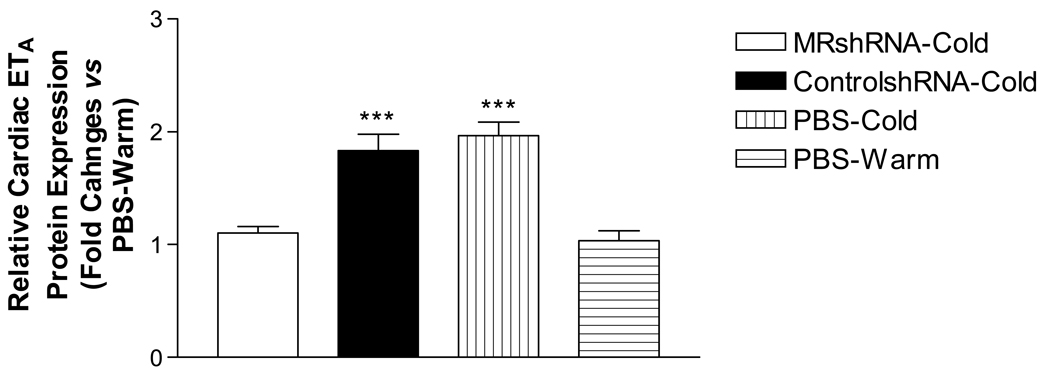
A recent study indicated that knockout of AT1A receptor gene significantly decreased the cardiac ET-1 in cold-exposed rats (Fig. 1), suggesting that the cold-induced increase in ET-1 production may be mediated by the RAS via AT1A receptors. The cold-induced down-regulation of ETB receptors in the heart and renal medulla was likely due to the increased ET-1 levels in these tissues. Interestingly, cold exposure up-regulated ETA receptors in the heart and renal cortex against the background of the increased ET-1 levels. This unique regulation of ETA receptors was probably mediated by mineralocorticoid receptors (MR). Indeed, RNAi inhibition of MR abolished the cold-induced up-regulation of ETA receptors in the heart (Fig. 2), suggesting an important role of MR in the regulation of ETA receptors during cold exposure. Future studies are warranted to evaluate the role of the ET system in the pathogenesis of CIH.
Figure 1.
Effects of knockout of AT1A receptor gene (AT1A-KO) on the cold-induced increase in cardiac endothelin-1 production. The measurement was done in mice expose to cold (5°C) for 5 weeks. AT1A-KO-Cold, AT1A-KO mice exposed to cold (5±2°C); AT1A-KO-Warm, AT1A-KO mice maintained at room temperatures (warm, 25±2°C); WT-Cold, wild type mice maintained at cold; WT-Warm, wild type mice maintained at room temperatures (warm). ***p<0.001 vs the WT-Warm group. N=6.
Figure 2.
Effects of RNAi inhibition of mineralocorticoid receptors (MR) on the cold-induced increase in cardiac ETA receptor protein expression. The measurement was done in mice exposed to cold for 32 days. RNA interference (RNAi) is a molecular technique used to inhibit a target protein expression; siRNA, small interference RNA; MRshRNA-Cold, mice treated with short hairpin siRNA for MR and exposed to cold (5±2°C); ControlshRNA-Cold, mice treated with scrambled short hairpin siRNA sequence and exposed to cold; PBS-Cold, mice treated with phosphate buffered solution and exposed to cold; PBS-Warm, mice treated with PBS and maintained at room temperatures (warm, 25°C). ***p<0.001 vs the PBS-Warm group. N=6.
6. COLD-INDUCED CARDIAC HYPERTROPHY (CICH)
It is interesting to note that prevention or attenuation of CIH does not attenuate cold-induced cardiac hypertrophy (CICH) (31,32,39,40,41,43), indicating that CICH is independent of elevation in BP. Numerous studies have shown that blockade of α- or β-adrenergic receptors (19,31), inhibition of the RAS at different levels (39,40,41,43), or knockout of angiotensinogen or AT1A receptor gene (41,43) does not attenuate CICH although these treatments prevent or attenuate CIH and tachycardia. Therefore, the SNS or the RAS is not be involved in CICH although they may play a role in other types of cardiac hypertrophy. Therefore, CICH may be caused by changes in endocrine factors associated with cold exposure. Genetic deletion of AVP gene does not attenuate CICH, indicating that AVP may not be involved in the pathogenesis of CICH (38). Thus, the endocrine mechanism mediating CICH remains to be explored.
The protooncogene c-myc is involved in the regulation of cell proliferation and growth and its role in tumorigenesis has been studied extensively (73,74). A recent study showed that c-myc protein expression was increased significantly in the heart at 1, 3, and 5 weeks after exposure to cold, which was correlated with the development of CICH (72). This study further showed that heart-specific suppression of c-myc expression abolished the cold-induced increase in heart wall thickness, heart weight and cardiomyocyte size. Because myocytes are terminally differentiated cells, cellular hypertrophy is their unique response to hypertrophic stimuli (e.g., cold exposure). CICH was mainly characterized by myocyte hypertrophy (overgrowth of myocytes) (72). Antisense inhibition of cardiac c-myc expression prevented the cold-induced increase in myocyte size, suggesting that cold-induced myocyte hypertrophy was mediated by the increased c-myc expression. It has been reported that the circulating levels of thyroid hormones (T3 and T4) are increased in response to cold exposure (75). An increase in the production of thyroid hormones increases the non-shivering thermogenesis, which is essential for cold-exposed animals to maintain their body temperatures. Therapeutic inhibition of thyroid hormones attenuates CICH (75). A cell culture study indicated that thyroid hormone-induced myocyte hypertrophy requires p38 mitogen-activated protein kinase (p38MAPK) (76). p38MAPK activates transcription factors including c-myc (77). Therefore, a further study is warranted to test the hypothesis that the cold-induced increase in c-myc expression is mediated by the thyroid hormone-p38MAPK pathway.
7. CONCLUDING REMARKS
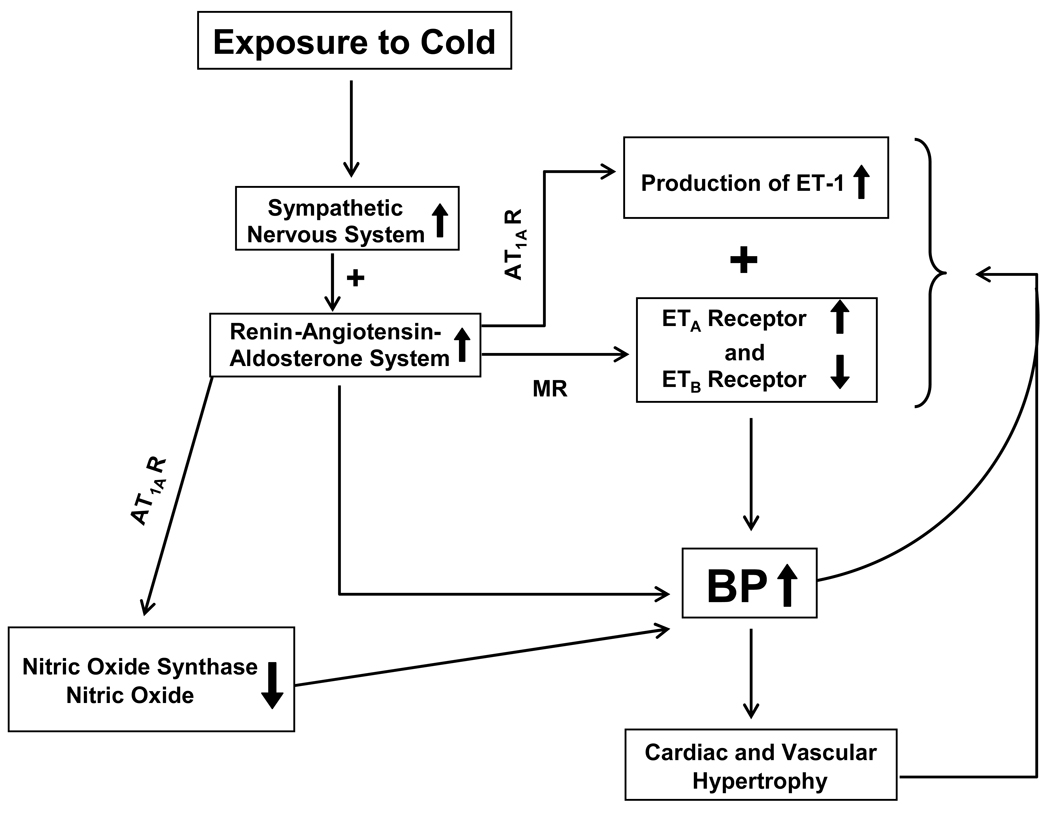
Cold temperatures have adverse effects on the human cardiovascular system. Animals develop hypertension and cardiac hypertrophy during exposure to cold. Cold exposure activates the SNS which, in turn, increases the activity of the RAS (Fig. 3). The RAS suppresses eNOS expression and decreases NO production which contributes the development of CIH. The RAS also mediates the cold-induced increase in ET-1 production. Cold exposure up-regulates ETA but down-regulates ETB receptors. This unique pattern of changes in the ET system may be involved in the development of CIH. The relationship of the SNS, the RAS, the ET system and the NO system in the development of CIH is summarized in Figure 3. The mechanism of CICH may be different from that of CIH. The development of CICH is disassociated with CIH and is independent of the SNS and the RAS. The protooncogene c-myc is up-regulated in the hearts of cold-exposed rats, which may mediate CICH. The potential role of thyroid hormones in the cold-induced up-regulation of c-myc needs to be evaluated. CIH and CICH are prototypic models of environmentally-induced hypertension and hypertrophy, which are induced without surgical intervention, genetic manipulation or large doses of drugs or hormones.
Figure 3.
A diagram to explain the development of cold-induced hypertension (CIH). AT1AR, angiotensin II type 1A receptor; ET-1, endothelin-1; ETA Receptor, endothelin type A receptor; ETB Receptor, endothelin type B receptor.
ACKNOWLEDGEMENTS
This work was supported by NIH R01 HL-077490 and AHA GIA 0655257B.
Abbreviations
- CIH
cold-induced hypertension
- RAS
renin-angiotensin system
- SNS
sympathetic nervous
- CICH
cold-induced cardiac hypertrophy
- MR
mineralocorticoid receptor
- siRNA
small interference RNA
- RNAi
RNA interference
- NE
norepinephrine
- eNOS
endothelial nitric oxide synthase
- VSMC
vascular smooth muscle cell
REFERENCES
- 1.Arjona-Castro A, Arjona J. Cerebrovascular stroke, the cause of the death of the caliph al-Hakam II. Neurologia. 1997;12(2):78–81. [PubMed] [Google Scholar]
- 2.Baker-Blocker A. Winter weather and cardiovascular mortality in Minneapolis-St. Paul. Am J Public Health. 1982;72(3):261–265. doi: 10.2105/ajph.72.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caicoya M, Rodriguez T, Lasheras C, Cuello R, Corrales C, Blazquez T. Stroke incidence in Austrias, 1990–1991. Rev Neurol. 1996;24:806–811. [PubMed] [Google Scholar]
- 4.He BL. Epidemiological characteristic of stroke in 1985–1989, Beijing. Chung Hua I Hsueh Tsa Chin Taipei. 1993;73:104–108. [PubMed] [Google Scholar]
- 5.Jakovljevic D, Salomaa V, Sivenius J. Seasonal variation in the occurrence of stroke in a Finnish adult population. Stroke. 1996;27:1774–1779. doi: 10.1161/01.str.27.10.1774. [DOI] [PubMed] [Google Scholar]
- 6.Lejeune JP, Vinchon M, Amouyel P, Escartin D, Christiaens JL. Association of occurrence of aneurysmal bleeding with meteorologic variations in the north of France. Stroke. 1994;25:338–341. doi: 10.1161/01.str.25.2.338. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth WT, Bond N. Environmental temperature and the risk of subarachnoid hemorrhage [Letter; comment] Stroke. 1994;25:1882-183. [PubMed] [Google Scholar]
- 8.Manfredini R, Gallerani M, Portaluppi F, Salmi R, Fersini C. Chronobiological patterns of onset of acute cerebrovascular diseases. Thromb Res. 1997;88:451–463. doi: 10.1016/s0049-3848(97)00286-7. [DOI] [PubMed] [Google Scholar]
- 9.Seretakis D, Lagiou P, Lipworth L, Signorello LB, Rothman KJ, Trichopoulos D. Changing seasonality of mortality from coronary heart disease. JAMA. 1997;278:1012–1014. [PubMed] [Google Scholar]
- 10.Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol. 1999;33:1916–1919. doi: 10.1016/s0735-1097(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 11.Fu S, Cao Y, Li Y. Epidemiological study of hypertension in Heilongjiang province. Zhonghua Nei ke Za Zhi. 2002;41:114–116. [PubMed] [Google Scholar]
- 12.Gyllerup S, Lanke J, Lindholm L, Schersten B. High coronary mortality in cold regions of Sweden. J Intern Med. 1991;230:479–485. doi: 10.1111/j.1365-2796.1991.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara T, Kawamura M, Nakajima J, Adachi T, Hiramori K. Seasonal differences in diurnal blood pressure of hypertensive patients living in a stable environmental temperature. J Hypertens. 1995;13:1747–1752. [PubMed] [Google Scholar]
- 14.Hata T, Ogihara T, Maruyama H, Mikami H, Nakamaru M, Naka T, Kumahara Y, Nugent CA. The seasonal variation of blood pressure in patients with essential hypertension. Clin. and Exper.-Theory and Practice. 1982;A4:341–354. doi: 10.3109/10641968209060747. [DOI] [PubMed] [Google Scholar]
- 15.Minami J, Kawano Y, Ishimitsu T, Yoshimi H, Takishita S. Seasonal variations in office, home and 24 h ambulatory blood pressure in patients with essential hypertension. J Hypertens. 1996;14:1421–1425. doi: 10.1097/00004872-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Verdon F, Boudry F, Chuat M, Studer JP, Truong CN, Jacot E. Seasonal variations in arterial blood pressure in hypertensive patients. Schwei Med Wochenschr. 1993;123:2363–2369. [PubMed] [Google Scholar]
- 17.Winnicki M, Canali C, Accurso V, Dorigatti F, Giovinazzo P, Palatini P. Relation of 24-hour ambulatory blood pressure and short-term blood pressure variability to seasonable changes in environmental temperature in stage I hypertensive subjects. Results of the Harvest Trial Clin Exp Hypertens. 1996;18:995–1012. doi: 10.3109/10641969609081031. [DOI] [PubMed] [Google Scholar]
- 18.Fregly MJ, Kikta DC, Threatte RM, Torres JL, Barney CC. Development of hypertension in rats during chronic exposure to cold. J Appl Physiol. 1989;66:741–749. doi: 10.1152/jappl.1989.66.2.741. [DOI] [PubMed] [Google Scholar]
- 19.Fregly MJ, Rossi F, Sun Z, Tumer N, Cade JR, Hegland D, Yurekli M. Effect of chronic treatment with prazosin and L-arginine on the elevation of blood pressure during cold exposure. Pharmacology. 1994;49:351–362. doi: 10.1159/000139254. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Cade R. Cold-induced hypertension and diuresis. J Therm Biol. 2000;25:105–109. [Google Scholar]
- 21.Sun Z, Cade R, Katovich MJ, Fregly MJ. Body fluid distribution in rats with cold-induced hypertension. Physiol Behav. 1999;65:879–884. doi: 10.1016/s0031-9384(98)00250-9. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z, Cade R, Morales C. Role of Central angiotensin II receptors in cold-induced hypertension. Am J Hypertens. 2002;15:85–92. doi: 10.1016/s0895-7061(01)02230-0. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, Cade R, Tatum C. Central imidazoline and angiotensin II receptors in cardiovascular responses to chronic cold exposure in rats. J Therm Biol. 2001;26:513–518. [Google Scholar]
- 24.Bell AW, Thompson GE. Effects of acute cold and feeding on circulation of young oxen. Res Vet Sci. 1974;17:384–389. [PubMed] [Google Scholar]
- 25.Thomas GE, Gardner JW, Bell AW. VO2, fatty acids and glycerol uptake by the liver in fed and fasted sheep in cold. Q J Exp Physiol. 1975;60:107–121. doi: 10.1113/expphysiol.1975.sp002297. [DOI] [PubMed] [Google Scholar]
- 26.Shechtman O, Papanek PE, Fregly MJ. Reversibility of cold-induced hypertension after removal of rats from cold. Can J Physiol Pharmacol. 1990;68:830–835. doi: 10.1139/y90-126. [DOI] [PubMed] [Google Scholar]
- 27.van Bergen P, Fregly MJ, Rossi F, Shechtman O. The effect of intermittent exposure to cold on the development of hypertension in the rat. Am J Hypertens. 1992;5:548–555. doi: 10.1093/ajh/5.8.548. [DOI] [PubMed] [Google Scholar]
- 28.Barney CC, Katovich MJ, Fregly MJ, Taylor PE. Changes in beta-adrenergic responsiveness of rats during chronic cold exposure. J Appl Physiol. 1980;49:923–929. doi: 10.1152/jappl.1980.49.6.923. [DOI] [PubMed] [Google Scholar]
- 29.Fregly MJ, Field FP, Nelson EL, Tyler PE, Dasler R. Effect of chronic exposure to cold on some responses to catecholamines. J Appl Physiol. 1977;43:349–354. doi: 10.1152/jappl.1977.42.3.349. [DOI] [PubMed] [Google Scholar]
- 30.Papanek PE, Wood CE, Fregly MJ. Role of the sympathetic nervous system in cold-induced hypertension in rats. J Appl Physiol. 1991;71:300–306. doi: 10.1152/jappl.1991.71.1.300. [DOI] [PubMed] [Google Scholar]
- 31.Sun Z, Cade R, Fregly MJ, Rowland NE. Effect of chronic treatment with propranolol on the cardiovascular responses to chronic cold exposure. Physiol Behav. 1997;62:379–384. doi: 10.1016/s0031-9384(97)00033-4. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z, Fregly MJ, Cade JR. Effect of renal denervation on elevation of blood pressure in cold-exposed rats. Can J Physiol Pharmacol. 1995;73:72–78. doi: 10.1139/y95-010. [DOI] [PubMed] [Google Scholar]
- 33.Sun Z, Bello Roufai M, Wang X. RNAi inhibition of mineralocorticoid receptor prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;294:H1880–H1887. doi: 10.1152/ajpheart.01319.2007. [DOI] [PubMed] [Google Scholar]
- 34.Bryar BA, Fregly MJ, Field FP. Changes in vascular responsiveness following chronic exposure to cold in the rat. J Appl Physiol. 1983;55:823–829. doi: 10.1152/jappl.1983.55.3.823. [DOI] [PubMed] [Google Scholar]
- 35.Flaim SF, Hsieh CL. Effect of cold-acclimation on rabbit carotid artery: Altered response to norepinephrine. Gen Pharmacol. 1978;9:437–442. doi: 10.1016/0306-3623(78)90031-9. [DOI] [PubMed] [Google Scholar]
- 36.Fregly MJ, Brummermann M. Effect of chronic exposure to cold on vascular responsiveness to phenylephrine and angiotensin II. Pharmacol. 1993;47:237–243. doi: 10.1159/000139103. [DOI] [PubMed] [Google Scholar]
- 37.Fregly MJ, Field FP, Nelson EL, Tyler PE, Dasler R. Effect of chronic exposure to cold on some responses to catecholamines. J Appl Physiol. 1977;43:349–354. doi: 10.1152/jappl.1977.42.3.349. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z. Genetic AVP deficiency abolishes cold-induced diuresis but does not attenuate cold-induced hypertension. Am J Physiol Renal Physiol. 2006;290:F1472–F1477. doi: 10.1152/ajprenal.00430.2005. [DOI] [PubMed] [Google Scholar]
- 39.Fregly MJ, Rossi F, van Bergen P, Brummermann M, Cade R. Effect of chronic treatment with losartan potassium (Dup 753) on the elevation of blood pressure during chronic exposure of rats to cold. Pharmacology. 1993;46:198–205. doi: 10.1159/000139046. [DOI] [PubMed] [Google Scholar]
- 40.Shechtman O, Fregly MJ, van Bergen P, Papanek PE. Prevention of cold-induced increase in blood pressure of rats by captopril. Hypertension (Dallas) 1991;17:763–770. doi: 10.1161/01.hyp.17.6.763. [DOI] [PubMed] [Google Scholar]
- 41.Sun Z, Zhang Z, Cade R. Angiotensinogen gege knockout delays and attenuates cold-induced hypertension. Hypertension. 2003;41:322–327. doi: 10.1161/01.hyp.0000050964.96018.fa. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Sun Z, Cade R. Prolonged attenuation of cold-induced hypertension by adenoviral delivery of renin antisense. Kidney Int. 2005;68:690–687. doi: 10.1111/j.1523-1755.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 43.Sun Z, Cade R, Wang X, Wood CE, Cade R. Genetic AT1A receptor deficiency attenuates cold-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R433–R439. doi: 10.1152/ajpregu.00466.2004. [DOI] [PubMed] [Google Scholar]
- 44.Sun Z, Bello Roufai M, Wang X. RNAi inhibition of mineralocorticoid receptor prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;294(4):H1880–H1887. doi: 10.1152/ajpheart.01319.2007. [DOI] [PubMed] [Google Scholar]
- 45.Arima S, Ito S. New insights into actions of the renin-angiotensin system in the kidney: concentrating on the ANG II receptors and the newly described Ang-(1–7) and its receptor. Semin Neprhrol. 2001;21:535–543. doi: 10.1053/snep.2001.26792. [DOI] [PubMed] [Google Scholar]
- 46.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol. 2001;281:H2337–H2356. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Cade R, Sun Z. Human eNOS gene delivery attenuates cold-induced elevation of blood pressure in rats. Am J Physiol Heart & Cir Physiol. 2005;289:H1161–H1168. doi: 10.1152/ajpheart.01306.2004. [DOI] [PubMed] [Google Scholar]
- 48.Harada S, Tokunaga S, Momohara M, Masaki H, Imaizumi T, Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ Res. 1993;72:511–516. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- 49.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med. 2001;226:814–824. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- 50.Feleder C, Perlik V, Blatteis CM. Preoptic nitric oxide attenuates endotoxic fever in guinea pigs by inhibiting the POA release of norepinephrine. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1144–R1151. doi: 10.1152/ajpregu.00068.2007. [DOI] [PubMed] [Google Scholar]
- 51.Abassi ZA, Ellahham S, Winaver J, Hoffman A. The intrarenal endothelin system and hypertension. News Physiol Sci. 2001;16:152–157. doi: 10.1152/physiologyonline.2001.16.4.152. [DOI] [PubMed] [Google Scholar]
- 52.Hanes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16:1081–1098. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- 53.D’Orieans-Juste P, Plante M, Honore E, Carrier E, Labonte J. Synthesis and degradation of endothelin-1. Can J Physiol Pharmacol. 2003;81:503–510. doi: 10.1139/y03-032. [DOI] [PubMed] [Google Scholar]
- 54.Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 55.Schiffrin EL. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J Cardiovascular Pharmacol. 2001;38:S3–S6. doi: 10.1097/00005344-200111002-00002. [DOI] [PubMed] [Google Scholar]
- 56.Mateo AO, Artinano AA. Highlights on endothelins: A review. Pharmacol Res. 1997;36:339–351. doi: 10.1006/phrs.1997.0246. [DOI] [PubMed] [Google Scholar]
- 57.D’Orleans-Juste P, Labonte J, Bkaily G, Choufani S, Plante M, Honore JC. Function of the endothelinB receptor in cardiovascular physiology and pathophysiology. Pharmacol Thera. 2002;95:221–238. doi: 10.1016/s0163-7258(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 58.Chen GF, Sun Z. Effects of chronic cold exposure on the endothelin system. J Appl Physiol. 2006;100:1719–1726. doi: 10.1152/japplphysiol.01407.2005. [DOI] [PubMed] [Google Scholar]
- 59.Lepert J, Ringqvist A, Karlberg BE, Ringqvist I. Whole-body cooling increases plasma endothelin-1 levels in women with primary Raynaud's phenomenon. Clin Physiol. 1998;18:420–425. doi: 10.1046/j.1365-2281.1998.00105.x. [DOI] [PubMed] [Google Scholar]
- 60.Mangiafico RA, Malatino LS, Santonocito M, Spada RS, Tamburino G. Plasma endothelin-1 concentrations during cold exposure in essential acrocyanosis. Angiology. 1996;47:1033–1038. doi: 10.1177/000331979604701102. [DOI] [PubMed] [Google Scholar]
- 61.Sun Z, Cade JR, Fregly MJ. Cold-induced hypertension. A model of mineralocorticoid-induced hypertension. Ann N Y Acad Sci. 1997;15(813):682–688. doi: 10.1111/j.1749-6632.1997.tb51767.x. [DOI] [PubMed] [Google Scholar]
- 62.Cullen JP, Bell D, Kelso EJ, McDermott BJ. Use of A-192621 to provide evidence for involvement of endothelin ET(B)-receptors in endothelin-1-mediated cardiomyocyte hypertrophy. Eur J Pharmacol. 2001;417:157–168. doi: 10.1016/s0014-2999(01)00905-0. [DOI] [PubMed] [Google Scholar]
- 63.Ito H, Hiroe M, Hirata Y, Fujisaki H, Adachi S, Akimoto H, Ohta Y, Marumo F. Endothelin ETA receptor antagonist blocks cardiac hypertrophy provoked by hemodynamic overload. Circulation. 1994;89:2198–2203. doi: 10.1161/01.cir.89.5.2198. [DOI] [PubMed] [Google Scholar]
- 64.Ito H, Hirata Y, Hiroe M, Tsujino M, Adachi S, Takamoto T, Nitta M, Taniguchi K, Marumo F. Endothelin-1 induces hypertrophy with enhanced expression of muscle-specific genes in cultured neonatal rat cardiomyocytes. Cir Res. 1991;69:209–215. doi: 10.1161/01.res.69.1.209. [DOI] [PubMed] [Google Scholar]
- 65.Shohet RV, Kisanuki YY, Zhao X, Siddiquee Z, Franco F, Yanagisawa M. Mice with cardiomyocyte-specific disruption of the endothelin-1 gene are resistant to hyperthyroid cardiac hypertrophy. Proc Natl Acad Sci. 2004;101:2088–2093. doi: 10.1073/pnas.0307159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumura Y, Hashimoto N, Taira S, Kuro T, Kitano R, Ohkita M, Opgenorth TJ, Takaoka M. Different contributions of Endothelin-A and Endothelin-B receptors in the pathogenesis of deoxycorticosterone acetate-salt-induced hypertension in rats. Hypertension. 1999;33:759–765. doi: 10.1161/01.hyp.33.2.759. [DOI] [PubMed] [Google Scholar]
- 67.Dvorak P, Kramer HJ, Backer A, Maly J, Kopkan L, Vaneckova I, Vernerova Z, Opocensky M, Tesar V, Bader M, Ganten D, Janda J, Cervenka L. Blockade of Endothelin receptors attenuates end-organ damage in homozygous hypertensive ren-2 transgenic rats. Kidney Blood Press Res. 2004;27:248–258. doi: 10.1159/000080052. [DOI] [PubMed] [Google Scholar]
- 68.Kohan DE, Padilla E. Endothelin-1 production by rat inner medullary collecting duct: effect of nitric oxide, cGMP, and immune cytokines. Am J Physiol. 1994;266:F291–F297. doi: 10.1152/ajprenal.1994.266.2.F291. [DOI] [PubMed] [Google Scholar]
- 69.Kohan DE. Endothelins in the normal and diseased kidneys. Am J Kidney Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 70.Abassi ZA, Gurbanov K, Rubinstein I, Better OS, Hoffman A, Winaver J. Regulation of intrarenal blood flow in experimental heart failure: role of endothelin and nitric oxide. Am J Physiol Renal Physiol. 1998;274:F766–F744. doi: 10.1152/ajprenal.1998.274.4.F766. [DOI] [PubMed] [Google Scholar]
- 71.Sun Z, Cade R, Katovich MJ, Fregly MJ. Body fluid distribution in rats with cold-induced hypertension. Physiol Behav. 1999;65:879–884. doi: 10.1016/s0031-9384(98)00250-9. [DOI] [PubMed] [Google Scholar]
- 72.Bello Roufai M, Li H, Sun Z. Heart-specific inhibition of protooncogene c-myc attenuates cold-induced cardiac hypertrophy. Gene Ther. 2007;14:1406–1416. doi: 10.1038/sj.gt.3302995. [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Liu J, Zhao CQ, Diwan BA, Merrick BA, Waalkes MP. Association of cmyc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol Appl Pharmacol. 2001;175:261–268. doi: 10.1006/taap.2001.9253. [DOI] [PubMed] [Google Scholar]
- 74.Ponzielli R, Katz S, Barsyte-Lovejov D, Penn LZ. Cancer therapeutics: targeting the dark side of Myc. Eur J Cncer. 2005;41:2485–2501. doi: 10.1016/j.ejca.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 75.Fregly MJ, Rossi F, Cade R. A role of thyroid hormones in cold-induced elevation of blood pressure and cardiac hypertrophy. Can J Physiol Pharmacol. 1994;72:1066–1074. doi: 10.1139/y94-149. [DOI] [PubMed] [Google Scholar]
- 76.Kinugawa K, Jeong MY, Bristow MR, Long CS. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor alpha1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol. 2005;19:1618–1628. doi: 10.1210/me.2004-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang Q, Molkentin JD. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animals models. J Mol Cell Cardiol. 2005;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]