Abstract
Furan is a rodent hepatotoxicant and carcinogen. Since this compound is an important industrial intermediate and has been detected in heat-processed foods and smoke, humans are likely exposed to this toxic compound. Characterization of urinary metabolites of furan will lead to the development of biomarkers to assess human health risks associated with furan exposure. Previous studies indicate that furan is oxidized to a reactive α, β-unsaturated dialdehyde, cis-2-butene-1,4-dial (BDA), in a reaction catalyzed by cytochrome P450. Five previously characterized metabolites are derived from the reaction of BDA with cellular nucleophiles such as glutathione and protein. They include the mono-glutathione reaction product, N-[4-carboxy-4-(3-mercapto-1H-pyrrol-1-yl)-1-oxobutyl]-L-cysteinylglycine cyclic sulfide and its downstream metabolite, S-[1-(1,3-dicarboxypropyl)-1H-pyrrol-3-yl]methylthiol as well as R-2-acetylamino-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid and N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine and its sulfoxide. The last two compounds are downstream metabolites of a BDA-derived cysteine-lysine crosslink, S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine. In this report, we present the characterization of seven additional urinary furan metabolites, all of which are derived from this crosslink. The cysteinyl residue is subject to several biotransformation reactions, including N-acetylation and S-oxidation. Alternatively, it can undergo β-elimination followed by S-methylation to a methylthiol intermediate that is further oxidized to a sulfoxide. The lysine portion of the crosslink is either N-acetylated or undergoes an oxidative transamination reaction to generate an α-ketoacid metabolite that undergoes oxidative decarboxylation. Some of these metabolites are among the most abundant furan metabolites present in urine as judged by LC-MS/MS analysis, indicating that the oxidation of furan to BDA and BDA’s subsequent reaction with cellular cysteine and lysine residues may represent a significant in vivo pathway of furan biotransformation. Since they are derived from cellular BDA reaction products, these metabolites are markers of furan exposure and bioactivation and could be explored as potential biomarkers in human studies.
Keywords: furan, metabolism
Introduction
Furan is an important industrial chemical. It has also been detected in cigarette smoke, wood smoke and engine exhaust (1) as well as in heat processed foods such as canned fruits and vegetables (2). Furan causes hepatocellular tumors and cholangiocarcinomas in rodents (3). As a result, the International Agency for Research on Cancer and the National Toxicology Program listed it as a possible human carcinogen (Group 2B) (1,3). Currently, there are no studies linking furan exposure to adverse events in humans. Accurate human risk assessment for this compound is limited by the high volatility of furan as well as the lack of appropriate biomarkers. Biomarkers are valuable tools for assessing human exposure to environmental chemicals as well as for assessing human health risks associated with exposure (4). Development of mechanism-based biomarkers for furan exposure and toxicity will significantly aid risk assessment of furan exposure in humans. It is therefore of considerable interest to understand the metabolic fate of furan in a species susceptible to the harmful effects of furan (i.e. the rat) since this understanding can lead to the development of valuable biomarkers for use in human studies to assess health risks associated with furan exposure.
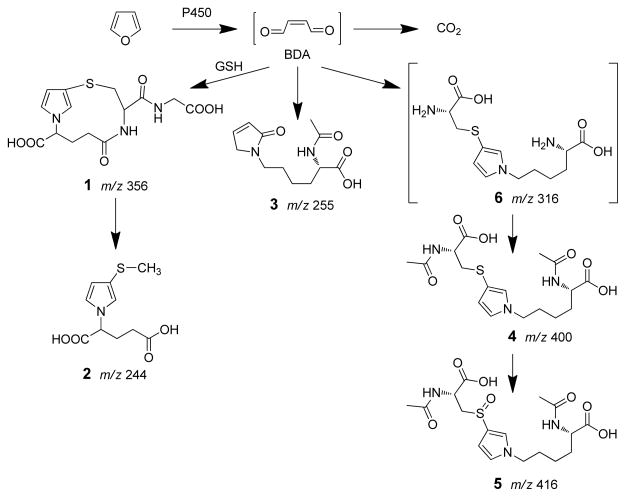
Furan’s toxic effects require biotransformation (5–7). It is extensively metabolized in rats; 84% of an 8 mg/kg [14C]furan dose is converted to metabolites within 24 h (5). The major metabolite is carbon dioxide, representing 26% of the total dose. A significant portion of the dose was eliminated in the urine (22%) and feces (20%). A substantial amount of radioactivity remains in the liver bound to proteins at 24 h. The metabolism of furan is initiated by P450 catalyzed oxidation to cis-2-butene-1,4-dial (BDA1, Scheme 1) (8,9). The metabolic pathway to carbon dioxide likely involves subsequent oxidation of BDA to maleic acid followed by rearrangement to fumaric acid. This latter compound can enter the citric acid cycle leading to the generation of carbon dioxide. BDA is also reactive and will alkylate with cellular nucleophiles such as GSH, amino acids and DNA (10–15). Products of these reactions have been observed in the urine of furan-treated rats (11,16,17). The mono-glutathione-BDA reaction product, N-[4-carboxy-4-(3-mercapto-1H-pyrrol-1-yl)-1-oxobutyl]-L-cysteinylglycine cyclic sulfide (1) and a downstream metabolite of this product, S-[1-(1,3-dicarboxypropyl)-1H-pyrrol-3-yl]methylthiol (2) have been detected (Scheme 1) (16,17). Three additional urinary metabolites of furan have also been identified: R-2-acetylamino-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid (3), N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (4) and its sulfoxide (5) (17). Metabolite 3 results from the reaction of BDA with lysine whereas metabolites 4 and 5 result from the crosslinking of cysteine and lysine by BDA (10). Metabolites 4 and 5 have several possible sources (11). One source is BDA-derived protein-protein crosslinks involving cysteine and lysine residues. Another source is BDA-derived crosslinks between glutathione and either free or protein bound lysine.
Scheme 1.
Identified pathways of furan metabolism.
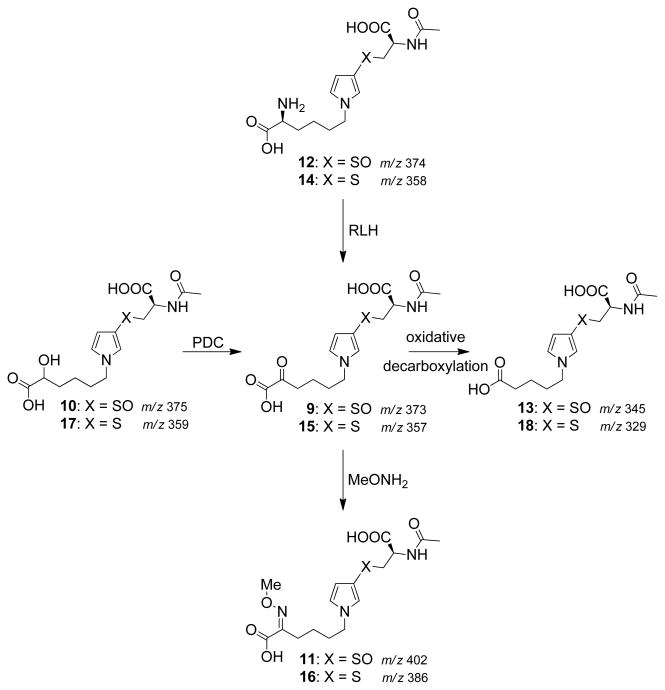
In this report, we present the characterization of seven additional urinary metabolites (Scheme 2 and Scheme 3) whose structural features reveal that they are all derived from a common precursor, S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (6). These observations indicate that the formation of cysteine-BDA-lysine crosslinks is an important component in the overall metabolism of furan in rats. Given the strong possibility that these urinary metabolites are degraded protein adducts (11), these metabolites could serve as markers of furan exposure and bioactivation.
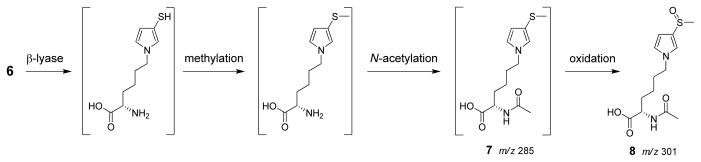
Scheme 2.
Proposed cysteine biotransformation pathway in rats. It is not known whether N-acetylation precedes or follows β-elimination. Only one possibility is shown for ease of presentation.
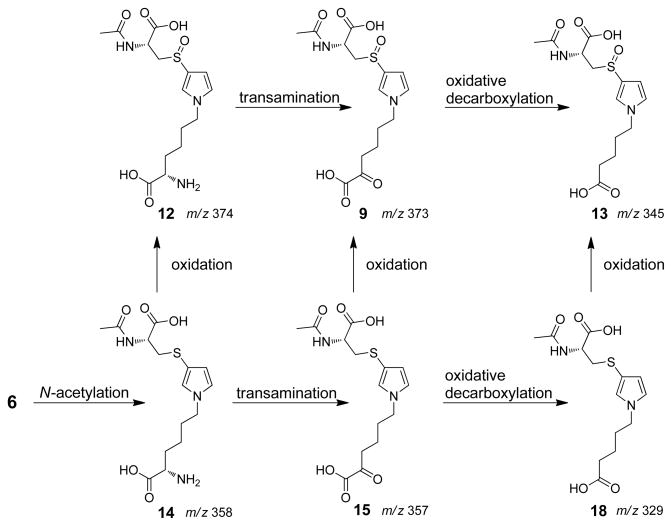
Scheme 3.
Proposed lysine biotransformation pathway in rats.
Materials and Methods
Caution
Furan is toxic and carcinogenic in laboratory animals. All laboratory procedures involving this chemical should be performed with safety gloves in a well ventilated fume hood.
Chemicals
Furan and trifluoroacetic acid were purchased from Acros Organics (Pittsburgh, PA). [13C4]Furan, cis-2-butene-1,4-dial (BDA), 6-amino-2-hydroxyhexanoic acid and N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine were synthesized as previously described (11,14,18,19). All chemicals and reagents were of the highest purity available and were used without further purification unless noted otherwise.
Instrumentation
HPLC purifications were carried out on a Shimadzu LC-10AD system coupled to a Shimadzu SCL-10A UV-Vis detector, using a Phenomenex (Torrence, CA) Synergi 4μ Hydro-RP column (250 × 10 mm, 4 micron) with a flow rate of 4 mL/min. Six different HPLC methods were employed. These methods are described in Table 1.
Table 1.
HPLC Methods
| Method | Solvent A | Solvent B | Gradienta |
|---|---|---|---|
| 1 | 50 mM ammonium formate, pH 2.8 | acetonitrile with 50% water | 10 min at 100% A; 26 min gradient to 75% A, 25% B; 10 min gradient to 50% A, 50% B; 4 min gradient to 100% B |
| 2 | 20 mM ammonium acetate, pH 6.8 | methanol with 5% water | 30 min gradient from 100% A to 50% A, 50% B; 20 min gradient to 100% B |
| 3 | 10 mM ammonium formate, pH 2.8 | acetonitrile with 50% water | 10 min at 100% A; 26 min gradient to 75% A, 25% B; 10 min gradient to 50% A, 50% B; 4 min gradient to 100% B |
| 4 | 50 mM ammonium formate, pH 2.8 | acetonitrile with 50% water | 3 min at 100% A; 3 min gradient to 50% A, 50% B; 4 min gradient to 10% A, 90% B; 13 min gradient to 100% B |
| 5 | 10 mM ammonium formate, pH 2.8 | acetonitrile with 50% water | 4 min at 100% A; 10 min gradient to 78% A, 22% B; 14 min gradient to 50% A, 50% B; 2 min gradient to 100% B |
| 6 | 10 mM ammonium formate, pH 2.8 | acetonitrile with 50% water | 3 min at 100% A; 7 min gradient to 78% A, 22% B; 12 min gradient to 50% A, 50% B; 4 min gradient to 100% B |
All gradients were linear.
Collision-induced mass spectra of standards were obtained on an Agilent 1100 series LC/MSD Trap SL mass spectrometer operating in positive ion mode. Each compound was dissolved in 10 mM ammonium formate, pH 2.8, and directly infused into the ion source. Helium was the nebulizing and drying gas (15 psi, 5 L/min) which had a temperature set at 200 °C. High resolution mass spectral data for the metabolites were obtained on a Thermo Ultra AM Triple Quadrupole mass spectrometer. High resolution mass spectral data for the synthetic standards were obtained on a Bruker BioTOF II mass spectrometer in the Department of Chemistry, University of Minnesota.
1H, 13C NMR and two-dimensional NMR (COSY, HMQC) spectra were recorded on a 500 or 600 MHz Varian Inova spectrometer or a Bruker Avance 700 MHz spectrometer in the Department of Biochemistry Molecular Biology and Biophysics, University of Minnesota. Chemical shifts are reported in parts per million (ppm) as referenced to the residual solvent peak.
General procedure for reaction between thiols, BDA and amines
Thiols (sodium thiomethoxide or Nα-acetyl-L-cysteine, 130 μmol) and BDA (130 μmol) were incubated in 1 M sodium phosphate (pH 7.4, 1.5 mL) at 37 °C for 25 min before the addition of amines (N-acetyl-L-lysine,5-aminovaleric acid or 6-amino-2-hydroxyhexanoic acid, 130 μmol; total volume: 2 mL). The reaction was stirred for 15 hours and products were purified by semi-preparative HPLC. The organic solvent of peak collections was removed under reduced pressure and buffer salts were removed by solid phase extraction using Strata-X cartridges (Phenomenex, Torrance, CA). All standards were then characterized by NMR and MS.
S-[1-(5-Acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol (7)
Sodium thiomethoxide, BDA and N-acetyl-L-lysine were combined to generate S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol. The product eluted at 53.8 min with HPLC Method 1 (yield: 16 mg, 43%). 1H NMR (600 MHz, DMSO-d6): δ 7.97 (d, 1H, J=7.8 Hz, NH), 6.75 (s, 1H, H2′), 6.70 (s, 1H, H5′), 5.99 (s, 1H, H4′), 4.11 (dd, 1H, J=8.4, 13.2 Hz, H5), 3.77 (t, 2H, J=7.2 Hz, H1), 2.21 (s, 3H, SCH3), 1.80 (s, 3H, COCH3), 1.68-1.6 (m, 3H, H2 and H4a), 1.6-1.5 (m, 1H, H4b), 1.24-1.21 (m, 2H, H3); 13C NMR (150 MHz, DMSO-d6): δ 122.7, 122.2, 111.4, 52.5, 49.4, 31.3, 31.2, 23.2, 23.1, 20.7; ESI-MS/MS: m/z 285 [M+H+], 243, 197, 180. Molecular formula: C13H20N2O3S, molecular weight: 284.4 g/mol.
N-Acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (17)
Reaction of N-acetyl-L-cysteine, 6-amino-2-hydroxyhexanoic acid and BDA generated N-acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine. This compound eluted at 49.4 min using HPLC Method 1 (yield: 24 mg, 52%). 1H NMR (600 MHz, DMSO-d6): δ 8.14 (d, 1H, J=7.8 Hz, NH), 6.81 (t, 1H, J=2.4 Hz, H2′), 6.72 (t, 1H, J=2.4 Hz, H5′), 6.00 (dd, 1H, J=2.4, 2.4 Hz, H4′), 4.21 (ddd, 1H, J=4.8, 8.4, 12.6 Hz, Cys αCH), 3.86 (dd, 1H, J=4.2, 7.8 Hz, H5), 3.78 (t, 2H, J=7.2 Hz, H1), 2.85 (dd, 1H, J=4.8, 13.8 Hz, Cys β-CHa), 2.68 (dd, 1H, J=9.0, 13.2 Hz, Cys β-CHb), 1.81 (s, 3H, Me), 1.66-1.55 (m, 3H, H2, H4a), 1.51-1.46 (m, 1H, H4b), 1.27-1.22 (m, 2H, H3); 13C NMR (150 MHz, DMSO-d6): δ 125.6, 122.5, 113.5, 70.3, 52.5, 49.7, 39.2, 34.1, 31.4, 23.2, 22.6. ESI-MS/MS: m/z 359 [M + H+], 341, 295, 230. Molecular formula: C15H22N2O6S; molecular weight: 358.4 g/mol.
N-Acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine (18)
BDA, N-acetyl-L-cysteine and 5-aminovaleric acid were combined to generate N-acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine. The product eluted at 51.2 min when HPLC Method 1 was employed (yield: 30 mg, 69%). 1H NMR (500 MHz, DMSO-d6): δ 8.19 (d, 1H, J=8 Hz, NH), 6.85 (s, 1H, H2′), 6.76 (t, 1H, J=2.5 Hz, H5′), 6.05 (dd, 1H, J=1.5, 2.5 Hz, H4′), 4.24 (ddd, 1H, J=5.0, 9.5, 13.5 Hz, Cys α-CH), 3.84 (t, 2H, J=6.5 Hz, H1), 2.89 (dd, 1H, J=5.0, 13.5 Hz, Cys β-CHa), 2.72 (dd, 1H, J=9.0, 13.0 Hz, Cys β-CHb), 2.21 (t, 2H, J=7.5 Hz, H4), 1.85 (s, 3H, Me), 1.70-1.64 (m, 2H, H2), 1.44-1.38 (m, 2H, H3); 13C NMR (125 MHz, DMSO-d6): δ 124.9, 121.8, 112.8, 51.6, 48.9, 38.5, 33.3, 30.3, 23.3, 21.6. Mass spectral data is presented in Table 2.
Table 2.
Mass spectral data and retention times for the urinary furan metabolites and their synthetic standards.
| m/z | RT (min) | MS2 (fragment ions, m/z) | Molecular Formula | HRMS (calculated) | HRMS (measured) | ||
|---|---|---|---|---|---|---|---|
| 8 | Standard | 301 | 47.6 | 283, 238, 213, 196, 179 | C13H20N2O4S | 301.1217 (M+H)+ | 301.1221 (M+H)+ |
| [12C4]furan | 301 | 47.7 | 283, 238, 213, 196, 179 | 301.1202 (M+H)+ | |||
| [13C4]furan | 305 | 47.1 | 287, 242, 217, 200, 183 | n.d. | |||
| 9 | Standard | 373 | 18.2a | 296, 278, 226, 196, 178 | C15H20N2O7S | 373.1064 (M+H)+ | 373.1074 (M+H)+ |
| [12C4]furan | 373 | 25.2b (18.0a) | 296, 278, 226, 196, 178 | 373.1056 (M+H)+ | |||
| [13C4]furan | 377 | 25.2b | 300, 282, 230, 200, 182 | n.d. | |||
| 11 | Standard | 402 | 29.7a | 384, 325, 307, 255, 225 | C16H23N3O7S | 402.1329 (M+H)+ | 402.1333 (M+H)+ |
| [12C4]furan | 402 | 48.3 | 384, 325, 307, 255, 225 | 402.1329 (M+H)+ | |||
| [13C4]furan | 406 | 48.4 | 388, 329, 311, 259, 229 | 406.1463 (M+H)+ | 406.1467 (M+H)+ | ||
| RLH | 402 | 30.0a | 384, 325, 307, 255, 225 | 402.1324 (M+H)+ | |||
| 12 | Standard | 374 | 30.9 | 297, 279, 227, 197 | C15H23N3O6S | 374.1380 (M+H)+ | 374.1262 (M+H)+ |
| [12C4]furan | 374 | 31.2 | n.d. | n.d. | |||
| 13 | Standard | 345 | 28.1 (25.9a) | 268, 250, 198, 168 | C14H20N2O6S | 367.0934 (M+Na)+ | 367.0944 ((M+Na)+ |
| [12C4]furan | 345 | 30.7 | 268, 250, 198, 168 | 345.1115 (M+H)+ | 345.1118 (M+H)+ | ||
| [13C4]furan | 349 | 30.0 | 272, 254, 202, 172 | n.d. | |||
| RLH | 345 | 24.3a | 268, 250, 198, 168 | 345.1113 (M+H)+ | |||
| 14 | Standard | 358 | 56.6 | 340, 316, 294, 260, 229 | C15H23N3O6S | 356.1286 (M−H)− | 356.1282 (M−H)− |
| [12C4]furan | 358 | 56.5 | n.d. | n.d. | |||
| 16 | Standard | 386 | 60.2c | 368, 339, 321, 292, 257, 181 | C16H23N3O6S | 386.1380 (M+H)+ | 386.1384 (M+H)+ |
| [12C4]furan | 386 | 57.9 (60.2c) | 368, 339, 321, 292, 257, 181 | 386.1374 (M+H)+ | |||
| [13C4]furan | 390 | 58.0 | 372, 343, 325, 296, 261, 185 | n.d. | |||
| RLH | 386 | 61.4c | 368, 339, 321, 292, 257, 181 | 386.1365 (M+H)+ | |||
| 18 | Standard | 329 | 51.2 | 311, 293, 200, 182 | C14H20N2O5S | 329.1166 (M+H)+ | 329.1160 (M+H)+ |
| [12C4]furan | 329 | 50.4 | 311, 293, 200, 182 | 329.1153 (M+H)+ | |||
| [13C4]furan | 333 | 49.9 | n.d. | n.d. | |||
Retention times were determined using HPLC Method 5.
Retention times were determined using HPLC Method 1. All other retention times were determined with HPLC Method 3.
Experiments were performed at the same day.
General procedure for the synthesis of sulfoxide standards
m-Chloroperbenzoic acid (10 μmol) was added to a stirred solution of the corresponding sulfide (10 μmol) in methanol-dichloromethane (1:1) at −78 °C (1 mL). The reaction was stirred for 1 hour before warming up to room temperature. Solvent was removed with nitrogen. The crude product was dissolved in water and purified by semi-preparative HPLC. The organic solvent was removed under reduced pressure and buffer salts were removed by solid phase extraction using Strata-X cartridges. The identities of products were established by MS and NMR analysis.
S-[1-(5-Acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol sulfoxide (8)
Oxidation of S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol generated S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol sulfoxide. This compound eluted at 10.7 min using HPLC Method 4 (yield: 2.1 mg, 68%). 1H NMR (600 MHz, DMSO-d6): δ 8.01 (d, 1H, J=7.2 Hz, NH), 7.27 (s, 1H, H2′), 6.90 (s, 1H, H5′), 6.40 (s, 1H, H4′), 4.09 (ddd, 1H, J=8.4, 8.4, 13.2 Hz, H5), 3.86 (t, 2H, J=6.6 Hz, H1), 2.68 (s, 3H, SOCH3), 1.80 (s, 3H, COCH3), 1.69-1.62 (m, 3H, H2, H4a), 1.55-1.52 (m, 1H, H4b), 1.24-1.20 (m, 2H, H3); 13C NMR (150 MHz, DMSO-d6): δ 124.0, 122.9, 105.9, 53.0, 49.8, 42.0, 31.4, 31.2, 23.3, 23.0. Mass spectral data is displayed in Table 2.
N-Acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (12)
Oxidation of N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine yielded N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide. Two diastereomers (m/z 374) were separated employing HPLC Method 1 in a ratio of 2:5 with retention times of 22.2 and 22.8 min, respectively (yield diastereomer 1: 1.1 mg, 27%; yield diastereomer 2: 1.5 mg, 40%). Diastereomer 1: 1H NMR (600 MHz, DMSO-d6): δ 8.32 (s, 1H, NH), 7.27 (s, 1H, H2′), 6.90 (s, 1H, H5′), 6.36 (s, 1H, H4′), 4.23-4.22 (m, 1H, Cys α-CH), 3.88-3.86 (m, 2H, H1), 3.38-3.35 (m, 1H, Cys β-CHa), 3.35-3.10 (m, 1H, H5), 2.99-2.97 (m, 1H, Cys β-CHb), 1.80 (s, 3H, Me), 1.64-1.56 (m, 4H, H4, H2), 1.22-1.16 (m, 2H, H3); 13C NMR (150 MHz, DMSO-d6): δ 124.5, 124.1, 107.2, 59.2, 55.1, 50.6, 50.4, 31.8, 31.8, 24.4, 23.9; ESI-MS/MS: m/z 374 [M+H+] 227, 197. Diastereomer 2: 1H NMR (600 MHz, DMSO-d6): δ 8.45 (d, 1H, NH), 7.23 (s, 1H, H2′), 6.92 (s, 1H, H5′), 6.34 (s, 1H, H4′), 3.94-3.85 (m, 2H, H5, Cys α-CH), 3.28-3.12 (m, 4H, Cys β-CH2, H1), 1.79 (s, 3H, Me), 1.65-1.57 (m, 4H, H2, H4), 1.15-1.06 (m, 2H, H3); 13C NMR (150 MHz, DMSO-d6): δ 124.2, 124.0, 106.3, 58.2, 54.4, 49.2, 30.8, 30.8, 23.0, 22.6: ESI-MS/MS: m/z 374 [M+H+] 227, 197, 194. Molecular formula: C15H23N3O6S; molecular weight: 373.4 g/mol.
N-Acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (10)
N -acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine was oxidized to N-acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide. The product had a retention time of 33.1 min when HPLC Method 1 was employed (yield: 2.3 mg, 62%). 1H NMR analysis showed the presence of two diastereomers in a 3:2 ratio. The major isomer: 1H NMR (500 MHz, DMSO-d6): δ 8.44 (d, 1H, J=7.5 Hz, NH), 7.33 (t, 1H, J=1.5 Hz, H2′), 7.00 (t, 1H, J=2.0 Hz, H5′), 6.43-6.42 (m, 1H, H4′), 4.21 (ddd, 1H, J=6.5, 6.5, 14.0 Hz, Cys α-CH), 4.01-3.90 (m, 3H, H5, H1), 3.31-3.18 (m, 2H, Cys β-CH2), 1.84 (s, 3H, Me), 1.76-1.68 (m, 2H, H2), 1.67-1.61 (m, 1H, H4a), 1.58-1.52 (m, 1H, H4b), 1.34-1.28 (m, 2H, H3); 13C NMR (125 MHz, DMSO-d6): δ 127.0, 126.8, 108.3, 72.6, 59.1, 52.1, 51.1, 35.6, 33.6, 25.6, 25.1. The minor isomer: 1H NMR (500 MHz, DMSO-d6): δ 8.44 (d, 1H, J=7.5 Hz, NH), 7.39 (s, 1H, H2′), 6.98 (t, 1H, J=2.5 Hz, H5′), 6.45-6.44 (m, 1H, H4′), 4.51-4.48 (m, 1H, Cys α-CH), 4.01-3.90 (m, 3H, H5, H1), 3.41-3.36 (m, 1H, Cys β-CHa), 3.00-2.95(m, 1H, Cys β-CHb), 1.88 (s, 3H, Me), 1.76-1.68 (m, 2H, H2), 1.67-1.61 (m, 1H, H4a), 1.58-1.52 (m, 1H, H4b), 1.34-1.28 (m, 2H, H3). 13C NMR (125 MHz, DMSO-d6): δ 126.7, 126.2, 108.7, 72.6, 59.4, 52.1, 51.0, 35.6, 33.6, 25.5, 25.1. ESI-MS/MS: m/z 375 [M + H+], 298, 280, 252, 228, 198, 178. Molecular formula: C15H23N3O6S; molecular weight: 373.4 g/mol.
N-Acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (13)
N-acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine was oxidized with m-chloroperbenzoic acid to generate N-acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide. This product eluted at 35.9 min when HPLC Method 1 was employed (yield: 2.1 mg, 61%). 1H NMR analysis showed the presence of two diastereomers in a 1:2 ratio. The minor diastereomer: 1H NMR (500 MHz, DMSO-d6): δ 8.43 (t, 1H, J=7.0 Hz, NH), 7.39 (s, 1H, H2′), 7.00-6.97 (m, 1H, H5′), 6.45 (s, 1H, H4′), 4.50-4.46 (m, 1H, Cys α-CH), 3.93 (t, 2H, J=6.5 Hz, H1), 3.42-3.37 (m, 1H, Cys β-CHa), 2.96 (t, 1H, J=12.0 Hz, Cys β-CHb), 2.22 (t, 2H, J=7.5 Hz, H4), 1.87 (s, 3H, Me), 1.74-1.68 (m, 2H, H2), 1.43-1.40 (m, 2H, H3). The major diastereomer: 1H NMR (500 MHz, DMSO-d6): δ 8.43 (t, 1H, J=7.0 Hz, NH), 7.33 (s, 1H, H2′), 7.00-6.97 (m, 1H, H5′), 6.42 (s, 1H, H4′), 4.20-4.15 (m, 1H, Cys α-CH), 3.93 (t, 2H, J=6.5 Hz, H1), 3.25-3.23 (m, 2H, Cys β-CH2), 2.22 (t, 2H, J=7.5 Hz, H4), 1.83 (s, 3H, Me), 1.74-1.68 (m, 2H, H2), 1.43-1.40 (m, 2H, H3). 13C NMR (125 MHz, DMSO-d6): δ 174.6, 172.4, 171.9, 169.9, 169.4, 123.8, 123.7, 123.4, 105.4, 56.5, 49.0, 48.2, 33.3, 30.4, 22.6, 21.7. Mass spectral data is displayed in Table 2.
Reaction of MeONH2 with compounds containing an α-keto acid moiety
Pyridinium dichromate (1.3 mg, 3.5 μmol) was added directly to a solution of either N-acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine or N-acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide(3.5 μmol) in 0.5 mL dichloromethane-dimethylformamide (1:1) at 0 °C (20). The reaction was stirred for 1 hour before adding methoxyamine (1.5 mg, 17.5 μmol)directly ( 21). After 2 h, the reaction was stopped by removing all the solvent under a nitrogen stream. The crude product was dissolved in water and purified by semi-preparative HPLC. Organic solvent of the product peak collection was removed under reduced pressure and buffer salts were removed by solid phase extraction using Strata-X cartridges. The identities of the product were established by direct infusion for MS and verified by NMR analysis.
N-Acetyl-S-[1-(5-methoxyimino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (16)
This compound eluted at 56.6 min using HPLC Method 1 (yield: 0.22 mg, 16%). 1H NMR (700 MHz, DMSO-d6): δ 6.80 (s, 1H, H2′), 6.68 (s, 1H, H5′), 6.00 (s, 1H, H4′), 4.08-4.03 (m, 1H, Cys α-CH), 3.82-3.78 (m, 2H, H1), 3.75 (s, 3H, OCH3), 3.01-2.99 (m, 1H, Cys β-CHa), 2.76-2.72 (m, 1H, Lys β-CHb), 2.39-2.37 (m, 2H, H4), 1.81 (s, 3H, Me), 1.64-1.60 (m, 2H, H2), 1.37-1.33 (m, 2H, H3). 13C NMR (175 MHz, DMSO-d6): 124.4, 121.6, 112.7, 61.3, 54.4, 49.0, 41.1, 31.2, 23.3, 23.0. Mass spectral data is displayed in Table 2.
N-Acetyl-S-[1-(5-methoxyimino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (11)
This compound eluted at 50.5 min using HPLC Method 1. 1H NMR analysis showed the presence of two diastereomers in a 2:1 ratio (yield: 0.25 mg, 18%). The major isomer: 1H NMR (700 MHz, D2O): δ 7.26 (s, 1H, H2′), 6.88 (s, 1H, H5′), 6.46 (s, 1H, H4′), 4.06-4.02 (m, 1H, Cys α-CH), 3.88-3.84 (m, 2H, H1), 3.74 (s, 3H, OMe), 3.58-3.54 (m, 1H, Cys β-CHa), 3.43-3.39 (m, 1H, Cys β-CHb), 2.41-2.38 (m, 2H, H4), 1.79 (s, 3H, Me), 1.69-1.65 (m, 2H, H2), 1.33-1.30 (m, 2H, H3). The minor isomer: 1H NMR (700 MHz, D2O): δ 7.26 (s, 1H, H2′), 6.86 (s, 1H, H5′), 6.46 (s, 1H, H4′), 4.38-4.34 (m, 1H, Cys α-CH), 3.88-3.84 (m, 2H), 3.74 (s, 3H, OMe), 3.66-3.63 (m, 1H, Cys β-CHa), 3.21-3.18 (m, 1H, Cys β-CHb), 2.41-2.38 (m, 2H, H4), 1.77 (s, 3H, Me), 1.69-1.65 (m, 2H, H2), 1.33-1.30 (m, 2H, H3). Mass spectral data is displayed in Table 2.
In vivo furan metabolites
Furan treatment of rats and LC-MS/MS analyses of their urine samples were performed as previously described (16). In some cases, urine (100 μL) from control rats and [12C4]furan- or [13C4]furan-treated rats was reacted with methyoxyamine (10 mg) at 37 °C for 2 hours before centrifugation at 5,000 rpm for 3 min to remove any solids. The supernatants were stored at 4 °C until LC-MS/MS analysis.
LC-MS/MS analyses were conducted in an Agilent 1100 series LC/MSD Trap SL mass spectrometer equipped an Agilent (Palo Alto, CA) Zorbax SB-C18 column (5 μm, 150 × 0.5 mm) or Phenomenex Synergi 4 μ Hydro-RP 80A column (250 × 0.50 mm, 4 micron). The column was eluted with HPLC Methods 1, 2, 3 or 5 with a flow rate of 15 μL/min. Neutral loss scans of 95, 129 and 147 Da were performed on a Triple Quadrupole LC mass spectrometer (Thermo Electron Quantum Ultra AM, San Jose, CA) coupled to a Waters NanoAcquity UPLC (Milford, MA). A Phenomenex Synergi 4 μ Hydro-RP 80A column (250 × 0.50 mm, 4 micron) was eluted with HPLC Method 3 with a flow rate of 15 μL/min. Argon was used as the collision gas with a collision energy set at 15 eV.
Preparation of rat liver homogenate (RLH)
Male F344 rat liver (1.2 g) from Harlan Laboratories (Indianapolis, IN) was homogenized in 4 mL of 0.1 M Tris buffer containing 0.1 M KCl, 1 mM EDTA, pH 7.4, at 0 °C in a handheld homogenizer. The homogenate was then sonicated for 6 seconds at 30% power. Protein concentrations were determined with the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin as the reference protein.
Incubations of standards with RLH
N-Acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine or its diastereomeric sulfoxides (2 mM) were incubated with RLH (2.7 mg) in the presence of 10 mM pyridoxal 5′-phosphate, 10 mM α-ketoglutarate in 0.1 M phosphate buffer, pH 7.4 at 37 °C for 2 hours (total volume: 0.5 mL). Controls were performed in the presence or absence of pyridoxal 5′-phosphate and α-ketoglutarate, RLH or substrate. In some controls, heat inactivated RLH (80 °C for 5 min) was substituted for active RLH. The incubations were quenched by adding 0.3 N Ba(OH)2 and 0.3 N ZnSO4 (75 μL each). Solids were removed by centrifugation. In some cases, methoxyamine (20 mmol) was added after 1 hour to derivatize all carbonyl groups. The samples were analyzed by LC-MS/MS analysis as described above.
Results
Previous studies indicated that metabolites 4 and 5 were significant urinary metabolites of furan (11, 17). A likely precursor to these metabolites is a cysteine-BDA-lysine crosslink 6 (Scheme 1). In addition to acetylation, both amino acid residues are predicted to undergo other pathways of biotransformation. We explored the ability of both the cysteine and the lysine residues of 6 to undergo further metabolism. Compound 6, itself, was not observed as a urinary metabolite of furan (data not shown).
Biotransformation of cysteine residue of 6
In a previous report, S-[1-(1,3-dicarboxypropyl)-1H-pyrrol-3-yl]methylthiol (2) was reported as a downstream metabolite of BDA-glutathione conjugates (17). In a similar reaction, one might predict that 6 could be metabolized by the concerted action of cysteine S-conjugate β-lyase, S-methyltransferase and N-acetyltransferase to S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol (7, Scheme 2). Oxidation of the thiol group will led to S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]methanethiol sulfoxide (8, Scheme 2), another potential metabolite. Therefore, we prepared 7 by reacting sodium methylthiolate with BDA and Nα-acetyl-L-lysine. Structural confirmation was obtained by NMR and MS analysis of the purified product. Oxidation of this compound with m-chloroperbenzoic acid led to the formation of the anticipated sulfoxide product, 8. The structural assignment was supported by NMR analysis. As expected for the oxidation of the sulfur group, the proton signal for 8′s 3-methyl group (2.68 ppm) was shifted downfield relative to that of 7 (2.21 ppm) whereas the methylene group directly connected to the pyrrole nitrogen display similar chemical shifts for both compounds (3.86 ppm and 3.77 ppm, respectively). Similarly, the 13C-NMR signal for the 3-methyl group of 8 resonated at 42.0 ppm whereas the same carbon atom in 7 appeared at 20.7 ppm; there was little difference in the chemical shift of the methylene carbon attached to the pyrrole nitrogen (7: 49.4 ppm; 8: 49.8 ppm).
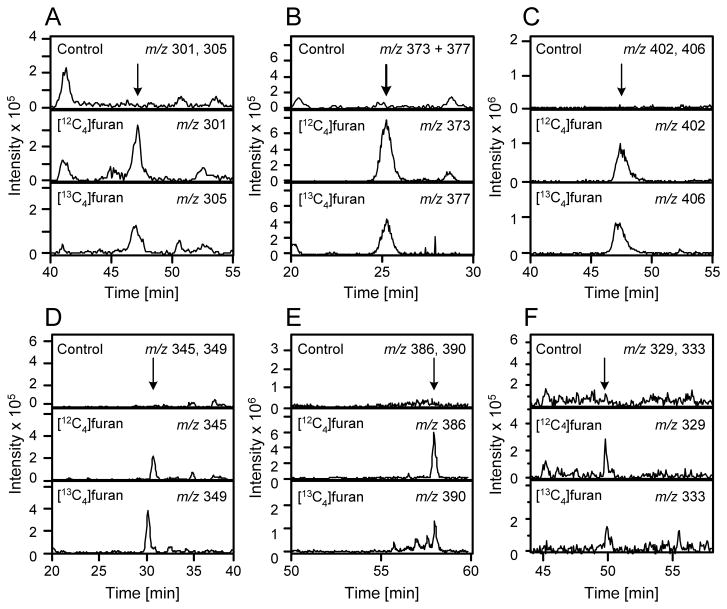
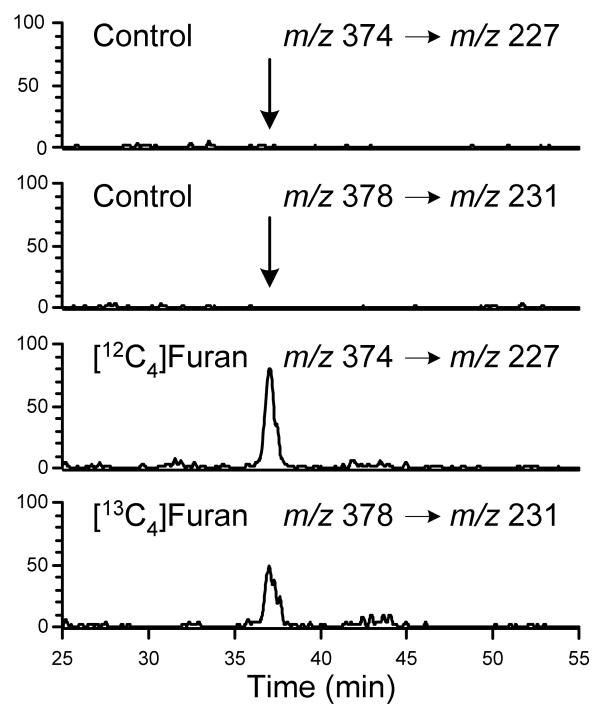
LC-MS/MS analysis of the urine from furan-treated rats did not display a peak at m/z 285 with a retention time similar to 7 (data not shown). A peak was observed at m/z 301 in [12C4]furan-treated rat urine and at m/z 305 in [13C4]furan-treated rat urine with a retention time similar to 8 (47.7 min, Figure 1A). The metabolite co-eluted with the synthetic standard and had an identical daughter ion spectrum (Table 2 and Supplemental Figure 1). In addition, high resolution MS analysis indicated that the metabolite had the expected exact mass for compound 8 (Table 2).
Figure 1.
Extracted mass chromatograms obtained for urine from [12C4]furan-treated rats, [13C4]furan-treated rats or untreated controls. A. m/z 301 and 305; B. m/z 373 and 377; C. m/z 402 and 406; D. m/z 345 and 349; E. m/z 386 and 390; F. m/z 329 and 333.
Biotransformation of lysine residue of 6
Kellert et al has previously reported the detection of a metabolite with m/z 371 in LC-MS/MS analyses performed in negative ion mode (17). They proposed that the structure of this adduct might be derived from the transamination of an oxidized N-acetylcysteine-BDA-lysine reaction product. We also observed a similar product in the urine of furan-treated rats. There is a major peak at m/z 373 in the urine from [12C4]furan-treated rats when LC-MS/MS analyses were performed in positive ion mode (Figure 1B). This metabolite had a mass of m/z 377 in urine from [13C4]furan-treated rats and was absent in the urine of untreated rats (Figure 1B). The collision induced fragmentation mass spectrum of this metabolite contained fragment ions that were characteristic for an N-acetylcysteine sulfoxide substituted pyrrole moiety (neutral loss of 77, 95, 147 and 177, Table 2, Supplemental Figure 2A) (11). Therefore, we proposed a structure of N-acetyl-S-[1-(5-oxo-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (9, Scheme 3). High resolution MS analysis of the metabolite indicated that 9 was a likely candidate for this metabolite (Table 2).
A synthetic standard for this metabolite was generated by oxidizing N-acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (10) with pyridinium dichromate (Scheme 4). LC-MS/MS analysis of the reaction mixture indicated the formation of a compound with m/z of 373 which displayed similar retention time and daughter ion mass spectrum to those of the urinary metabolite (Table 2, Supplemental Figure 2A). However, the synthetic material was not stable to preparative isolation for NMR analysis. The source of this instability was likely the α-ketoacid functionality of this compound (22,23). To assist in the chemical characterization, the oxidation product was reacted with methoxyamine prior to its isolation (21). This derivatization converted the α-ketone to an O-methyl oxime and resulted in the shift of the molecular ion by 29 Da to m/z 402. This compound was stable to purification. One- and 2-dimensional NMR analyses demonstrated that this derivatized product is N-acetyl-S-[1-(5-methoxyimino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (11, Scheme 4 and Supplemental Figure 3). Therefore, we also concluded that the product of pyridinium dichromate oxidation of 10 generated 9.
Scheme 4.
The chemical and biochemical synthesis of metabolites 9 and 15 and their subsequent chemical transformations.
Synthetic 9 coeluted with the urinary metabolite supporting our initial conclusion (Supplemental Figure 2B). Treatment of urine with methoxyamine led to the disappearance of the m/z 373 metabolite and the formation of a new compound with a molecular ion (m/z 402) and retention time similar to the synthetic standard for 11 (Figure 1C, Table 2). This derivatized metabolite was shifted by 4 Da in the urine from [13C4]furan-treated rats (Figure 1C). Consistent with the structural assignment, the O-methyl oxime derivatized metabolite co-eluted with the synthetic standard (Supplemental Figure 4A). This structure was further supported by high resolution mass spectral analysis (Table 2).
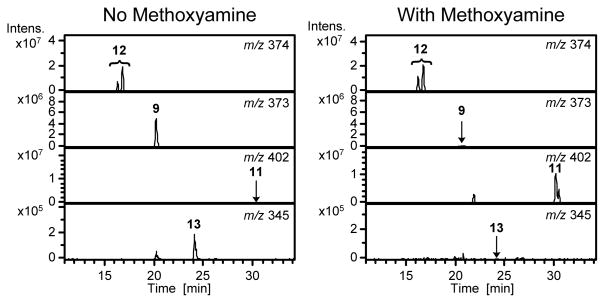
A likely precursor to metabolite 9 is N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (12, Scheme 4). This compound would produce 9 in a transamination reaction. Incubation of 12 in the presence of RLH led to the formation of 9 which was converted to 11 upon reaction with methoxyamine (Figure 2). This reaction product did not form in the presence of boiled RLH. Its levels were enhanced when the incubation mixture was supplemented with 10 mM pyridoxal 5′-phosphate and 10 mM α-ketoglutarate (Supplemental Figure 5). The metabolically generated 11 co-eluted with the synthetic standard (Supplemental Figure 4B). These observations support the hypothesis that metabolite 9 is derived from metabolite 12 in vivo.
Figure 2.
Representative extracted mass chromatograms for the incubation of 12 with RLH. Incubation mixtures were either analyzed directly or following reaction with methoxyamine.
Compound 9 was somewhat unstable. In the absence of methoxyamine, 9 disappeared and a new metabolite with a molecular ion at m/z 345 increased in abundance. In the RLH incubations, the formation of the new metabolite could be blocked by the inclusion of methoxyamine (Figure 2). The mass difference between 9 and this new metabolite (28 Da) is consistent with the oxidative decarboxylation of 9 to yield N-acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide (13). The mass spectra of the metabolite contained a fragment resulting from the neutral loss of 177 (Table 2 and Supplemental Figure 6A). This fragmentation is consistent with the loss of the N-acetylcysteine sulfoxide residue demonstrating that N-acetyl-cysteine portion of the molecule was intact. Urine samples from [12C4]furan-treated rat also contained a metabolite with a molecular ion of m/z 345 (Figure 1D). This metabolite was shifted by 4 Da in the urine from [13C4]furan-treated rats (Figure 1D). Consistent with the structural assignment of 13, it produced a similar mass spectrum as the corresponding synthetic standard (Table 2 and Supplemental Figure 6A). In addition, the urinary and RLH metabolite co-eluted with the synthetic standard for 13 (Supplemental Figure 6B) and had the expected exact mass for this structure (Table 2).
As demonstrated in the RLH incubations, metabolite 12 is a precursor of metabolites 9 and 13. Metabolite 12 was not observed when the urine from furan-treated rats was analyzed by LC-MS/MS using an ion trap mass spectrometer even when operated in selected ion monitoring mode. Since all compounds containing the N-acetylcysteine sulfoxide moiety (5, 9, 13 and 12) lost 147 Da (C5H9N05) as a neutral fragment, the urine samples were re-analyzed by tandem mass spectrometry with a constant neutral loss scan of 147. When this approach was employed, a peak was observed with a retention time comparable to synthetic 12 (Figure 3). There was a corresponding 4 Da increase in the metabolite (m/z 378) detected in the urine from [13C4]furan-treated rats (Figure 3). Similar data was obtained when the neutral loss of 95 (C2H9NO3) was monitored (data not shown). The identity of the metabolite was also verified by coelution with synthetic standard 12 (Supplement Figure 7). Further studies will be required to determine why this compound was so difficult to detect. Preliminary studies indicate that co-eluting materials in the urine dramatically interfere with the detection of 12 (data not shown).
Figure 3.
Extracted mass chromatograms obtained metabolite 12 in urine from [12C4]furan-treated rats, [13C4]furan-treated rats or untreated controls obtained through LC-MS/MS analysis of rat urine samples using neutral loss of 147 Da scanning.
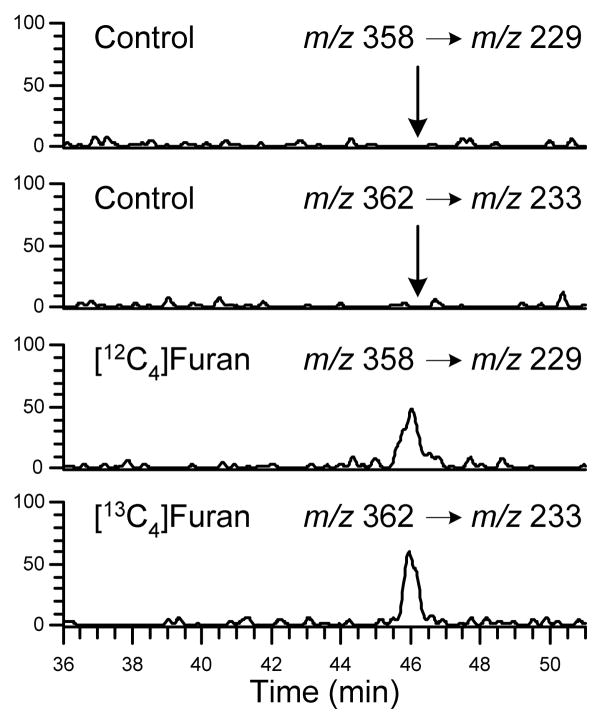
Since metabolites 9 and 13 were urinary metabolites of furan, it is also likely that the N-acetylcysteine precursors to 9, 12 and 13 are also present in the urine of furan-treated rats. Oxidation of N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (14) would lead to 12 (Scheme 3). Compound 14 was detected as a metabolite of furan in hepatocytes (11). As with 12, metabolite 14 was not detected when the urine was analyzed with an ion trap mass spectrometer. However, this metabolite was observed when the urine was re-analyzed by tandem mass spectrometry with a constant neutral loss scan of 129 (C5H7NO3). This is a characteristic neutral loss observed in N-acetylcysteine conjugates (Figure 4) (24). This metabolite co-eluted with the synthetic standard 14 (Supplemental Figure 8). As with metabolite 12, the reasons for the detection difficulties require further investigation but may involve the presence of co-eluting chemicals that suppress the ionization of 14 (data not shown).
Figure 4.
Extracted mass chromatograms obtained metabolite 14 in urine from [12C4]furan-treated rats, [13C4]furan-treated rats or untreated controls obtained through LC-MS/MS analysis of rat urine samples using neutral loss of 129 Da scanning.
Metabolism of 14 by a transaminase leads to N-acetyl-S-[1-(5-oxo-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (15, Scheme 3). This metabolite, in turn, could be oxidized to generate 9. Compound 15 was not detected in the urine of furan-treated rats. However, when the urine was treated with methoxyamine, a peak with a molecular ion at m/z 386 was observed (Figure 1E). The mass of this peak was increased by 4 Da in the urine of [13C4]furan-treated rats (Figure 1E). The mass of this metabolite is 16 Da lighter than that of the O-methyl oxime of 9. On the basis of this information, we proposed that this metabolite was the O-methyl oxime derivative of 15, N-acetyl-S-[1-(5-methoxyimino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (16, Scheme 4). A synthetic standard for this compound was obtained by oxidizing N-acetyl-S-[1-(5-hydroxy-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine (17) with pyridinium dichromate followed by reaction with methoxyamine (Scheme 4). The synthetic standard coeluted with the urinary metabolite derivative 16 and their exact masses are within 5 ppm error of the theoretical value (Supplemental Figure 9A and Table 2). As with the sulfoxide derivatives, incubation of 14 with RLH followed by reaction with methoxyamine led to the formation of 16 (data not shown). This compound had the expected exact mass (Table 2) and coeluted with synthetic 16 (Supplemental Figure 9B).
In the absence of methoxyamine, both the synthetic or RLH-generated 15 underwent oxidative decarboxylation to N-acetyl-S-[1-(4-carboxybutyl)-1H-pyrrol-3-yl]-L-cysteine (18, Scheme 4, Supplemental Figure 10A). Extracted chromatograms at m/z 329 and 333 demonstrated the presence of metabolite 18 in the urine of [12C4]furan- and [13C4]furan-treated rats (Figure 1F). Both the RLH metabolite and the urinary metabolite had the correct exact mass (Table 2) and coeluted with the synthetic standard (Supplemental Figure 10A and B). In addition, the MS/MS spectra were comparable to that of synthetic 18 (Table 2 and Supplemental Figure 10C).
Discussion
The identification of 8, 9, 12, 13, 14, 15 and 18 as urinary metabolites of furan in addition to the previously identified metabolites, 4 and 5, indicates that the cysteine-BDA-lysine crosslink 6 is an important intermediate in the biotransformation of furan. Their structures indicate that both the cysteine and lysine residues of 6 can undergo further transformations. The cysteine residue can be N-acetylated by cysteine conjugate N-acetyltransferases. Alternatively, it can undergo β-lyase catalyzed β-elimination followed by S-methylation in a reaction likely catalyzed by S-methyltransferase (Scheme 2). Both metabolic routes produce sulfide metabolites that are oxidized to sulfoxides in reactions catalyzed by either cytochrome P450 or flavin mono-oxygenase (25,26). The order in which cysteine N-acetylation and S-oxidation occurs is not known. The lysine moiety of 6 also has several biotransformation pathways. If it is not N-acetylated, the lysine residue is converted to an α-ketoacid (9 and 15) by transaminases (Scheme 3). This metabolite can undergo oxidative decarboxylation to 13 or 18. This latter reaction can occur non-enzymatically. We did not observe any products resulting from both the β-elimination and transamination reaction pathways (data not shown).
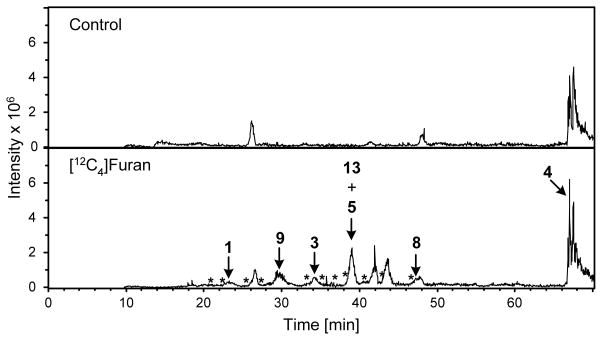
In the LC-MS/MS analyses, the intensity of the peaks was greatest for metabolites 4, 5 and 9 (Figure 5). These three compounds were also the dominant metabolites detected by Kellert et al (17). These observations indicate that, if they are not the most abundant urinary metabolites, they are at least the easiest to detect within the urine matrix by LC-MS/MS. A more accurate assessment of their overall contribution to the metabolism of furan will require the use of internal standards that correct for likely differences in ionization efficiency of each of the metabolites in urine.
Figure 5.
Extracted ion current for all identified metabolites in urine from furan-treated rats. Top trace: urine from untreated rats. Bottom trace: urine from [12C4]furan-treated rats. * denote the retention times of detected but as yet unidentified furan metabolites. The peaks present at 41.9 and 43.5 min in the urine from [12C4]furan-treated rats were not observed in urine from [13C4]furan-treated rats (data not shown) so these two peaks are not likely metabolites of furan.
Collectively, these metabolites indicate that the formation of cross-link 6 represents a significant biotransformation pathway for furan in vivo. There are multiple sources for this compound. Studies in hepatocytes indicate that a GSH-BDA-lysine cross-link is a metabolite of furan (11). It is formed when BDA reacts with GSH and the subsequent conjugate reacts with either free or protein-bound lysine residues. Enzymatic processing of the glutathione moiety by γ-glutamyltranspeptidase and cysteinyl-glycine dipeptidase or aminopeptidase M yields 6 (27). An alternative source of 6 is degraded protein-protein cysteine-lysine crosslinks. Consistent with the proposal that 6 is derived from degraded protein adducts is the observation that approximately 10% of a 8 mg/kg dose of [14C]furan remained covalently associated with liver proteins 24 h post exposure (5). Our observations indicate that a portion of these protein adducts are GSH- or protein cysteinyl-BDA-lysine crosslinks. Therefore, any of the nine identified urinary metabolites could be markers for the activation of furan to protein reactive metabolites. Metabolites of 4, 5 and 9 were the easiest to detect in urine of furan-treated rats (Figure 5), indicating that they may be good targets for human biomarker development.
In summary, structural characterization of furan urinary metabolites supports the hypothesis that furan is oxidized to reactive BDA, which triggers the formation of cysteine-BDA-lysine crosslinks. These metabolites will be explored as biomarkers of furan exposure in humans to aid in the determination of health risks associated with this exposure.
Supplementary Material
Acknowledgments
We thank Patrick Kinney, Dr. Fekadu Kassie, and Dr. Michael Byrns for their assistance with the animal studies, Dr. Peter Villalta and Brock Matters for their assistance with the mass spectral analyses, Choua Vu for the synthesis of [13C4]furan and Meredith Cummings for her assistance in the preliminary characterization of the furan metabolites. The mass spectral analyses were performed in the Analytical Biochemical Core at the Masonic Cancer Center, University of Minnesota, which is funded by National Cancer Institute Center Grant CA-77598. This research is funded by ES-10577 from the National Institutes of Health.
Footnotes
Abbreviations: BDA, cis-2-butene-1,4-dial; RLH, rat liver homogenate
Supporting Information Available: Collision-induced dissociation mass spectra of the metabolites and standards as well as co-elution data are displayed in the supporting information. The NMR spectrum of compound 11 is also provided. This information is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.International Agency for Research on Cancer. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC; Lyon, France: 1995. Furan; p. 393. [Google Scholar]
- 2.Crews C, Castle L. A review of the occurrence, formation and analysis of furan in heat-processed foods. Trends Food Sci Technol. 2007;18:365–372. [Google Scholar]
- 3.National Toxicology Program. Toxicology and carcinogenesis studies of benzofuran in F344/N rats and B6C3F1 mice vol. US Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 1989. NTP Technical Report No. 370. [Google Scholar]
- 4.Albertini R, Bird M, Doerrer N, Needham L, Robison S, Sheldon L, Zenick H. The use of biomonitoring data in exposure and human health risk assessments. Environ Health Perspect. 2006;114:1755–1762. doi: 10.1289/ehp.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burka LT, Washburn KD, Irwin RD. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health. 1991;34:245–257. doi: 10.1080/15287399109531564. [DOI] [PubMed] [Google Scholar]
- 6.Parmar D, Burka LT. Studies on the interaction of furan with hepatic cytochrome P-450. J Biochem Toxicol. 1993;8:1–9. doi: 10.1002/jbt.2570080103. [DOI] [PubMed] [Google Scholar]
- 7.Kedderis GL, Carfagna MA, Held SD, Batra R, Murphy JE, Gargas ML. Kinetic analysis of furan biotransformation by F-344 rats in vivo and in vitro. Toxicol Appl Pharmacol. 1993;123:274–282. doi: 10.1006/taap.1993.1246. [DOI] [PubMed] [Google Scholar]
- 8.Chen LJ, Hecht SS, Peterson LA. Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem Res Toxicol. 1995;8:903–906. doi: 10.1021/tx00049a001. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LA, Cummings ME, Vu CC, Matter BA. Glutathione trapping to measure microsomal oxidation of furan to cis-2-butene-1,4-dial. Drug Metab Dispos. 2005;33:1453–1458. doi: 10.1124/dmd.105.004432. [DOI] [PubMed] [Google Scholar]
- 10.Chen LJ, Hecht SS, Peterson LA. Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 1997;10:866–874. doi: 10.1021/tx9700174. [DOI] [PubMed] [Google Scholar]
- 11.Lu D, Sullivan MM, Phillips MB, Peterson LA. Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan. Chem Res Toxicol. 2009;22:997–1007. doi: 10.1021/tx800377v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gingipalli L, Dedon PC. Reaction of cis- and trans-2-butene-1,4-dial with 2′-deoxycytidine to form stable oxadiazabicyclooctaimine adducts. J Am Chem Soc. 2001;123:2664–2665. doi: 10.1021/ja0056421. [DOI] [PubMed] [Google Scholar]
- 13.Byrns MC, Predecki DP, Peterson LA. Characterization of nucleoside adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 2002;15:373–379. doi: 10.1021/tx0101402. [DOI] [PubMed] [Google Scholar]
- 14.Byrns MC, Vu CC, Peterson LA. The formation of substituted 1,N6-etheno-2′-deoxyadenosine and 1,N2-etheno-2′-deoxyguanosine adducts by cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 2004;17:1607–1613. doi: 10.1021/tx049866z. [DOI] [PubMed] [Google Scholar]
- 15.Byrns MC, Vu CC, Neidigh JW, Abad JL, Jones RA, Peterson LA. Detection of DNA adducts derived from the reactive metabolite of furan, cis-2-butene-1,4-dial. Chem Res Toxicol. 2006;19:414–420. doi: 10.1021/tx050302k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson LA, Cummings ME, Chan JY, Vu CC, Matter BA. Identification of a cis-2-butene-1,4-dial-derived glutathione conjugate in the urine of furan-treated rats. Chem Res Toxicol. 2006;19:1138–1141. doi: 10.1021/tx060111x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellert M, Wagner S, Lutz U, Lutz WK. Biomarkers of furan exposure by metabolic profiling of rat urine with liquid chromatography-tandem mass spectrometry and principal component analysis. Chem Res Toxicol. 2008;21:761–768. doi: 10.1021/tx7004212. [DOI] [PubMed] [Google Scholar]
- 18.Vu CC, Peterson LA. Synthesis of [13C4]furan. J Label Compounds Radiopharm. 2005;48:117–121. [Google Scholar]
- 19.Shin I, Lee M, Lee J, Jung M, Lee W, Yoon J. Synthesis of optically active phthaloyl D-aminooxy acids from L-amino acids or L-hydroxy acids as building blocks for the preparation of aminooxy peptides. J Org Chem. 2000;65:7667–7675. doi: 10.1021/jo0006573. [DOI] [PubMed] [Google Scholar]
- 20.Corey EJ, Schmidt G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Letters. 1979;20:399–402. [Google Scholar]
- 21.Yang L, Kasumov T, Kombu RS, Zhu SH, Cendrowski AV, David F, Anderson VE, Kelleher JK, Brunengraber H. Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle: II. Heterogeneity of metabolite labeling pattern. J Biol Chem. 2008;283:21988–21996. doi: 10.1074/jbc.M803455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AJL, Ginos JZ, Meister A. Synthesis and properties of the α-keto acids. Chem Rev. 1983;83:321–358. [Google Scholar]
- 23.Hayashi T, Tsuchiya H, Naruse H. The stabilization of α-keto acids in biological samples using hydrazide gel column treatment. Clin Chim Acta. 1983;132:321–325. doi: 10.1016/0009-8981(83)90011-6. [DOI] [PubMed] [Google Scholar]
- 24.Levsen K, Schiebel HM, Behnke B, Dotzer R, Dreher W, Elend M, Thiele H. Structure elucidation of phase II metabolites by tandem mass spectrometry: an overview. J Chromatogr. 2005;A1067:55–72. doi: 10.1016/j.chroma.2004.08.165. [DOI] [PubMed] [Google Scholar]
- 25.Sheffels P, Schroeder JL, Altuntas TG, Liggitt HD, Kharasch ED. Role of cytochrome P4503A in cysteine S-conjugates sulfoxidation and the nephrotoxicity of the sevoflurane degradation product fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether (compound A) in rats. Chem Res Toxicol. 2004;17:1177–1189. doi: 10.1021/tx049899e. [DOI] [PubMed] [Google Scholar]
- 26.Krause RJ, Glocke SC, Elfarra AA. Sulfoxides as urinary metabolites of S-allyl-L-cysteine in rats: evidence for the involvement of flavin-containing monooxygenases. Drug Metab Dispos. 2002;30:1137–1142. doi: 10.1124/dmd.30.10.1137. [DOI] [PubMed] [Google Scholar]
- 27.Anders MW, Dekant W. Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol. 1998;38:501–537. doi: 10.1146/annurev.pharmtox.38.1.501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.