Abstract
Background
Quantification of lymphoedema (LE) has been problematic, and the reported incidence of LE varies greatly among women treated with surgery and radiation for breast cancer.
Aims
This study aims to describe LE occurrence over time among breast cancer survivors using four diagnostic criteria based on three measurement techniques.
Methods
Limb volume and symptom assessment data were followed after surgery every three months for 12 months, then every six months for 30 months. Limb volume changes (LVC) were measured by circumferences and by perometry, and by symptom experience via interview. Standard survival analysis methods identified when the criteria indicating LE were met.
Results
Trends in LE occurrence are reported for data from 211 participants. At 30 months post-treatment, LE incidence ranged from 41–91%, with 2cm being the highest estimation method and self-reported signs and symtoms (SS) the lowest.
Conclusions
This 30-month analysis supports the previous 12-month analysis in finding the 2cm criteria as the most liberal definition of LE. Self-reporting of heaviness and swelling, along with 10% LVC, represented the most conservative definitions (41% and 45%, respectively).
Keywords: Breast cancer, Secondary lymphoedema, Diagnostic criteria, Lymphoedema occurrence, Survival analysis
Almost 200,000 American women are newly affected by breast cancer each year (American Cancer Society, 2008a). It comprises the most common cancer for women (outside of skin cancer) in developed parts of the world (American Cancer Society, 2007a). Worldwide, more than a million women are newly diagnosed with breast cancer every year, accounting for a tenth of all new cancers and nearly one-quarter (23%) of all female cancer cases (American Cancer Society, 2007a). In addition, more than two million breast cancer survivors are living in the US (American Cancer Society, 2008b), and the five-year survival rate in Europe is 76% (American Cancer Society, 2007b). Of those affected by breast cancer, up to 40% will develop lymphoedema (LE), depending on the criteria applied. However, all survivors are at risk for the condition (Casley-Smith, 1992; American Cancer Society, 2006, 2009) and the number of survivors affected and potentially affected by secondary LE is staggering, comprising potentially one to five million people.
LE is the accumulation of protein-rich fluid in the interstitial spaces of the affected body part due to a blockage or malfunction in the lymph system. This is different than swelling which may occur immediately after surgery and may be present at the post-operative visit (Mortimer, 1998). LE swelling causes discomfort and sometimes disability; later, it can cause cellulitis and lymphangitis, predisposing the patient to systemic and sometimes life-threatening infection. The physical and psychological aspects of the condition have a considerable impact on the daily lives of LE patients (Hull, 1998; Geller et al, 2003; Radina and Armer, 2001, 2004).
Earlier scientific literature reported anywhere from 6 to 30% (Petrek and Heelan, 1998) or 6 to 62.5% (Passik and McDonald, 1998) of the breast cancer population has LE. Medical literature, however, has narrowed the number, reporting that 15 to 20% of the breast cancer population has LE (Disa and Petrek, 2001). A common estimate is that 20 to 40% of breast cancer survivors develop LE (Coen et al, 2003; Deutsch and Flickinger, 2003; Geller et al, 2003; Voogd et al, 2003; Ozaslan and Kuru, 2004; Hayes et al, 2008), and the number does not significantly vary between Caucasians and African-Americans (Meeske et al, 2008). The discrepancies among the reported percentages stem from difficulties in measurement, diagnosis, and follow-up (Meek, 1998; Petrek and Heelan, 1998; Passik and McDonald,1998; Rockson, 1998; Armer and Stewart, 2005; Hayes et al, 2008).
Traditionally, finding 2 or more cm difference in limb girth between the affected and non-affected limbs warranted clinical diagnosis of LE (Callaway, 1988; Armer and Stewart, 2005). However, other methods are also commonly used. Measuring a 200ml limb volume difference or a 10% limb volume change (LVC) from baseline and/or between the affected and non-affected limb are both documented methods of LE diagnosis (Petlund, 1991; Armer and Stewart, 2005). Self-reported signs and symptoms (SS) are also identified as predictive of LE (Armer et al, 2003; Armer and Stewart, 2005).
The reported incidence of LE fluctuates greatly among each participant group at risk for LE. It has been reported that most often breast cancer patients are not made aware of the risk of LE post-operatively (Radina et al, 2004; Ridner, 2006). This lack of information may cause them to take longer to recognise and report possible symptoms of LE. Likewise, some survivors may not report symptoms because they may not know what LE is or how to detect it (Radina et al, 2004). Other survivors are well-aware of their risk and detect LE via self-assessment. Overall, though, lack of sufficient knowledge about LE and its effects contributes to variance in survivors’ reported incidence of LE (Armer and Stewart, 2005).
While numerous studies have reported LE incidence during the first 12 months following breast cancer treatment, little is known regarding long-term LE diagnosis. Very few studies have examined LE incidence past one year post-treatment, and most that have are retrospective or cross-sectional, not prospective in nature. In fact, in one analysis of existing literature, the authors found the study with the shortest follow-up (12 months) reported the lowest LE incidence (Petrek and Heelan, 1998). Likewise, the study with the longest follow-up (11 years) reported the highest incidence (Petrek and Heelan, 1998).
Aims
The current study aimed to compare three measurement techniques using four diagnostic criteria to quantify LE occurrence up to 30 months post-breast cancer treatment. This study is unique in its prospective design, examining LE prevalence through 2.5 years with baseline data collection occurring before treatment. The preliminary findings will add new insight regarding the duration and technique for optimal observation of LE incidence.
Methodology
The study was designed to use prospective, repeated measures on 211 female participants newly-diagnosed with breast cancer. Participant recruitment and data collection took place at a Midwestern university-affiliated state cancer centre. Consent was obtained and the participants were then enrolled. They were assessed pre-and post-treatment, every three months for 12 months, then every six months thereafter, for a total of 30 months.
Two objective measurement techniques were used at each visit to quantify limb volume characteristics: circumferential measurement and infra-red perometery. Traditional anthropometric measurements recorded limb girth every 4cm on each arm using a non-stretch, flexible tape measure. Infra-red perometry (Perometer 400T/350S, Juzo, Cuyahoga Falls, OH) was used to record three-dimensional images of each limb, which were used to calculate limb volume. A detailed description of these techniques has been previously published (Armer and Stewart, 2005).
In addition to the two objective measures, one subjective analysis of LE symptoms was administered each visit through the LE and Breast Cancer Questionnaire (LBCQ) (Armer et al, 2003). The LBCQ, which has previously been validated, consists of 57 questions examining 19 signs and symptoms drawn from the literature and clinical observation (Armer et al, 2003). Based on these previous findings, self-report of heaviness or swelling ‘now’ or ‘in the past year’, was included as one definition for LE.
From those measurements, four criteria for identifying LE were used:
2cm circumferential change at any measured location
200ml perometry LVC of the affected arm
10% perometry LVC of the affected arm
Self-report of limb heaviness and swelling, either ‘now’ or ‘in the past year.’
The objective-based criteria for identifying LE (the first three items above) were based on change from baseline measurements and/or versus unaffected limb.
Certain participants met the definitions for LE before treatment at the baseline (pre-treatment) measurement for one or more of the four criteria used. Those participants were included in the study, but not for analysis in that particular criteria, resulting in different numbers of participants for a given criteria. For example, 16 of the 211 participants met the definition for LE at baseline based on the criteria of self-reported limb heaviness and swelling, resulting in 195 participants in the subsequent self-report analysis. Also, those participants who had both right and left limbs affected due to bilateral mastectomies, lumpectomies, or for prophylactic reasons at any point during the study, were not included for this analysis.
Sample description
The mean age of participants was 57 years old, ranging from 30 to 89 years of age. The treatment characteristics of study participants varied greatly. The greatest number of the participants, 48%, had a mastectomy to treat their breast cancer. Thirty-nine percent had had a lumpectomy and 11% both surgical treatments. Sixty percent underwent chemotherapy and 51% underwent radiation treatment. Forty-three percent of participants had sentinel lymph node biopsy (SLNB) treatment and 30% underwent axillary lymph node dissection (ALND). Eleven percent underwent both SLNB and ALND treatments, while 16% had neither treatment. All of these treatment characteristics illustrate the diverse yet representative treatment of the study sample.
Survival analysis
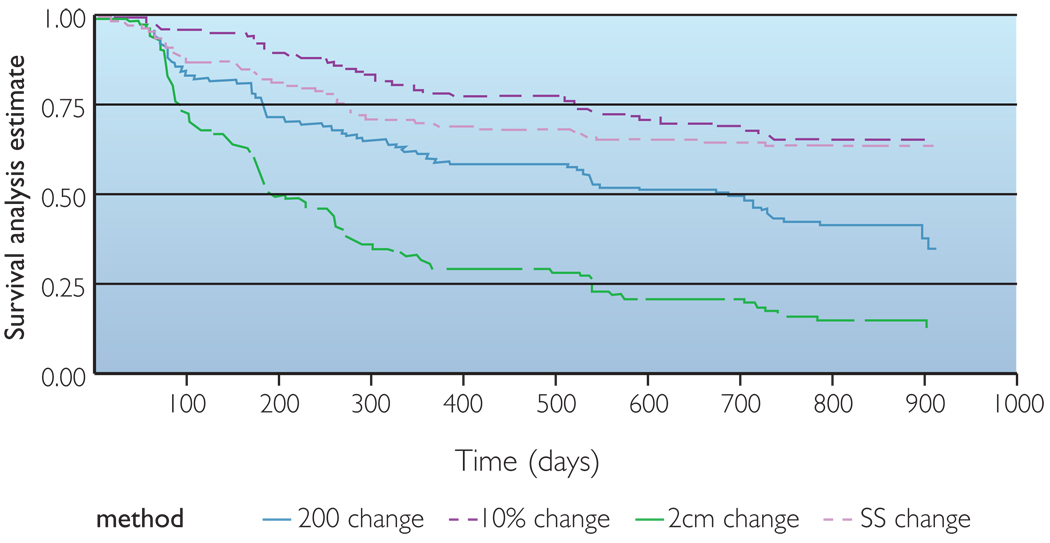
The Lifetest procedure in Statistical Analysis Software (SAS) v9 (SAS Institute Inc, Cary, NC, USA) was used to estimate the survival distributions under each of the definitions of LE using the product-limit or Kaplan-Meier method, a non-parametric technique for estimating time-related events. Survival analysis allows one to estimate the probability of distribution of time to a specified event, such as development of lymphoedema. Specifically, the survival curve gives the probability that the event of interest will occur later than a given time. The event of interest in this analysis is the diagnosis of LE. At time 0 (baseline), the probability is 1.00 (or 100%) that no one has met the criteria for diagnosis. Time was measured from initial treatment to the first diagnosis of LE. Kaplan-Meier estimates of the survival curves were obtained separately for each of the four definitions of LE (Figure 1).
Figure 1.
Comparison of four methods for estimating lymphedema using observations at 6, 12, 18, 24 and 30 months post-treatment using survival analysis.
For any subject for whom the LE diagnosis was met, the actual time is not known precisely since limb volume was measured every three months for the first year and then every six months to 30 months, not every day. When present, we know LE occurred since the previous measurements were taken but we do not know precisely when it emerged. In addition, although subjects were scheduled for measurement visits at three- or six-month intervals, the actual time between visits varied.
Limitations
Only data from subjects with pre-operative baseline measurements were used in this analysis. Also, immediate post-treatment data, which may provide insight to the earliest possible onset of LE, were excluded from analysis due to potential confounding results from post-treatment swelling. In addition, changes in body weight were not considered in this analysis.
Results
The 2cm identifying criteria was the most liberal of the four methods examined, resulting in the highest estimation of LE at the end of the 30 months (91%). The 200ml LVC criteria was the second most likely to identify LE at the end of 30 months (67%). Meanwhile, 10% LVC and SS were the most conservative of the criteria, identifying LE in 45% and 41% of the participants, respectively. The estimated lymphoedema incidence rates for each method over time are presented in Table 1. In addition, the survival analysis is displayed in Figure 1.
Table 1.
Estimated lymphoedema occurence rates using four diagnostic criteria
| Percent occurrence of LE (95% CI) | |||||
|---|---|---|---|---|---|
| Identifying criteria | 6 months | 12 months | 18 months | 24 months | 30 months |
| 200 ml LVC = 182 | 24 (18–31) | 40 (33–48) | 49 (41–57) | 56 (48–64) | 67 (58–76) |
| 10% LVC n = 182 | 9 (5–14) | 22 (16–29) | 28 (21–35) | 34 (27–42) | 45 (33–59) |
| 2 cm n = 166 | 45 (37–53) | 72 (64–79) | 79 (72–85) | 85 (78–90) | 91 (84–96) |
| SS n = 195 | 17 (13–24) | 31 (25–39) | 35 (28–43) | 37 (30–45) | 41 (31–54) |
When examined over smaller time frames, the overall 30-month trends continued. At any six-month point of time during the study, the 2cm criteria identified participants with LE at the highest frequency. Similarly, 200ml LVC was the second most likely to identify LE at any point in the study. However, while 10% LVC and SS had similar occurrence rates at 30 months, they had somewhat different rates of identification of LE over that time period. SS was more likely to identify LE in the first 12 months (31%), then slowed (41% at 30 months). In contrast, 10% LVC was slow to identify LE (22%) in the first 12 months, but more than doubled the rate of identifying LE thereafter (45% at month 30).
Discussion
Previous work in our laboratory has shown that identification of LE in the first year post-treatment for breast cancer varies greatly depending on four commonly used criteria to define LE (Armer and Stewart, 2005). The present study expands on these findings by measuring LE occurrence through two and a half years, the only work to prospectively measure LE occurrence for that length of time. As with the 12-month findings, LE incidence at 30 months varied greatly depending on the criteria used. Nearly all participants (91%) met the criteria for LE based on the 2cm method, by far the highest incidence rate in this analysis. In contrast, SS identified the fewest participants (41%). This wide range of occurrence rates (50%) is identical to the high and low range of occurrence rates at 12 months (72–22% using 2cm and 10% LVC, respectively). This finding represents a common occurrence, since, in the absence of a ‘gold standard’ to diagnose LE, a wide range of criteria exists that is used by nurses, therapists, physicians and researchers. This analysis provides evidence that the discrepancies noted in the literature regarding the identification of LE are due, in part, to the variety of criteria used.
A unique aspect of the design of this study is the use of pre-treatment measurements as baseline data. The majority of the literature available is based on LE occurrence using post-treatment data as the comparison criteria (McLaughlin et al, 2008). By using pre-treatment measurements, this study was able to identify those participants who met the criteria for, but did not have, LE at baseline. Those participants were not included for further analysis in that particular criterion, eliminating a confounding factor for later LE identification. Using this method, up to 21% of the participants at baseline were identified to meet one of the criteria for LE before treatment due to reasons other than LE (i.e. limb volume differences due to arm dominance).
The objective measurements (2cm, 10% LVC, 200ml LVC) were the more frequently met criteria (14–21%), while the subjective criterion (SS) was met at pre-treatment far less frequently (8%). The lack of pre-treatment measurements in other studies most likely results in erroneous estimations of LE, and contributes to the wide ranging discrepancies in LE occurrence rates across the literature. These findings document the importance of pre-treatment anthropometric and symptom data collection.
These preliminary findings provide additional evidence that breast cancer survivors are at a long-term risk for developing LE. Indeed, the findings of this study show that LE identification, regardless of the method used for estimation, continued to increase past the first year post-treatment. From months 12 to 30, LE identification increased by an additional 10–27%, depending on the criteria used. This increase after the first 12 months underscores the need for long-term measurement of limbs, and monitoring of patient signs and symptoms in breast cancer survivors by healthcare professionals.
The preliminary results of this 30-month analysis of LE occurrence provide unique insight into one aspect of breast cancer survivorship. Future research in our laboratory will continue to examine LE incidence, providing additional evidence that breast cancer survivors are at risk of developing LE up to seven years post-breast cancer treatment. In addition, a prospective longitudinal risk-reduction intervention for the treatment and management of LE is currently underway. Finally, a psychosocial analysis of the impact of breast cancer, an equally neglected area of LE research, is ongoing.
Key points
Breast cancer survivors are at life-time risk of developing lymphoedema (LE).
Quantification of LE has been problematic, despite the fact that various methods have been used to measure the lymphoedematous limb.
In part because of difficulties and variability in measurement and diagnosis, the reported incidence of LE varies greatly among women treated with surgery and radiation for breast cancer.
From these preliminary 30-month data, in combination with earlier 12-month data analysis, it appears that 10% limb volume change corresponds to a more conservative definition of LE, while the 2cm difference corresponds to a more liberal definition.
In the absence of a ‘gold standard’ in clinical lymphoedema measurement, it is only possible to say that the different LE criteria are not equivalent, but not which of the four definitions is best.
These preliminary findings also document the importance of baseline (pre-operative) anthropometric and symptom data and monitoring of changes over time.
Conclusions
These preliminary findings provide information about additional evidence that breast cancer survivors are at a risk for developing LE beyond the first year following treatment. This 30-month analysis supports the previous 12-month analysis in finding the 2cm criteria as the most liberal definition of LE. Self-reporting of heaviness and swelling, along with 10% LVC, represented the most conservative definitions (41% and 45%, respectively). Furthermore, the variety of criteria used to identify LE, along with the lack of pre-treatment measurements, are likely to be responsible for the wide range of lymphoedema rates available in the literature.
Footnotes
Declaration of interest: None.
References
- American Cancer Society. Lymphedema: Understanding and managing lymphedema after cancer treatment. Atlanta: ACS; 2006. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2007. Atlanta: American Cancer Society; 2007a. [Google Scholar]
- American Cancer Society. Global Cancer Facts & Figures. Atlanta: American Cancer Society; 2007b. [Google Scholar]
- American Cancer Society. Breast Cancer Facts & Figures. Atlanta: American Cancer Society; 2008a. [Google Scholar]
- American Cancer Society. Lymphedema: What every woman with breast cancer should know. Atlanta: ACS; 2009. [last accessed 23 March, 2009]. Available online at: www.cancer.org/docroot/MIT/content/MIT_7_2x_Lymphedema_and_Breast_Cancer.asp. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2008b. [Google Scholar]
- Armer JM. The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer Investigation. 2005;23:76–83. [PubMed] [Google Scholar]
- Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Armer JM, Stewart BR. A Comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphatic Res Biol. 2005;3:208–217. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- Callaway CW. Circumferences. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Illinois: Human Kinetics Books; 1988. [Google Scholar]
- Casley-Smith JR. Modern treatment of lymphoedema. Modern Med Australia. 1992;35(5):70–83. [Google Scholar]
- Coen JJ, Taghian AG, Kachnic LA, Assad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiation Oncol Biol Physics. 2003;55:1209–1215. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Flickinger JC. Arm edema after lumpectomy and breast irradiation. Am J Clin Oncol. 2003;26:229–231. doi: 10.1097/01.COC.0000018177.75673.06. [DOI] [PubMed] [Google Scholar]
- Disa JJ, Petrek J. Rehabilitation after treatment for cancer of the breast. In: Devita VT jr, Hellman S, Rosenberg SA, editors. Cancer Principles and Practice of Oncology. 6th edn. Philadelphia PA: Lippincott,Williams & Wilkins; 2001. [Google Scholar]
- Geller BM, Vacek PM, O’Brien P, Secker-Walker RH. Factors associated with arm swelling after breast cancer surgery. J Women’s Health. 2003;12:921–930. doi: 10.1089/154099903770948159. [DOI] [PubMed] [Google Scholar]
- Hayes S, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema secondary to breast cancer: how choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology. 2008;41:10. [PubMed] [Google Scholar]
- Hull MM. Functional and psychosocial aspects of lymphedema in women treated for breast cancer. Innovations Breast Cancer Care. 1998;3:97–100. [Google Scholar]
- McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek AG. Breast Radiotherapy and Ly mphedema. Cancer Suppl. 1998;83(12):2788–2797. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2788::aid-cncr27>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Meeske KA, Sullivan-Halley J, Smith AW, McTiernan A, Baumgartner KB, Harlan LC, Bernstein L. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat. 2008;113(2):383–391. doi: 10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]
- Mortimer P. Pathophysiology of lymphedema. Cancer Suppl. 1998;83(12):2798–2802. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. doi: 10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- Passik S, McDonald M. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. American Cancer Society Lymphedema Workshop. Cancer Suppl. 1998;83(12):2817–2820. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Petlund CF. Volumetry of limbs. In: Olszewski WL, editor. Lymph stasis: Pathophysiology, diagnosis and treatment. Boca Raton, Florida, USA: CRC Press; 1991. [Google Scholar]
- Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Radina ME, Armer JM. Surviving breast cancer and living with lymphedema: Resiliency among women and their families. J Family Nurs. 2004;10(4):485–505. [Google Scholar]
- Radina ME, Armer JM. Post-breast cancer lymphedema and the family: A qualitative investigation of families coping with chronic illness. J Family Nurs. 2001;7(3):281–299. [Google Scholar]
- Radina ME, Armer JM, Culbertson SD, Dusold JM. Post-breast cancer lymphedema: understanding women’s knowledge of their condition. Oncol Nurs Forum. 2004;31:97–104. doi: 10.1188/04.ONF.97-104. [DOI] [PubMed] [Google Scholar]
- Ridner SH. Pretreatment lymphedema education and identified educational resources in breast cancer patients. Patient Ed Counseling. 2006;61:72–79. doi: 10.1016/j.pec.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Diagnosis and management of lymphedema. Cancer. 1998;83:2882–2885. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2882::aid-cncr45>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90:76–81. doi: 10.1002/bjs.4010. [DOI] [PubMed] [Google Scholar]