Abstract
Remineralization of demineralized dentin lesions adjacent to glass-ionomer cements (GICs) has been reported in the literature. This study tested the hypothesis that a strontium-based GIC can remineralize completely demineralized dentin by nucleation of new apatite crystallites within an apatite-free dentin matrix. Human dentin specimens were acid-etched, bonded with Fuji IXGP, and immersed in a calcium-and-phosphate-containing 1.5X simulated body fluid (SBF) for 1-4 months. Polyacrylic acid and polyvinylphosphonic acid biomimetic analogs were added to the SBFs to create 2 additional remineralization media. Specimens were processed by transmission electron microscopy (TEM). No apatite deposition could be identified in the completely demineralized dentin in any of the specimens immersed in the 3 remineralization media, despite TEM/EDX evidence of diffusion of ions specific to the strontium-based GIC into the demineralized dentin. The hypothesis was rejected; mineral concentration alone is not a sufficient endpoint for assessing the success of contemporary remineralization strategies.
Keywords: glass-ionomer cement, demineralized dentin, remineralization, apatite, ion diffusion
Introduction
Glass-ionomer cements (GICs) have been widely used in restorative dentistry (Mount, 1998), due to their self-adhesive and fluoride-releasing properties (Naasan and Watson, 1998). Indirect evidence of the ability of GICs to remineralize artificial caries lesions was based on the results of microradiography and micro-computed tomography (Creanor et al., 1998; Exterkate et al., 2005; Lee et al., 2008). The use of energy-dispersive x-ray microanalysis (EDS) (Massara et al., 2002) and electron probe microanalysis (EPMS) (Kitasako et al., 2003; Ab-Ghani et al., 2007) further revealed changes in mineral profiles of specific elements across the GIC-tooth interface. Despite evidence of ion exchange between GICs and dentin at a chemical level (Coutinho et al., 2007), there is no direct ultrastructural evidence of interfibrillar or intrafibrillar remineralization of the demineralized collagen matrix. Intrafibrillar remineralization, in particular, is crucial for restoring the mechanical properties of partially mineralized dentin to those of mineralized dentin (Kinney et al., 2003).
A recent report presented a successful strategy for in situ remineralization of phosphoric-acid-etched dentin in the presence of biomimetic analogs (Tay and Pashley, 2008). In that study, transmission electron microscopy (TEM) and electron diffraction were used to establish definitive evidence of interfibrillar and intrafibrillar apatite deposition within the collagen fibrils of the demineralized dentin matrix. These ultrastructural techniques were indispensible in discriminating the dimensions and ordered arrangement of apatite platelets in mineralized tissues (Traub et al., 1989). Incorporation of biomimetic analogs of non-collagenous proteins into the remineralization medium helped to stabilize amorphous calcium phosphate precursors in their nanoscale dimensions (Zhang and Webster, 2009). The analogs further served as templates that guided the self-assembly of the stabilized nanoprecursors along specific sites of the collagen fibrils (Gajjeraman et al., 2007), their subsequent transformation into nanoapatites, and fusion into conventional apatite crystallites. These phenomena were thought to proceed via the non-classic crystallization pathways of “bottom-up” nanoparticle assembly and mesocrystalline transformation (Niederberger and Cölfen, 2006).
Fuji IXGP (GC International, Tokyo, Japan) is a strontium-based GIC developed for the Atraumatic Restorative Treatment of carious dentin (Massara et al., 2002; Amaral et al., 2006). It has been demonstrated that ions specific to Fuji IXGP penetrated deeply into caries-affected dentin (Ngo et al., 2006). Those authors considered the presence of strontium and aluminum ions in the demineralized dentin matrix as evidence of a remineralization process. In this study, we challenged the ability of Fuji IXGP to remineralize completely demineralized dentin at an ultrastructural level by testing the hypothesis that the strontium-based GIC can successfully remineralize completely demineralized dentin by nucleation of new apatite crystallites within an apatite-free dentin matrix.
Materials & Methods
Specimen Preparation
Twenty-one non-carious human third molars were collected after donors’ informed consents were obtained under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. For each tooth, a flat surface was created in mid-coronal dentin by means of a slow-speed Isomet diamond saw (Buehler Ltd, Lake Bluff, IL, USA) under water cooling. The surface was polished with 600-grit silicon carbide paper under running water. A second parallel cut was made at the cemento-enamel junction to create a dentin block that was devoid of pulpal tissues.
Placement of GIC
Each coronal dentin surface was etched with 32% phosphoric acid gel (Bisco Inc., Schaumburg, IL, USA) for 1 min to create a completely demineralized dentin layer on top of a mineralized dentin base. The etched surface was rinsed with de-ionized water and covered with moistened lint-free tissues to prevent desiccation of the demineralized collagen matrix.
Fuji IXGP capsules were triturated for 10 sec and dispensed over the acid-etched dentin to produce 5-mm-thick cores, resulting in 16 GIC-bonded specimens. After initial setting, the exposed GICs were coated with nail varnish, leaving only the dentinal tubules along the pulpal side of the dentin blocks as pathways of communication between the remineralization media and the bonded dentin (Czarnecka et al., 2002). After storage at 37°C and 100% relative humidity for 24 hrs, the bonded specimens were immersed in the designated remineralization medium. One of the Fuji IXGP specimens was not immersed in any of the 3 remineralization media, but was analyzed as a 24-hour baseline specimen.
Remineralization Media
We prepared a 1.5X simulated body fluid (SBF; Kokubo and Takadama, 2006) by dissolving 205.2 mM NaCl, 6.3 mM NaHCO3, 4.5 mM KCl, 1.5 mM K2HPO4·3H2O, 2.25 mM MgCl2·6H2O, 3.75 mM CaCl2, and 0.75 mM Na2SO4 in de-ionized water and adding 3.08 mM sodium azide to prevent bacterial growth. This 1.5X SBF served as the classic remineralization medium, which contained no biomimetic analog. Two additional biomimetic remineralization media were prepared. For the first medium, 200-800 µg/mL of polyvinylphosphonic acid (PVPA; Sigma-Aldrich, St. Louis, MO, USA) was added to the 1.5X SBF as the sole biomimetic analog. Polyacrylic acid (PAA), being a component of the GIC liquid, was not used in this medium. For the other medium, the 1.5X SBF was supplemented with 200-800 µg/mL of PVPA and 200-1,500 µg/mL of PAA (MW 1,800; Sigma-Aldrich) as dual biomimetic analogs. All solutions were buffered to pH 7.2 with 0.1 M Tris Base or 0.1 M HCl.
Remineralization of GIC-bonded Dentin
The GIC-bonded dentin specimens were randomly divided into 3 groups (N = 5) according to the remineralization medium. Each specimen was placed in a glass scintillation vial that was filled with 15 mL of the respective medium. The medium was changed every mo. Specimens were retrieved after 1-4 mos for ultrastructural examination of the extent of remineralization. Each specimen was sectioned occluso-gingivally into a 0.9-mm-thick slab containing the GIC-dentin interface after 1, 2, 3, or 4 mos. The exposed GIC-dentin interface of the intact specimen was protected with nail varnish after being sectioned. The assembly was returned to the vial for continuous remineralization prior to being re-sectioned at the second, third, and fourth mos.
Portland Cement-based Biomimetic Remineralization Control
The remaining 5 teeth were subjected to a biomimetic remineralization scheme that was slightly modified from the one reported previously (Tay and Pashley, 2008). Briefly, white Portland cement was mixed at a water-to-powder ratio of 0.35:1, and placed on top of an unetched dentin disk with a piece of Saran wrap placed between the dentin surface and the cement to obtain an impression of the dentin disk. After the mixture set, the Saran wrap was removed. The dentin surface was etched with 32% phosphoric acid for 60 sec. The set cement block was placed over the acid-etched dentin, and the assembly was immersed in 15 mL of the aforementioned second biomimetic remineralization medium (1.5X SBF with dual biomimetic analogs PAA and PVPA). Monthly sectioning of the specimen slabs followed the manner described for the GIC specimens, with the exception that the Portland cement-dentin assemblies were stabilized with sewing threads prior to being sectioned. The exposed interfaces were protected with varnish.
Transmission Electron Microscopy
The specimen slabs were prepared for TEM according to a previously reported protocol (Tay and Pashley, 2008). Briefly, the specimens were dehydrated in ethanol (70-100%), immersed in propylene oxide, and embedded in epoxy resin. Non-demineralized, 90- to 110-nm-thick sections were prepared and examined unstained in a JEM-1230 TEM (JEOL, Tokyo, Japan) operated at 110 kV. Selected area electron diffractions (SAEDs) were performed at specific locations of the TEM sections, with centered dark-field imaging.
Elemental Distribution
Representative 4-month-old specimens from the 3 GIC remineralization groups were examined by TEM-EDS in a Tecnai G2-20 TEM equipped with a scanning transmission attachment (FEI Co., Hillsboro, OR, USA). Line scans were performed for the distribution of calcium, phosphorus, strontium, aluminum, and fluorine across the GIC-dentin interfaces.
Results
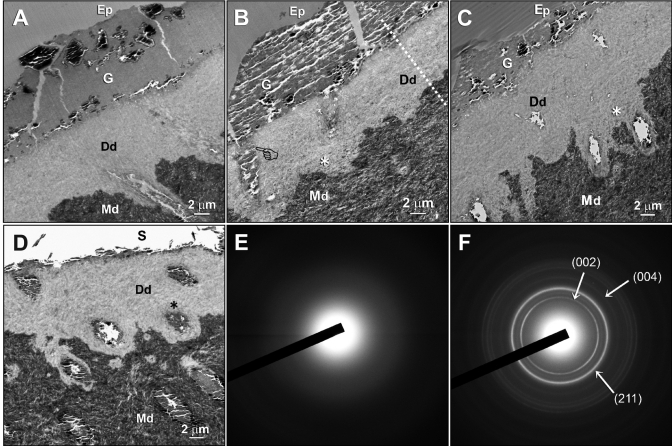
The baseline specimen revealed an 8- to 12-µm-thick layer of completely demineralized dentin created by 60 sec of phosphoric-acid-etching. The GIC adapted well to the apatite-depleted dentin surface (Fig. 1A). Apatite re-deposition was not apparent within the demineralized dentin matrices of any of the 1- to 4-month-old GIC-restored specimens, regardless of their medium type and immersion times. Nevertheless, a zone of electron-dense grains (Ed) containing demineralized dentin was identified adjacent to the mineralized dentin base after the specimens were immersed in the 3 remineralization media (Figs. 1B-1D, 2B). Diffuse SAED patterns derived from the Ed-containing demineralized dentin (Fig. 1E) indicated the absence of crystalline phases within these regions. By contrast, SAED ring patterns collected from the mineralized dentin base were characteristic of polycrystalline apatite (Fig. 1F).
Figure 1.
Transmission electron micrographs (TEMs) and selected area electron diffraction (SAED) taken from unstained, non-demineralized sections of glass-ionomer cement (GIC)-dentin interfaces. (A) A 24-hour baseline specimen that had not been immersed in any of the 3 remineralization media. A layer of apatite-depleted, completely demineralized dentin was produced by 60 sec of phosphoric-acid-etching. Desiccation artifacts could be seen within the GIC. (B) Low-magnification view of a representative specimen that had been immersed in a simulated body fluid (SBF) devoid of biomimetic analogs for 4 mos. The GIC formed tags (pointer) within the dentinal tubules. A zone of demineralized dentin containing electron-dense grains (Ed) was seen above the mineralized dentin base (asterisk). (C) A representative specimen that had been immersed in a polyvinylphosphonic acid (PVPA)-containing SBF for 4 mos. A basal Ed zone (asterisk) could be seen adjacent to the mineralized dentin base. (D) A representative specimen that had been immersed in a polyacrylic acid- and PVPA-containing SBF for 4 mos. The epoxy-resin-embedded GIC dislodged during sectioning, leaving a space (S) on top of the GIC remnants. Sectioning artifacts could be observed within the GIC glass particles. The Ed appeared diffuse throughout the demineralized dentin, although they were more concentrated around the periphery of dentinal tubules (asterisk). (E) A SAED pattern that is representative of the Ed-containing demineralized dentin. The diffuse electron diffraction pattern was indicative of the absence of crystalline phases within the demineralized dentin. (F) SAED collected from the mineralized dentin base revealed discrete rings indicative of the polycrystalline nature of the mineral phases. The indices confirmed the presence of apatites within the naturally mineralized dentin. Generic abbreviations: Ep, epoxy resin; G, GIC; Dd, demineralized dentin; Md, mineralized dentin.
Figure 2.
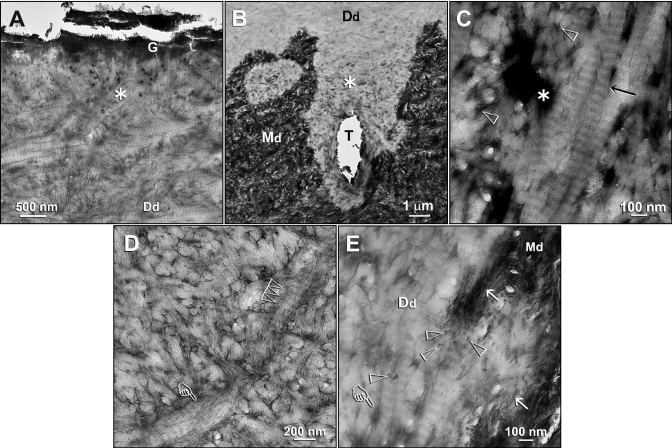
Ultrastructural characterization of the electron-dense grains (Ed)-containing zones in the demineralized dentin layers derived from specimens that had been immersed in the remineralization media. (A) A specimen that had been immersed in a PVPA-containing SBF for 4 mos. The electron-dense grainy materials were not exclusively found along the base of the demineralized dentin layer. A higher-magnification view of the junction between the fractured GIC (G) and the underlying demineralized dentin surface revealed an Ed-containing superficial region (asterisk). Although no additional staining was used, excellent banding characteristics of the demineralized dentin collagen matrix (Dd) could be observed. (B) An Ed-containing zone (asterisk) at the base of the demineralized dentin layer. The specimen had been immersed in a polyacrylic acid- and PVPA-containing SBF for 4 mos. T: dentinal tubule. (C) A high-magnification view of the Ed-containing regions depicted by the “asterisk” in Figs. 2A and 2B revealed a strong negative staining effect, in this bright-field micrograph, that was created in the absence of the use of an extrinsic negative stain. Banding could be identified within the collagen fibrils (arrow) even in the absence of extrinsic staining. No mineral crystallites could be identified around or within these fibrils. Open arrowheads: interfibrillar spaces between the collagen fibrils. (D) A high-magnification view of the area labeled with “Dd” in Fig. 2A. Dark interfibrillar spaces were present despite the absence of the larger, dark terminal tubular channels seen in Fig. 2C. Apart from banding of the collagen fibrils (multiple open arrowheads), the rope-like subfibrillar architecture of the collagen fibril (pointer) was highlighted by the negative staining. No intrafibrillar or interfibrillar crystallites could be identified in these fibrils. (E) A high-magnification view of the junction between the Ed-containing zone and the mineralized dentin base in Fig. 2B. Remnants of apatite platelets (open arrowheads) and needles (arrows) were present along the demineralization front. The Ed within the interfibrillar spaces and terminal tubular branches (pointer) appeared less electron-dense, due to the auto-contrast function of the microscope in the presence of the highly mineralized dentin base along the right side of the micrograph. The absence of apatite remineralization in the negatively stained collagen fibrils and the adjacent interfibrillar spaces is clearly evident. Generic abbreviations: Dd, demineralized dentin; Md, mineralized dentin.
Higher-magnification views of the unstained, Ed-containing demineralized dentin revealed excellent banding characteristics and the rope-like subfibrillar architecture of the collagen fibrils (Figs. 2A, 2D). Although interfibrillar spaces and the terminal branches of dentinal tubules were highlighted by an apparent negative staining effect (Figs. 2C, 2E), intrafibrillar and interfibrillar mineral crystallites were absent throughout the collagen matrix.
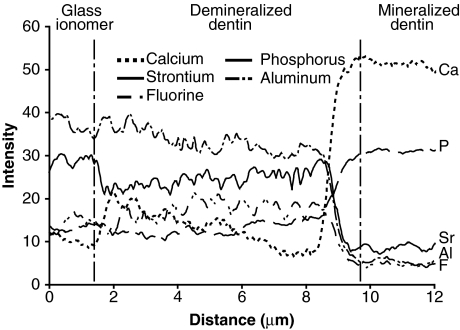
Elemental distributions across the GIC-dentin interface from a representative specimen that had been immersed in 1.5X SBF for 4 mos are shown in Fig. 3. Fuji IXGP-specific elements (aluminum and strontium) and fluorine were present in the demineralized dentin layer. These elements had intensity levels similar to or slight lower than those present in the GIC material. Compared with mineralized dentin, calcium and phosphorus intensity levels were lower in the demineralized dentin layer. Similar results were seen in specimens retrieved from the other 2 remineralization media (not shown).
Figure 3.
TEM-EDS line scans of the distribution of calcium (Ca), phosphorus (P), aluminum (Al), strontium (Sr), and fluorine (F) across a representative GIC-dentin interface in a specimen that had been immersed in the SBF devoid of biomimetic analogs for 4 mos. The white dotted line in Fig. 1B represents the generic location upon which elemental analyses were performed across the GIC-dentin interfaces. The changes in elemental profiles may be divided into 3 distinct zones, beginning at the left: (A) In the GIC restoration, strontium, aluminum, and fluorine intensity levels were higher than those in the sound dentin; (B) within the demineralized dentin layer, the 2 GIC-specific elements (strontium, aluminum) were similar to and slightly lower than their corresponding levels in the GIC proper, while, conversely, calcium and phosphorus intensities were low within this demineralized layer; and (C) within the mineralized dentin base, the intensity levels of the 2 GIC-specific elements and fluorine decreased to their lowest levels, while those of calcium and phosphorus increased to their maximum levels.
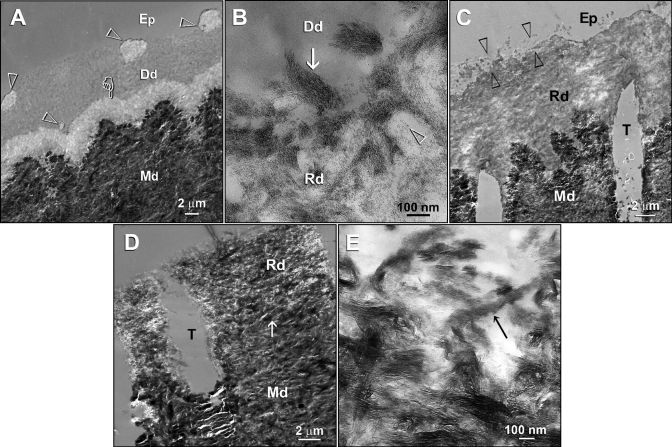
For the Portland cement control, specimens that were immersed in PAA- and PVPA-containing remineralization medium exhibited foci of remineralization within the demineralized dentin matrix at 2 mos (Fig. 4A). Coalescence of these foci resulted in heavier, but incomplete, remineralization along the entire thickness of the matrix at 3 (Fig. 4C) and 4 mos (Fig. 4D). There was clear evidence of intrafibrillar remineralization within the collagen fibrils (Figs. 4B,4E).
Figure 4.
TEMs taken from unstained, non-demineralized sections of acid-etched dentin in the Portland cement-based biomimetic remineralization control group. (A) A specimen retrieved after 2 mos of biomimetic remineralization in the polyacrylic acid- and PVPA-containing SBF revealed discrete islands of partial remineralization (open arrowheads) and a 3-µm-thick zone of partial remineralization (pointer) over the original demineralization front. (B) A high-magnification view of partially remineralized dentin depicted in Fig. 4A. Collagen fibrils along the dentin surface (arrow) were heavily remineralized with nanocrystals. Individual nanocrystals (open arrowhead) could also be seen in the less-highly-remineralized regions. (C) A 3-month-old specimen retrieved from the polyacrylic acid- and PVPA-containing SBF revealed heavier remineralization than extended from the dentin surface to the mineralized dentin base. Remineralization was absent or sparse in some locations (between open arrowheads). (D) A 4-month-old specimen retrieved from the polyacrylic acid- and PVPA-containing SBF showing more extensive but incomplete remineralization of the acid-etched dentin. The original demineralization front was difficult to be discerned (arrow). (E) A high-magnification view of Fig. 4D. Intrafibrillar remineralization of a collagen fibril could be seen in an incompletely remineralized region (arrow). Generic abbreviations: Dd, demineralized dentin; Rd, remineralized dentin; Md, naturally mineralized dentin; Ep, epoxy resin; T, dentinal tubule.
Discussion
In the present study, we used a model consisting of an 8- to 12-µm-thick layer of completely demineralized dentin to evaluate the status of remineralization. This model is different from the more conventionally used artificial caries model that consists of 200- to 300-µm-thick layers of partially demineralized dentin (ten Cate, 2001). Both models have inherent flaws, in that they do not simulate the tubular occlusion characteristics of caries-affected dentin (Zavgorodniy et al., 2008). From a thickness perspective, the former model is less taxing, in that one is unlikely to be able to remineralize a thick caries lesion if one could not even remineralize a demineralized dentin layer that is 1/20-1/30 its thickness. The former model is more challenging from a thermodynamic perspective, since it would have been easier to remineralize partially demineralized dentin that contains remnant apatite seed crystallites (Liu et al., 1997) for epitaxial growth (i.e., heterogeneous nucleation). Conversely, remineralization of a completely demineralized collagen matrix via a homogeneous nucleation mechanism is more thermodynamically and kinetically demanding (Wang and Nancollas, 2009). Nevertheless, the major reason for adopting the completely demineralized dentin model was to eliminate the ambiguity in differentiating partially demineralized apatite seed crystallites from remineralized apatite crystallites. This procedure is virtually impossible, even at the ultrastructural level, without resorting to high-precision chemo-analytical techniques for bulk analysis of ion-substituted apatites (Pietak et al., 2007).
The Fuji IXGP adapted well to the completely demineralized dentin. Glass particles within the polyalkenoate matrices were enclosed within a hydrogel layer indicative of a GI reaction. It is generally accepted that GICs bond chemically to tooth substrates through ionic bonds with calcium ions of apatite (Mount, 1991; Lin et al., 1992). This chemical bonding was confirmed by x-ray photoelectron spectroscopy (Yoshida et al., 2000). The present study provided evidence that GICs adapted well to apatite-depleted dentin surfaces.
The collagen banding and rope-like subfibrillar features identified in unstained bright-field TEM micrographs of the aged-Fuji IXGP specimens represent an unusual self-generated negative staining phenomenon. Penetration of Fuji IXGP-specific mineral ions (aluminum or strontium) into the collagen matrix probably accounted for the negative staining of the collagen fibrils. Although salts of heavy metals such as molybdenum, tungsten, and uranium have been conventionally used as negative stains, the salts of certain lighter metals, such as sodium, magnesium, and aluminum, may also be used for similar purposes (Massover, 2008). Electron-dense grainy regions were universally identified, but were more prominently seen along the base of the demineralized dentin. Presumably, the collagen fibrils and terminal branches of the dentinal tubules at these locations were less collapsed than those that are more superficially located, facilitating better infiltration of the mineral ions.
In Fuji IXGP, conventional calcium glass powder is substituted by a strontium-based glass to enhance its radiopacity. Strontium is similar to calcium in both chemical and physical properties, so that it can replace calcium without disrupting the apatite structure. Moreover, strontium has been perceived to play a pivotal role in chemical bond formation with tooth apatite (Wilson et al., 1983). In the present study, diffusion of strontium, aluminum, and fluorine ions into the completely demineralized dentin matrix was consistent with previously reported findings (Ngo et al., 2006) on partially demineralized caries-affected dentin. Diffusion of GIC-specific ions into the demineralized dentin probably occurs as a result of the ion concentration gradient that exists between GICs and dentin (Ferrari and Davidson, 1997; Knight et al., 2007).
The contributions of non-collagenous proteins to dentin remineralization had seldom been considered in classic remineralization studies, except by a few authors (Clarkson et al., 1998; Saito et al., 2003). It is well-known in biomineralization that non-collagenous proteins or their biomimetic analogs are required for generating apatites of the dimensions and order that can fit into the gap zones of type I collagen fibrils. The results of this study indicated that, apart from the uptake of GIC-specific ions, there was no ultrastructural evidence of mineral deposition of a crystalline nature in the Fuji IXGP-bonded acid-etched, completely demineralized dentin, regardless of whether a classic (non-biomimetic) or a biomimetic remineralization strategy (single- or dual-biomimetic analogs) was utilized. Moreover, microradiography-demonstrated reduction of lesion size in “remineralized” artificial carious dentin (Ten Bosch and Angmar-Månsson, 1991) does not imply that the radiopaque mineral precipitates are small enough to permit carbonated apatite to form inside and around collagen fibrils in demineralized dentin. Indeed, the instrumentation and resolutions used in some combined microradiography/morphologic studies on dentin remineralization (Kawasaki et al., 1999) were insufficient to permit such an intricate relation to be determined.
Based on the results of the Portland cement-based biomimetic remineralization control, we have to reject the hypothesis that a strontium-based GIC can remineralize completely demineralized dentin by nucleation of new apatite crystallites within an apatite-free dentin matrix. It is premature to extrapolate the results of the present study to caries-affected partially demineralized dentin, since the latter contains remaining apatite crystals which may constitute centers for heterogeneous nucleation (thus requiring less thermodynamic drive) and remineralization (which possibly involves epitaxial growth mechanisms of remaining crystals). Nevertheless, this study raises the issue of the possibility of non-remineralization of a dentin caries lesion that may contain apatite-depleted collagen matrices along the surface of the lesion. The results of the present study support the challenge raised (Kinney et al., 2003) to classic remineralization studies, that the use of mineral uptake alone is not a sufficient endpoint for assessing the success of contemporary remineralization strategies.
Acknowledgments
The Fuji IXGP capsule kits were generous gifts from GC America, Inc.
Footnotes
This study was supported by Grant R21 DE019213-01 from the National Institute of Dental and Craniofacial Research (PI. F.R. Tay). We thank Bob Smith for TEM technical assistance and Michelle Barnes for secretarial support.
References
- Ab-Ghani Z, Ngo H, McIntyre J. (2007). Effect of remineralization/demineralization cycles on mineral profiles of Fuji IX Fast in vitro using electron probe microanalysis. Aust Dent J 52:276-281 [DOI] [PubMed] [Google Scholar]
- Amaral MT, Guedes-Pinto AC, Chevitarese O. (2006). Effects of a glass-ionomer cement on the remineralization of occlusal caries - an in situ study. Braz Oral Res 20:91-96 [DOI] [PubMed] [Google Scholar]
- Clarkson BH, Chang SR, Holland GR. (1998). Phosphoprotein analysis of sequential extracts of human dentin and the determination of the subsequent remineralization potential of these dentin matrices. Caries Res 32:357-364 [DOI] [PubMed] [Google Scholar]
- Coutinho E, Yoshida Y, Inoue S, Fukuda R, Snauwaert J, Nakayama Y, et al. (2007). Gel phase formation at resin-modified glass-ionomer/tooth interfaces. J Dent Res 86:656-661 [DOI] [PubMed] [Google Scholar]
- Creanor SL, Awawdeh LA, Saunders WP, Foye RH, Gilmour WH. (1998). The effect of a resin-modified glass ionomer restorative material on artificially demineralised dentine caries in vitro. J Dent 26:527-531 [DOI] [PubMed] [Google Scholar]
- Czarnecka B, Limanowska-Shaw H, Nicholson JW. (2002). Buffering and ion-release by a glass-ionomer cement under near-neutral and acidic conditions. Biomaterials 23:2783-2788 [DOI] [PubMed] [Google Scholar]
- Exterkate RA, Damen JJ, ten Cate JM. (2005). Effect of fluoride-releasing filling materials on underlying dentinal lesions in vitro. Caries Res 39:509-513 [DOI] [PubMed] [Google Scholar]
- Ferrari M, Davidson CL. (1997). Interdiffusion of a traditional glass ionomer cement into conditioned dentin. Am J Dent 10:295- 297; erratum in Am J Dent 11:28, 1998. [PubMed] [Google Scholar]
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A. (2007). Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282:1193-1204 [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Ruben J, Stokroos I, Takagi O, Arends J. (1999). The remineralization of EDTA-treated human dentine. Caries Res 33:275-280 [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. (2003). The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res 82:957-961 [DOI] [PubMed] [Google Scholar]
- Kitasako Y, Nakajima M, Foxton RM, Aoki K, Pereira PN, Tagami J. (2003). Physiological remineralization of artificially demineralized dentin beneath glass ionomer cements with and without bacterial contamination in vivo. Oper Dent 28:274-280 [PubMed] [Google Scholar]
- Knight GM, McIntyre JM, Craig GG, Mulyani (2007). Electron probe microanalysis of ion exchange of selected elements between dentine and adhesive restorative materials. Aust Dent J 52:128-132 [DOI] [PubMed] [Google Scholar]
- Kokubo T, Takadama H. (2006). How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27:2907-2915 [DOI] [PubMed] [Google Scholar]
- Lee HS, Berg JH, Garcia-Godoy F, Jang KT. (2008). Long-term evaluation of the remineralization of interproximal caries-like lesions adjacent to glass-ionomer restorations: a micro-CT study. Am J Dent 21:129-132 [PubMed] [Google Scholar]
- Lin A, McIntyre NS, Davidson RD. (1992). Studies on the adhesion of glass-ionomer cements to dentin. J Dent Res 71:1836-1841 [DOI] [PubMed] [Google Scholar]
- Liu Y, Sethuraman G, Wu W, Nancollas GH, Grynpas M. (1997). The crystallization of fluorapatite in the presence of hydroxyapatite seeds and of hydroxyapatite in the presence of fluorapatite seeds. J Colloid Interface Sci 186:102-109 [DOI] [PubMed] [Google Scholar]
- Massara ML, Alves JB, Brandão PR. (2002). Atraumatic restorative treatment: clinical, ultrastructural and chemical analysis. Caries Res 36:430-436 [DOI] [PubMed] [Google Scholar]
- Massover WH. (2008). On the experimental use of light metal salts for negative staining. Microsc Microanal 14:126-137 [DOI] [PubMed] [Google Scholar]
- Mount GJ. (1991). Adhesion of glass-ionomer cement in the clinical environment. Oper Dent 16:141-148 [PubMed] [Google Scholar]
- Mount GJ. (1998). Clinical performance of glass-ionomers. Biomaterials 19:573-579 [DOI] [PubMed] [Google Scholar]
- Naasan MA, Watson TF. (1998). Conventional glass ionomers as posterior restorations. A status report for the American Journal of Dentistry. Am J Dent 11:36-45 [PubMed] [Google Scholar]
- Ngo HC, Mount G, McIntyre J, Tuisuva J, Von Doussa RJ. (2006). Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent 34:608-613 [DOI] [PubMed] [Google Scholar]
- Niederberger M, Cölfen H. (2006). Oriented attachment and mesocrystals: non-classical crystallization mechanisms based on nanoparticle assembly. Phys Chem Chem Phys 8:3271-3287 [DOI] [PubMed] [Google Scholar]
- Pietak AM, Reid JW, Stott MJ, Sayer M. (2007). Silicon substitution in the calcium phosphate bioceramics. Biomaterials 28:4023-4032 [DOI] [PubMed] [Google Scholar]
- Saito T, Toyooka H, Ito S, Crenshaw MA. (2003). In vitro study of remineralization of dentin: effects of ions on mineral induction by decalcified dentin matrix. Caries Res 37:445-449 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2008). Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 29:1127-1137 [DOI] [PubMed] [Google Scholar]
- Ten Bosch JJ, Angmar-Månsson B. (1991). A review of quantitative methods for studies of mineral content of intra-oral caries lesions. J Dent Res 70:2-14 [DOI] [PubMed] [Google Scholar]
- ten Cate JM. (2001). Remineralization of caries lesions extending into dentin. J Dent Res 80:1407-1411 [DOI] [PubMed] [Google Scholar]
- Traub W, Arad T, Weiner S. (1989). Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc Natl Acad Sci USA 86:9822-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Nancollas GH. (2009). Pathways to biomineralization and biodemineralization of calcium phosphates: the thermodynamic and kinetic controls. Dalton Trans 15:2665-2672 [DOI] [PubMed] [Google Scholar]
- Wilson AD, Prosser HJ, Powis DM. (1983). Mechanism of adhesion of polyelectrolyte cements to hydroxyapatite. J Dent Res 62:590-592 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Van Meerbeek B, Nakayama Y, Snauwaert J, Hellemans L, Lambrechts P, et al. (2000). Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res 79:709-714 [DOI] [PubMed] [Google Scholar]
- Zavgorodniy AV, Rohanizadeh R, Swain MV. (2008). Ultrastructure of dentine carious lesions. Arch Oral Biol 53:124-132 [DOI] [PubMed] [Google Scholar]
- Zhang L, Webster TJ. (2009). Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4:66-80 [Google Scholar]