Abstract
We used nondenaturing polyacrylamide gradient gel electrophoresis to examine the associations of high-density lipoprotein (HDL) subclasses with adiposity, physical activity, resting heart rate (an indicator of sympathetic drive), and plasma insulin and glucose levels in 97 men with angiographically documented coronary artery disease. These men neither smoked nor used medications known to affect lipoproteins. The absorbency of protein stain was used as an index of mass concentrations at intervals of 0.01 nm within five HDL subclasses: HDL3c (7.2 to 7.8 nm), HDL3b (7.8 to 8.2 nm), HDL3a (8.2 to 8.8 nm), HDL2a (8.8 to 9.7 nm), and HDL2b (9.7 to 12 nm). HDL peak diameter was determined from the predominant peak of the HDL particle distribution when plotted against particle diameter. Four men who were non-insulin-dependent diabetics as defined by a fasting glucose exceeding 140 mg/dL had significantly higher plasma HDL3b levels and significantly smaller HDL peak diameters than nondiabetic men, and were therefore excluded from further analyses. In the remaining 93 nondiabetic men, plasma HDL3b levels correlated positively with indices of truncal obesity (waist to hip ratio and subscapular skinfold), whereas plasma HDL2b levels correlated negatively with indices of total adiposity (body mass index [BMI]) and truncal obesity (subscapular and abdominal skinfold). Fasting plasma insulin levels correlated negatively with HDL3a, HDL2a, and HDL2b. Obesity significantly affected the relationships of resting heart rate with insulin and HDL subclasses. In heavier men (BMI > 25.8 kg/m2) but not in the less-obese men (BMI < 25.8 kg/m2), resting heart rate was negatively correlated with HDL3a, HDL2a, and HDL2b levels and HDL peak diameter and positively correlated with fasting plasma insulin concentrations. Although the reported physical activity in heavier men also correlated with HDL3a, HDL2a, and resting heart rate, the associations of resting heart rate with HDL3a, HDL2a, and HDL2b could not be attributed to activity level. These analyses suggest that the influences of plasma insulin, regional adiposity, physical activity, and resting heart rate on HDL involve specific HDL subclasses. In the presence of increased adiposity, sympathetic drive and physical inactivity may reduce levels of larger HDL and the peak diameter of the major HDL subspecies.
INTRODUCTION
High density lipoproteins (HDLs) can be characterized by particle size using nondenaturing polyacrylamide gradient gels [1]. Five HDL subclasses have so far been identified by this method: HDL3c (7.2 to 7.8 nm in diameter), HDL3b (7.8 to 8.2 nm), HDL3a (8.2 to 8.8 nm), HDL2a (8.8 to 9.7 nm), and HDL2b (9.7 to 12 nm) [1]. Prior studies show that different HDL subclasses exhibit specific relationships to exercise [2], weight loss [2], age [3], menstrual status [4], adiposity [3], alcohol intake [3], post-menopausal estrogen replacement [3], other lipoproteins [5,6], and shared genetic and environmental influences within families [7]. Case-control and angiographic studies suggest that coronary heart disease risk may be increased when HDL2b is decreased relative to HDL3c and HDL3b [8–10].
Adiposity, insulin resistance, increased sympathetic drive, and dyslipoproteinemia often occur concurrently and are therefore thought to have a common etiology [11,12]. Their association is reported to be greatest in the presence of obesity [13]. The manifestations of increased sympathetic activity include elevated resting heart rate and blood pressure. Prior studies have shown that high resting heart rate is associated with dyslipoproteinemia {decreased concentrations of HDL2 and increased concentrations of small low-density lipoproteins, intermediate-density lipoproteins, and very-low-density lipoproteins (VLDLs)} [14], elevated plasma insulin levels [15], increased development of atherosclerosis [16], and increased coronary heart disease risk [17–21].
This report examines the relationships of HDL subclasses to regional adiposity, resting heart rate, reported physical activity, and plasma insulin and glucose levels in nondiabetic men with coronary artery disease. The analyses to follow suggest that the influence of each of these factors on HDL involves specific subclasses. We also find that obesity may (1) exacerbate the dyslipoproteinemia associated with physical inactivity; and (2) be a necessary condition for sympathetic drive to affect plasma insulin and HDL concentrations.
SUBJECTS AND METHODS
Subject Selection
The data presented in this report were collected during the baseline visit for the Stanford Coronary Risk Intervention Project, a 4-year randomized clinical trial of multifactor risk reduction in 259 men and 41 women with angiographically documented coronary artery disease. The subjects were recruited among patients who received coronary arteriography at four local hospitals. All were free of congestive heart failure, severe pulmonary disease, intermittent claudication, and noncardiac life-threatening illness, and were less than 75 years old. All had at least one segment from a major coronary artery with lumen narrowing between 5% and 69% that was unaffected by revascularization procedures. Following arteriography, 145 of the 300 patients were medically treated, 139 received percutaneous transluminal coronary angioplasty, and 16 had coronary artery bypass graft surgery.
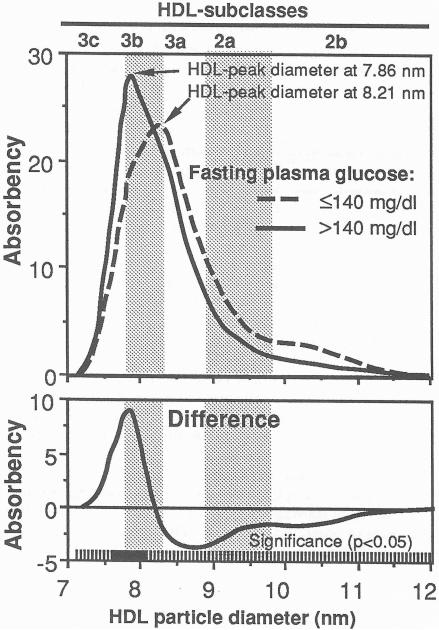
Too few women were free of cigarette usage or lipid-altering drugs to provide meaningful analyses. For this reason, women are excluded from the analyses to follow. Of the 259 men who entered the trial, 143 were excluded for using drugs that could potentially affect lipoprotein levels (lipid-towering drugs, β-blockers, diuretics, antiadrenergic drugs, antiarrhythmic drugs, hypoglycemics, tranquilizers, antidepressants, and barbiturates), 14 were excluded for smoking, and five for missing gradient gel electrophoresis of HDL. Four men who were non-insulin-dependent diabetics as defined by a fasting glucose exceeding 140 mg/dL had significantly higher plasma HDL3b levels and significantly smaller HDL peak diameters than nondiabetic men (Fig 1), and were therefore excluded from further analyses.
Fig 1.
Mean absorbency of protein-stained HDL by particle size in four untreated non-insulin-dependent diabetic men (fasting plasma glucose > 140 mg/dL) and 93 nondiabetic men (top)), and the mean differences between diabetic and nondiabetic men (bottom). The curves were computed by averaging the heights of the individual HDL distributions at each diameter value. The solid portions of the bar at the bottom of the graph designate those diameter values that achieve statistical significance for the differences (P≤ 0.05) for two sample t test. The predominant HDL peak diameters are indicated, and the subclass intervals defined by Blanche et al [1] are provided for reference. The untreated diabetics had significantly smaller HDL peak diameter (mean ± SD: 7.86 ± 0.06 vs. 8.21 ± 0.20 nm) than nondiabetic men and significantly higher HDL3b,
Clinical and Laboratory Measurements
Clinical measurements and fasting plasma samples were obtained at least 3 weeks after hospital discharge. Skinfolds were measured in triplicate at the subscapular, triceps, and suprailiac crest. Weight and height were measured with subjects wearing lightweight clothing or a hospital gown without shoes. Weight was measured to the nearest 0.1 kg using a balance scale. Height was measured to the nearest 0.5 cm with the patient standing erect, flat-footed, and with the head in the Frankfort horizontal plane. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Resting heart rate was the average of the last two values from three 15-second heart rate measurements taken with the subject seated and having rested for 5 minutes. BMI and resting heart rate were measured during two clinic visits (usually within 2 weeks of one another), which were averaged for each subject to represent the subject. BMI measurements were correlated (r = 0.99) and the resting heart rate measurements were correlated (r = 0.72) between the first and second visits; their respective standard deviations for the differences between the two visits were 0.30 kg/m2 and 7.37 beats per minute.
Reported smoking status was verified by measuring the carbon monoxide content of expired air and the thiocyanate concentration in plasma [23]. Dietary intake was determined by 4-day food records (Thursday through Sunday) using the Nutrition Coordinating Center protocols for data collection and coding and Version 13 data base for data analysis [24]. Participants were instructed and their records reviewed in person by trained diet coders.
Physical activity was reported using the Stanford Seven-Day Physical Activity Recall questionnaire [25]. Specifically, subjects were asked if they exercised regularly over the previous year and to recall the number of minutes over the previous week spent engaged in moderate, hard, and very hard activities. Activities were expressed in terms of total kilocalories per kilogram per day (1 kcal = 4.2 kJ).
Fasting plasma triglyceride and lipoprotein cholesterol levels were also measured during two clinic visits and averaged for each subject. Plasma concentrations of total cholesterol and triglycerides were measured in the Stanford Lipoprotein Research Laboratory by enzymatic procedures (ABA 200 instrument, Abbott Laboratories, Abbott Park, IL) [26,27]. The HDL cholesterol level was measured by dextran sulfate-magnesium precipitation [28] followed by enzymatic determination of cholesterol [26]. The laboratory remained certified by the Centers for Disease Control lipid standardization program throughout. Correlations between the first and second visit were r = 0.75 for triglycerides and r = 0.87 for HDL cholesterol, and their respective standard deviations for the differences between the two visits were 5.75 and 42.12 mg/dL. Plasma concentrations of HDL2 mass and HDL3 mass were measured by analytic ultracentrifugation [29]. The plasma glucose concentration was measured using the glucose oxidase method [30] and insulin concentration by radioimmunoassay [31] after an overnight fast and l hour after receiving a 100-g oral glucose load.
Electrophoresis of HDL in the ultracentrifuged d ≤ 1.20-g/mL fraction was performed on Pharmacia Electrophoresis Apparatus (GE 4-lI) using slab gradient gels (PAA 4/30, Pharmacia, Piscataway, NJ) [1]. The protein-stained gradient gels were scanned with a mode/ RFT densitometer (Transidyne, Ann Arbor, MI) at a wavelength of 603 nm. A mixture of four globular proteins (High Molecular Weight Calibration Kit, Pharmacia) was run on the central lane to calibrate for particle size. The HDL migration distances (Rf) were measured relative to the migration distance of the peak of bovine serum albumin, one of the protein standards [5]. The HDL distributions were converted from the migration distance scale to the particle diameter scale using a method previously described [5]. The HDL peak diameter was identified as the diameter of the maximum absorbency (highest point) of the HDL distribution when plotted along the diameter scale [5] (Fig 1).
Statistical Analysis
Student's t tests were used to evaluate differences in mean HDL levels between groups, and Pearson correlation coefficients were used to evaluate relationships between HDL levels and plasma insulin and glucose levels, adiposity, heart rate, and physical activity. The correlations and differences were computed at each 0.01 nm between 7.2 and 12 nm (ie, individually for all 481 diameter values). This approach enables us to study the relationship of HDL subclasses with other variables without necessarily assuming the shapes or particle size intervals of the HDL subclasses. Partial correlation coefficients were used for adjustment. All significance levels are two-tailed.
Conversion from absorbency to plasma concentration is not necessary for analyzing protein-stained HDL by particle diameter, because the significance levels used in this report (ie, for t tests, Pearson's correlation coefficients, and partial correlation coefficients) are invariant to translations of scale or location. This means that the significance levels for absorbency will be identical to those based on unknown plasma concentrations when the conversion involves the addition and/or multiplication of numerical constants. In fact, different constants may be used at each diameter, so that variation in chromogenicity across the HDL particle size spectrum will not affect the results.
RESULTS
The 93 men had a mean (±SD) age of 58.0 ± 9.3 years and a total cholesterol of 222.5 ± 39.4 mg/dL. Figure 1 displays the distribution of HDL by particle size. The curve was computed by averaging the heights of the individual HDL distributions at each diameter value. The approximate subclass intervals described by Blanche et al [1] are provided. Table 1 displays some of their other characteristics. Waist girth, hip girth, and abdominal skinfold measurements were introduced after the start of the study and therefore are missing in early recruits. Table 1 also includes the correlations of anthropometric and lipoprotein measurements with HDL cholesterol, HDL2 and HDL3 mass, triglycerides, BMI, and insulin and glucose levels when fasting and 1 hour after receiving a 100-g oral glucose load. The expected associations for HDL, triglyceride, and insulin levels were observed, namely (1) HDL cholesterol and HDI2 mass correlated negatively with triglycerides, BMI, and fasting insulin; (2) HDL2 mass also correlated negatively with abdominal and subscapular skinfolds and 1-hour insulin; and (3) plasma triglyceride levels correlated positively with fasting insulin and glucose, 1-hour insulin, and BMI. In addition, fasting and 1-hour insulin were positively correlated with BMI, and resting heart rate was positively correlated with fasting concentrations of triglycerides, insulin, and glucose. Table 1 also shows that the HDL peak diameter was negatively correlated with BMI and fasting concentrations of triglycerides, insulin, and glucose.
Table 1.
Sample characteristics and correlations with HDL cholesterol and mass concentrations, triglycerides, BMI, and plasma insulin and glucose concentrations
| Mean±SD | Correlations with: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HDL cholesterol | HDL2 mass | HDL3 mass | Triglycerides | BMI | Insulin | Glucose | ||||
| Fasting | 1-h | Fasting | 1-h | |||||||
| Triglycerides (mg/dL) | 123.84 ± 54.84 | −0.43‡ | −0.39‡ | −0.13 | 1.00 | 0.32† | 0.51‡ | 0.37‡ | 0.31† | 0.20 |
| HDL cholesterol (mg/dL) | 44.83 ± 10.85 | 1.00 | 0.77‡ | 0.61‡ | −0.43‡ | −0.23* | −0.35‡ | −0.16 | 0.11 | −0.07 |
| HDL peak diameter (nm) | 8.21 ± 0.20 | 0.49‡ | 0.51‡ | 0.25* | −0.49‡ | −0.24* | −0.43‡ | −0.20 | −0.20* | −0.14 |
| BMI (kg/m2) | 26.40 ± 3.31 | −0.23* | −0.29† | 0.04 | 0.32† | 1.00 | 0.39‡ | 0.37‡ | 0.07 | 0.19 |
| Waist to hip ratio | 0.94 ± 0.05 | −0.09 | −0.21 | 0.05 | 0.21 | 0.52‡ | 0.15 | 0.11 | 0.09 | 0.20 |
| Abdominal skinfold (mm) | 25.84 ± 8.61 | −0.15 | −0.31* | −0.16 | 0.22 | 0.71‡ | 0.16 | 0.09 | 0.02 | 0.24 |
| Subscapular skinfald (mm) | 18.95 ± 5.98 | −0.18 | −0.25* | −0.08 | 0.16 | 0.66‡ | 0.31† | 0.26* | 0.14 | 0.22* |
| Fasting insulin (pmol/L) | 14.40 ± 8.13 | −0.35‡ | −0.29† | −0.02 | 0.51‡ | 0.39‡ | 1.00 | 0.38‡ | 0.30† | 0.12 |
| 1-h insulin (pmol/L) | 119.01 ± 87.73 | −0.16 | −0.22* | 0.09 | 0.37‡ | 0.37‡ | 0.38‡ | 1.00 | 0.14 | 0.42‡ |
| Fasting glucose (mg/dL) | 96.71 ± 15.68 | 0.11 | 0.00 | 0.19 | 0.31† | 0.07 | 0.30† | 0.14 | 1.00 | 0.41‡ |
| 1-h glucose (mg/dL) | 147.71 ± 50.13 | −0.07 | −0.15 | −0.09 | 0.20 | 0.19 | 0.12 | 0.42† | 0.41‡ | 1.00 |
| Resting heart rate (bpm) | 66.18 ± 9.08 | −0.15 | −0.17 | −0.07 | 0.37‡ | 0.00 | 0.30† | −0.01 | 0.22* | −0.05 |
NOTE. Sample sizes are 93 men, except for subscapular skinfold and fasting waist to hip ratio (n = 59), and abdominal skinfold (n = 58).
P ≤ 0.05
P ≤ 0.01
P ≤ 0.001.
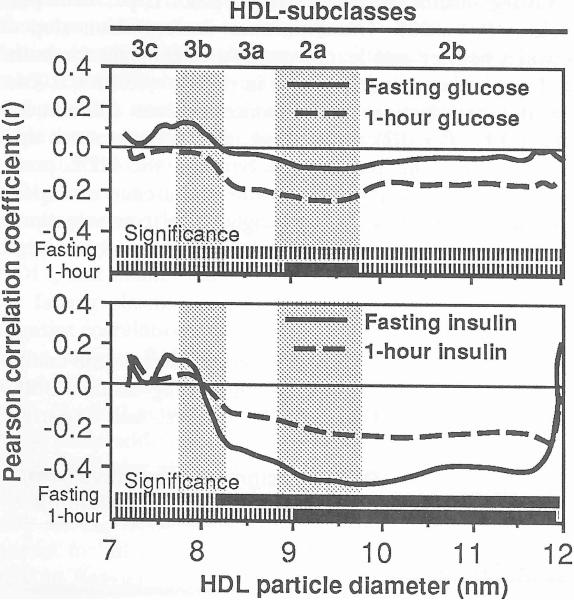
Figure 2 displays the correlations between HDL absorbency and plasma insulin and glucose concentrations. Significant correlations (P ≤ 0.05) are designated by the solid portions of the bars at the bottom of each graph. Plasma insulin concentrations were negatively correlated with plasma HDL3a, HDI2a, and HDL2b levels in fasting plasma and with fasting HDL2a and HDL2b levels 1 hour after receiving the glucose load. Postload plasma glucose levels but not fasting glucose were negatively correlated with fasting HDL2a.
Fig 2.
Pearson correlation coefficients between plasma insulin and glucose levels and the absorbency of protein-stained HDL by particle size, The solid portions of the bars at the bottom of the graphs designate the range of diameter values that correlate significantly at P ≤0.05. The subclass intervals defined by Blanche et al [1] are provided for reference.
Adiposity, Resting Heart Rate, and Exercise
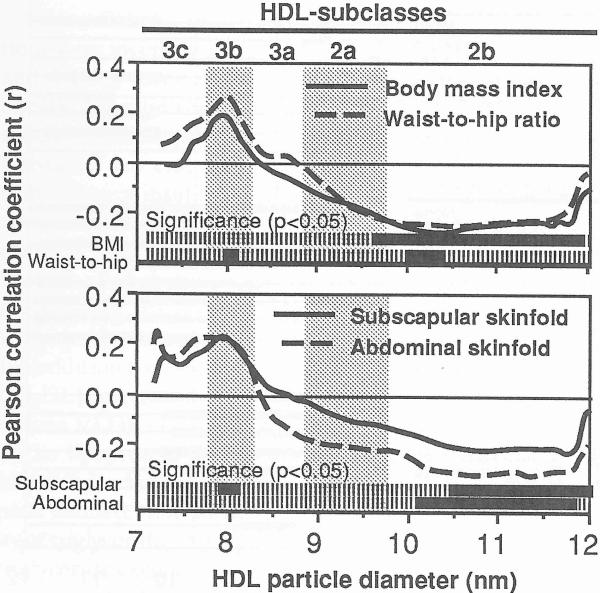
Figure 3 shows that plasma HDL2b levels were inversely correlated with BMI and subscapular and abdominal skinfolds. The ratio of waist to hip girths also correlated within the HDL2b, albeit significantly over a narrow diameter range. Both waist to hip girth and subscapular skinfolds correlated with portions of HDL3b.
Fig 3.
Pearson correlation coefficents between adiposity and absorbency of protein-stained HDL by particle size. The solid portions of the bars at the bottom of the graphs designate the range of diameter values that correlate significantly at P ≤0.05. The subclass intervals defined by Blanche et al [1] are provided for reference.
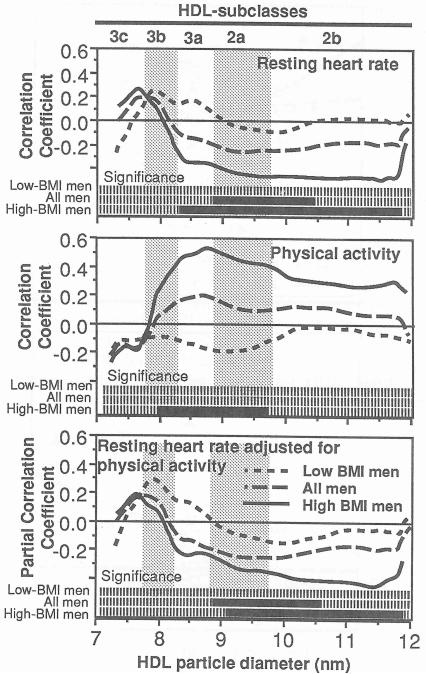
The correlations of Fig 4 show that resting heart rate was negatively correlated with HDL2a and HDL2b and that these relationships primarily involve the more obese men. Specifically, when the data were divided at the 50% percentile of BMI for this sample, resting heart rate in the heavier men (25.8 kg/m2 < BMI < 36.3 kg/m2) correlated significantly with HDL3a, HDL2a, and HDL2b, whereas resting heart rate in the leaner men (20.6 kg/m2< BMI < 25.8 kg/m2) showed no relationship to HDL subclasses. The relationships are in fact significantly different between the heavier and leaner men for HDL between 8.29 and 8.95 nm (analyses not displayed). Although reported physical activity also correlated with HDL3a and HDL2a (middle panel, Fig 4) and weakly with resting heart rate (r =−0.25 , P = .09) in heavier men, the bottom panel of Fig 4 suggests that physical activity does not account for the associations between resting heart rate and the HDL2a and HDL2b subclasses. Similar results were obtained when subscapular skinfold measurements were used to dichotomize the sample into the upper (17.6 to 40 mm) and lower (9.5 to 17.5 ram) halves of distribution, and HDL levels were correlated with resting heart rate (significant for HDL between 8.82 and 11.84 nm for men with thicker skinfolds and nonsignificant for those with thinner skinfolds) and reported physical activity (significant for HDL between 8.41 and 9.85 nm for men with thicker skinfolds and nonsignificant for those with thinner skinfolds, results not displayed).
Fig 4.
Pearson correlation coefficients of resting heart rate (top) and reported physical activity (middle) with absorbency of protein stained HDL by particle size. Partial correlations between resting heart rate and protein-stained HDL adjusted for physical activity are shown in the bottom panel. Results are presented for all 93 men, Low-BMI men (BMI≤ 25.8 kg/m2, n = 47), and high-BMI men (BMI ≥ 25.8 kg/m2, n = 46). The solid portions of the bars at the bottom of the graphs designate the range of diameter values that correlate significantly at P ≤ 0.05. The subclass intervals defined by Blanche et al [1] are provided for reference.
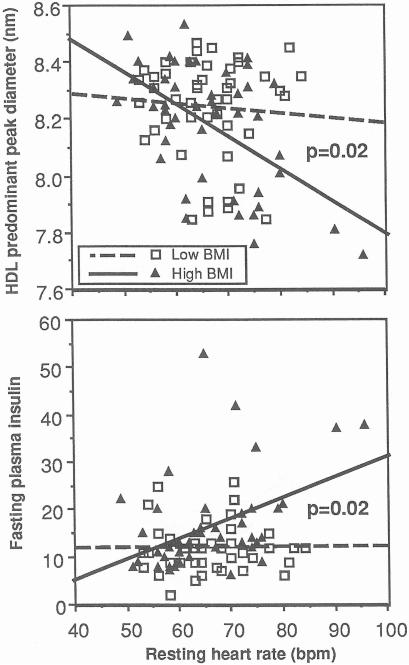
Figure 5 displays the relationships of resting heart rate with HDL peak diameter and fasting insulin levels. The data are again divided by the 50% percentile of BMI. In the leaner men, resting heart rate was unrelated to both peak diameter and fasting insulin, whereas in the heavier men resting heart rate was inversely related to HDL peak diameter (regression slope (β± SE: −0.011 ± 0.003 nm per beat per minute, P < .0001) and concordantly related to fasting insulin (β± SE: 0.425±0.132 pmol/L per beat per minute, P < 0.002). The difference in regression slopes between heavier and leaner men was significant for both HDL peak diameter (difference in slopes, −0.010 ±0.004, P = 0.02) and fasting insulin concentrations (difference, 0.42 ± 0.17, P = 0.02). In contrast, adiposity does not appear to affect the relationship between the HDL peak diameter and fasting plasma insulin (difference in slopes, −0.004±0.006, P = 0.56) or triglyceride concentrations (difference in slopes, −0.0003 ± 0.0007, P = 0.68, analyses not displayed).
Fig 5.
Regression lines depicting the relationships of resting heart rate with the predominant HDL peak diameter (top) and fasting insulin levels (bottom). Separate lines are shown for the Low-BMI man (BMI ≤25.8 kg/m2, n = 47) and high-BMI men (BMI ≥ 25.8 kg/m2, n = 46). Significance levels refer to the difference in slopes between the low-and high-BMI men.
Partial Correlation Analyses
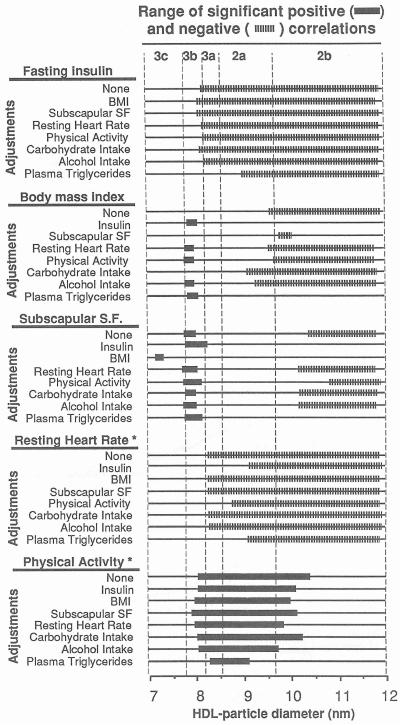
Figure 6 displays the results of partial correlation analyses between HDL absorbency and fasting insulin, BMI, subscapular skinfold, resting heart rate, and reported physical activity. The wide bars designate the range of diameter values that are significant when adjusted for fasting insulin, BMI, subscapular skinfold, resting heart rate, reported physical activity, alcohol intake (kcal/d), carbohydrate intake (% of total calories), or plasma triglyceride concentrations. The following results were observed: (1) insulin: Negative correlations with HDL3a, HDL2a, and HDL2b remain significant when adjusted for all variables except plasma triglyceride levels. Adjustment for plasma triglycerides eliminates the significant association with HDL3a and smaller-diameter HDL2a; (2) BMI: Adjustment for fasting plasma insulin or triglycerides eliminates the negative correlation with HDL2b levels. The positive correlation between BMI and HDL3b achieves significance when adjusted for plasma insulin or triglycerides, resting heart rate, physical activity, or alcohol intake, presumably because these additional sources of variation are eliminated; (3) subscapular skinfold: Adjustment for fasting plasma insulin or triglycerides eliminates the negative correlation with HDL2b levels. The positive correlations with HDL3b persist for all adjustments except BMI; (4) resting heart rate in heavier men: The correlations with HDL3a and smaller HDL2a are eliminated when adjusted for fasting plasma insulin or triglyceride levels or physical activity; however, the correlations with HDL2b levels are unaffected; and (5) physical activity in heavier men: The correlations with HDL3a and HDL2a are generally unaffected by the adjustments. The association is significant over a more restricted diameter range when adjusted for plasma triglyceride levels.
Fig 6.
Partial correlational analyses between HDL protein levels and fasting plasma insulin, BMI, subscapular skinfold, resting heart rate, and reported physical activity. Expanded portions of the lines designate the range of diameter values that correlate significantly at P ≤0.05 when adjusted. *In heavier men only (25.8 kg/m2 ≤ BMI ≤ 36.3 kg/m2).
DISCUSSION
Insulinemia and Adiposity
In nondiabetic men, fasting plasma insulin concentrations were inversely correlated with plasma HDL3a, HDL2a, and HDL2b levels (Fig 2). Presumably this reflects the effects of insulin resistance on HDL3a, HDL2a, and HDL2b subclasses, since plasma insulin concentrations and insulin resistance are essentially linearly related [32]. The relationship between insulin and the HDL particle distribution is apparently unaffected by adiposity, suggesting that insulin exerts a more direct effect on lipoprotein metabolism. Studies of diabetic subjects show that plasma levels of triglycerides are reduced and of HDL cholesterol are increased by oral hypoglycemic agents or insulin therapy [33]. In addition to increasing very-low-density lipoprotein (VLDL) production, hyperinsulinemia also appears to reduce VLDL clearance, possibly due to reduced synthesis of the lipoprotein lipase enzyme that hydrolyzes triglycerides [34,35]. Reductions in HDL3a, HDL2a, HDL2b, and HDL peak diameter may be due in part to the increased availability of triglyceride-enriched lipoproteins for cholesteryl ester-triglyceride exchange.
Figure 3 shows that three measures of upper-body adiposity (waist to hip girth, subscapular skinfold, and abdominal skinfold) and overall adiposity (BMI), were negatively correlated with HDL2b. The association between HDL2 and waist to hip girth has been observed cross-sectionally [36–38] and longitudinally [39]. The partial correlations of Fig 6 suggest that metabolic factors associated with insulin resistance and elevated triglycerides could mediate in part the reduction in HDL2b associated with adiposity. Salomma et al [40] have hypothesized the primacy of insulin resistance in mediating associations between adiposity and lipoproteins in men. Taskinen et al [41] reported that insulin therapy increases HDL2 and reduces HDL3 concomitant to a twofold increase in adipose tissue lipoprotein lipase activity. Eckel and Yost have reported that increases in HDL2 cholesterol after weight loss are strongly correlated with a change in the responsiveness of adipose tissue lipoprotein lipase to insulin [42].
Other studies report that the association between adiposity and HDL2 is only partially explained by fasting insulin [37,43], and it is unlikely that the adiposity-HDL2b relationship is solely the metabolic consequence of insulin resistance. Adipocytes may affect HDL concentrations directly, ie, plasma HDL cholesterol levels are inversely correlated with HDL2 and HDL3 binding to adipocytes, with the affinity for binding to adipocytes being greater for HDL2 than for HDL3 [44,45].
Sympathetic Drive
A Western life-style, characterized by high dietary intake of fat, low carbohydrate intake, and sedentary existence, appears to increase sympathetic drive [46–49]. Figures 4 and 5 suggest that resting heart rate (an indicator of sympathetic drive) was negatively correlated with HDL peak diameter and HDL2a and HDL2b levels, and positively correlated with fasting insulin levels in the presence of obesity.
There is disagreement as to whether sympathetic drive is increased by elevated plasma insulin concentrations stemming from peripheral insulin resistance [50] or whether the plasma insulin concentration is increased by elevated sympathetic drive [51]. Elevated plasma insulin may increase heart rate and stimulate the sympathetic nervous system [52]. O'Hara et al [53] showed that application of the euglycemic-hyperinsulinemic clamp to increase insulin without altering plasma glucose increases sympathetic drive equally in both nonobese and obese individuals [53]. This is in contrast to Fig 5, which suggests that the relationship of resting heart rate with plasma insulin levels is different in leaner and more obese individuals. The discrepancy between our results and theirs may argue against hyperinsulinemia as an antecedent to higher heart rate in our sample.
Increased sympathetic drive may increase plasma insulin levels, which in turn may affect lipoprotein metabolism. High sympathetic drive increases plasma catecholamine levels. Catecholamines stimulate the release of fatty acids by adipose tissue, particularly in intra-abdominal fat cells, which are more responsive to the lipolytic actions of catecholamines than the gluteal fat cell [54] and more resistant to the ability of insulin to inhibit catecholamine-induced lipolysis [55]. This may promote insulin resistance because the free fatty acids compete with glucose for oxidation by muscle, so that glucose uptake is reduced and higher serum glucose levels in plasma result [56]. Higher plasma insulin levels may follow through peripheral insulin resistance and islet cell dysfunction [57]. Excess free fatty acids also inhibit hepatic insulin uptake [54,58]. By releasing fatty acids directly into portal circulation, sympathetic stimulation of intra-abdominal fat cells may also lead to enhanced VLDL production [54,59]. This may account for the increases in VLDL production during stimulation of the α-adrenergic receptors [60]. Increased VLDL concentrations may reduce HDL2a, HDL2b, and HDL peak diameter through enhanced cholesteryl ester-triglyceride exchange [61]. Increased fatty acid concentrations in plasma could also directly affect cholesteryl ester-triglyceride exchange through increased binding of cholesteryl ester transfer protein to lipoproteins [62]. Thus, both insulin and plasma lipoproteins may be affected by sympathetic drive via the release of fatty acids by adipose tissue. As the direct intermediary, the amount of adipose tissue (particularly visceral or abdominal fat) could affect the insulin and lipoprotein responses to the primary effects of heightened sympathetic drive. The larger adipocytes of heavier men may also play a role, given that the release of free fatty acids from adipose tissue increases linearly with the size of the fat cell [63].
Physical Activity
Our previous intervention studies show that running increases both HDL3 and HDL2 concentrations. It is our belief that several metabolic effects may be involved. Specifically, we report elsewhere that initially sedentary men who ran an average of 13.4 km/wk for 1 year appear to increase HDL2b through metabolic processes associated with weight loss and to increase HDL2a and HDL3a through processes that are largely independent of weight loss [64]. There is a considerable difference in the level of strenuous physical activity between the men studied here and the men who participated in our previous running study. Nevertheless, the distinct effects of physical activity and BMI on HDL subclasses are suggested in the present report. Whereas adiposity appears to be associated with HDL2b (Fig 3), reported physical activity appears to be principally associated with HDL3a and HDL2a levels in heavier men (Fig 4). The correlations of Fig 4 are consistent with the report by Ruys et al [65] suggesting that HDL3 cholesterol production is increased in trained as compared with untrained arm muscles [66]. Although Keins and Lithell [66] reported increased arteriovenous HDL2 cholesterol production across exercising leg muscles, the increase may involve the HDL2a component [64].
Caveats and Summary
Caution is warranted when extrapolating our results to men without overt coronary artery disease. Men with coronary disease tend to have decreased levels of total HDL cholesterol, HDL2 cholesterol, and apolipoprotein A-I [67,68] and a smaller proportion of HDL protein within the HDL3a, HDL2a, and HDL2b intervals [8]. However, the correlations of Table 1 are all consistent with those reported for coronary heart disease-free men, suggesting that the factors affecting HDL levels in men with and without coronary artery disease may be similar. Our results suggest that the influences of insulin status, adiposity, resting heart rate, and physical activity on HDL involve specific HDL subclasses. Studies that report the absence of significant relationships of HDL3 to total adiposity [69], upper-body obesity [37,38], and non-insulin-dependent diabetes mellitus [70] could be missing important alterations in HDL3b. However, these results are derived on a specialized sample of men, those with coronary heart disease, and their generalizability to the larger population remains to be determined.
ACKNOWLEDGMENT
We wish to thank Laura Holl, Charlotte Brown, and Bahareh Sahami for laboratory analysis of gradient gel electrophoresis, and the staff of the Stanford Coronary Risk Intervention Project for their efforts in completing the clinical trial.
Supported in part by Grants No. HL-02183, HL-24462, and HL-28292 from the National Heart, Lung, and Blood Institute, and conducted at Stanford University and Lawrence Berkeley Laboratory (Department of Energy Grant No. DE-ACO3-76SFO0098 to the University of California).
REFERENCES
- 1.Blanche PJ, Gong EL, Forte TM, et al. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981;665:408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 2.Williams PT, Krauss RM, Vranizan KM, et al. Effects of weight loss by exercise and by diet on apolipoprotein A-I and A-II and the particle size distribution of high-density lipoproteins in men. Metabolism. 1992;41:441–449. doi: 10.1016/0026-0495(92)90082-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams PT, Vranizan KM, Austin MA, et al. Associations of age, adiposity, alcohol intake, menstrual status and estrogen therapy with high-density lipoprotein subclasses. Arterioscler Thromb. 1993;13:1654–1661. doi: 10.1161/01.atv.13.11.1654. [DOI] [PubMed] [Google Scholar]
- 4.Williams PT, Austin MA, Krauss RM. Variations in high density lipoprotein subclasses during the menstrual cycle. Metabolism. 1994;43:1438–1441. doi: 10.1016/0026-0495(94)90041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PT, Krauss RM, Nichols A, et al. Identifying the predominant peak diameter of high-density (HDL) and low density (LDL) lipoproteins by electrophoresis. J Lipid Res. 1990;31:1131–1139. [PubMed] [Google Scholar]
- 6.Williams PT, Krauss RM, Vranizan KM, et al. Associations of lipoproteins and apolipoproteins with gradient gel electrophoresis estimates of high-density lipoprotein subfractions in men and women. Arteriosclerosis. 1992;12:332–340. doi: 10.1161/01.atv.12.3.332. [DOI] [PubMed] [Google Scholar]
- 7.Williams PT, Vranizan KM, Austin MA, et al. Familial correlations of HDL-subclasses based on gradient gel electrophoresis. Arterioscler Thromb. 1992;12:1467–1474. doi: 10.1161/01.atv.12.12.1467. [DOI] [PubMed] [Google Scholar]
- 8.Wilson HM, Patel JC, Skinner ER. The distribution of high-density lipoprotein subfractions in coronary survivors. Biochem Soc Trans. 1990;18:1175–1176. doi: 10.1042/bst0181175. [DOI] [PubMed] [Google Scholar]
- 9.Johansson J, Carlson LA, Landou C, et al. High density lipoproteins and coronary atherosclerosis. A strong inverse relationship with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb. 1991;11:174–182. doi: 10.1161/01.atv.11.1.174. [DOI] [PubMed] [Google Scholar]
- 10.Cheung MC, Brown BG, Wolf AC, et al. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. J Lipid Res. 1991;32:383–394. [PubMed] [Google Scholar]
- 11.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan NM. The deadly quartet: Upper-body obesity, glucose intolerance, hypertriglyceridemia and hypertension. Arch Intern Med. 1989;149:1514–1520. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 13.Manicardi V, Camellini L, Bellodi G, et al. Evidence for an association of high blood pressure and hyperinsulinemia in obese men. J Clin Endocrinol Metab. 1986;62:1302–1304. doi: 10.1210/jcem-62-6-1302. [DOI] [PubMed] [Google Scholar]
- 14.Williams PT, Haskell WL, Vranizan KM, et al. Associations of resting heart rate with concentrations of lipoprotein subfractions in sedentary men. Circulation. 1985;71:441–449. doi: 10.1161/01.cir.71.3.441. [DOI] [PubMed] [Google Scholar]
- 15.Cambien F, Jacqueson A, Richard JL, et al. Associations between systolic blood pressure, heart rate, and post load glucose and insulin: The Paris Prospective Study. In: Eschwege E, editor. Advances in Diabetes Epidemiology. Elsevier; Amsterdam, The Netherlands: 1982. pp. 189–196. ISERM syrup no. 22. [Google Scholar]
- 16.Beerc PA, Glagov S, Zarins CK. Experimental atherosclerosis at the carotid bifurcation of the cynomolgus monkey. Localization, compensatory enlargement, and the sparing effect of lowering heart rate. Arterioscler Thromb. 1992;12:1245–1253. doi: 10.1161/01.atv.12.11.1245. [DOI] [PubMed] [Google Scholar]
- 17.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: Findings in three Chicago epidemiological studies. Am J Epidemiol. 1980;112:736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 18.Schroll M, Hagerup LM. Risk factors of myocardial infarction and death in men aged 50 at entry. A ten-year prospective study from the Glostrup Population Studies. Dan Med Bull. 1977;24:252–255. [PubMed] [Google Scholar]
- 19.Medalie JH, Kahn HA, Neufeld HN, et al. Five-year myocardial infarction incidence. II. Associations of single variables to age and birthplace. J Chronic Dis. 1973;26:325–349. doi: 10.1016/0021-9681(73)90036-2. [DOI] [PubMed] [Google Scholar]
- 20.Friedman GD, Klatsky AL, Siegelaub AB. Predictors of sudden cardiac death. Circulation. 1975;52(suppl 3):164–169. [PubMed] [Google Scholar]
- 21.Shurtleff D. Some characteristics related to the incidence of cardiovascular disease and death: Framingham study 18 year follow-up. In: Kannel WB, Gordon T, editors. The Framingham Study-An Epidemiological Investigation of Cardiovascular Diseases. US DHEW; Washington, DC: 1974. Section 30. [Google Scholar]
- 22.Fortmann SP, Rogers T, Vranizan KM, et al. Indirect measures of cigarette use: Expired air carbon monoxide vs. plasma thiocyanate. Prey Med. 1984;13:127–135. doi: 10.1016/0091-7435(84)90045-8. [DOI] [PubMed] [Google Scholar]
- 23.Butts WC, Kuehneman M, Widdowson GM. Automated method for determining serum thiocyanate to distinguish smokers from nonsmokers. Clin Chem. 1974;20:1344–1348. [PubMed] [Google Scholar]
- 24.Nutrient Data Base. version 13 Nutrition Coordinating Center, University of Minnesota; Minneapolis, MN: 1986. [Google Scholar]
- 25.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 26.National Heart, Lung, and Blood Institute . Lipid Research Clinics Program. In: Hainline A Jr, Karon J, Lippel K, editors. Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. ed 2. Government Printing Office; Washington, DC: 1982. DHEW publication no. NIH 75628 [revised] [Google Scholar]
- 27.Sampson EJ, Demers LM, Krieg AF. Faster enzymatic procedure for serum triglycerides. Clin Chem. 1975;21:1983–1985. [PubMed] [Google Scholar]
- 28.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 29.Lindgren FT, Jensen LC, Hatch FT. The isolation and quantitative analysis of serum lipoproteins. In: Nelson GJ, editor. Blood Lipids and Lipoproteins: Quantitation, Composition and Metabolism. Wiley-Interscience; New York, NY: 1972. pp. 181–274. [Google Scholar]
- 30.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–27. [Google Scholar]
- 31.Hales CN, Randle PJ. Immunoassay of insulin with insulin antibody precipitate. Biochem J. 1963;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobey TA, Greenfield M, Kraemer F, et al. Relationship between insulin resistance, insulin secretion, very low density lipoprotein kinetics and plasma triglyceride levels in normotriglyceridemic men. Metabolism. 1981;30:165–171. doi: 10.1016/0026-0495(81)90167-0. [DOI] [PubMed] [Google Scholar]
- 33.Agardh CD, Nilsson-Ehle P, Schersten B. Improvement of the plasma lipoprotein pattern after institution of insulin treatment in diabetes mellitus. Diabetes Care. 1982;5:322–325. doi: 10.2337/diacare.5.3.322. [DOI] [PubMed] [Google Scholar]
- 34.Arbeeny CM, Eder HA. Effects of diabetes on the metabolism of triglyceridol-rich lipoproteins. Biochem Soc Trans. 1989;17:51–53. doi: 10.1042/bst0170051. [DOI] [PubMed] [Google Scholar]
- 35.Pykalisto OJ, Smith PH, Brunzell JD. Determinants of human adipose tissue lipoprotein lipase: Effects of diabetes and obesity on basal and diet induced activity. J Clin Invest. 1975;56:1108–1117. doi: 10.1172/JCI108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostlund RE, Staten M, Kohrt WM, et al. The ratio of waist-to-hip circumference, plasma insulin levels, and glucose intolerance as independent predictors of the HDL2 cholesterol level in older adults. N Engl J Med. 1990;322:229–234. doi: 10.1056/NEJM199001253220404. [DOI] [PubMed] [Google Scholar]
- 37.Folsum AR, Burke GL, Ballew C, et al. Relation of body fatness and its distribution to cardiovascular risk factors in young blacks and whites. The role of insulin. Am J Epidemiol. 1989;130:911–924. doi: 10.1093/oxfordjournals.aje.a115424. [DOI] [PubMed] [Google Scholar]
- 38.Terry RB, Wood PD, Haskell WL, et al. Regional adiposity patterns in relation to lipids, lipoprotein cholesterol, and lipoprotein subfraction mass in men. J Clin Endocronol Metab. 1989;68:191–199. doi: 10.1210/jcem-68-1-191. [DOI] [PubMed] [Google Scholar]
- 39.Williams PT, Krauss RM, Vranizan KM, et al. Effects of exercise induced weight loss on low density lipoprotein subfractions in healthy men. Arteriosclerosis. 1989;9:623–632. doi: 10.1161/01.atv.9.5.623. [DOI] [PubMed] [Google Scholar]
- 40.Salomaa VV, Tuomilebto J, Jauhiainen M, et al. Hypertriglyceridemia in different degrees of glucose intolerance in a Finnish population-based study. Diabetes Care. 1992;15:657–665. doi: 10.2337/diacare.15.5.657. [DOI] [PubMed] [Google Scholar]
- 41.Taskinen MR, Kuusi T, Helve E, et al. Insulin therapy induces antiatherogenic change of serum lipoproteins in noninsulindependent diabetes. Arteriosclerosis. 1988;8:168–177. doi: 10.1161/01.atv.8.2.168. [DOI] [PubMed] [Google Scholar]
- 42.Eckel RH, Yost TJ. HDL-subfractions and adipose tissue metabolism in the reduced-obese state. Am J Physiol. 1989;256:E740–E746. doi: 10.1152/ajpendo.1989.256.6.E740. [DOI] [PubMed] [Google Scholar]
- 43.Haffner SM, Fong D, Hazuda HP, et al. Hyperinsulinemia, upper body adiposity and cardiovascular risk factors in nondiabetics. Metabolism. 1988;37:338–345. doi: 10.1016/0026-0495(88)90133-3. [DOI] [PubMed] [Google Scholar]
- 44.Salter AM, Fong BS, Jimenez J, et al. Regional variation in high-density lipoprotein binding to human adipocyte plasma membranes of massively obese subjects. Eur J Clin Invest. 1987;17:16–22. doi: 10.1111/j.1365-2362.1987.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez JG, Fong B, Julien P, et al. Effect of massive obesity on low and high density lipoprotein binding to human adipocytes plasma membranes. Int J Obes. 1989;13:699–709. [PubMed] [Google Scholar]
- 46.Krieger DR, Landsberg L. Neuroendocrine mechanisms in obesity-related hypertension: The role of insulin and catecholamines. In: Laragh JH, Brenner BM, Kaplan NM, editors. Endocrine Mechanisms in Hypertension. Raven; New York, NY: 1989. pp. 105–128. [Google Scholar]
- 47.De Haven J, Sherwin R, Hendler R, et al. Nitrogen and sodium balance and sympathetic nervous system activity in obese subjects treated with low calorie protein or mixed diet. N Engl J Med. 1980;302:477–482. doi: 10.1056/NEJM198002283020901. [DOI] [PubMed] [Google Scholar]
- 48.Landsberg L, Young JB. The influence of diet on the sympathetic nervous system. In: Muller EE, MacLeod RM, Frohman LA, editors. Neuroendocrine Perspectives. Elsevier; Amsterdam, The Netherlands: 1985. pp. 191–218. [Google Scholar]
- 49.Nelson L, Jennings GL, Esler MD, et al. Effect of changing levels of physical activity on blood pressure and hemodynamics in essential hypertension. Lancet. 1986;2:473–476. doi: 10.1016/s0140-6736(86)90354-5. [DOI] [PubMed] [Google Scholar]
- 50.DeFronzo RA, Ferrannini E. Insulin resistance--A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 51.Daly PA, Landsberg L. Hypertension in obesity and NIDDM-Role of insulin and sympathetic nervous system. Diabetes Care. 1991;14:240–248. doi: 10.2337/diacare.14.3.240. [DOI] [PubMed] [Google Scholar]
- 52.Rowe JW, Young JB, Minaker KL, et al. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981;30:219–225. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- 53.O'Hara JA, Minaker K, Young JB, et al. Insulin increases plasma norepinephrine (NE) and lowers plasma potassium equally in lean and obese men. Clin Res. 1985;33:441A. abstr. [Google Scholar]
- 54.Peiris AN, Mueller RA, Smith GA, et al. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest. 1986;78:1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kissebah AH, Evans DJ, Peiris A, et al. Endocrine characteristics in regional obesities: Role of sex steroids. In: Vague J, Bjorntorp P, Guy-Grand B, et al., editors. Metabolic complications of Obesity. Elsevier Science; New York, NY: 1985. p. 115. [Google Scholar]
- 56.Randle PJ, Garland PB, Hales CN, et al. The glucose fatty acid cycle: Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 57.Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: Implications for the management of diabetes. Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 58.Smith U. Regional differences in adipocyte metabolism and possible consequences in vivo. Recent Adv Obes Res. 1986;6:33–36. [PubMed] [Google Scholar]
- 59.Kissebab AH, Adams PW, Wynn V. Interrelationships between insulin secretion and plasma free fatty acids and triglyceride transport kinetics in maturity onset diabetes, and the effect of phenethylbiguanide (Phenformin) Diabetologia. 1974;10:119–130. doi: 10.1007/BF01219667. [DOI] [PubMed] [Google Scholar]
- 60.Dzau VJ, Sacks FM. Regulation of lipoprotein metabolism by adrenergic mechanisms. J Cardiovasc Pharmacol. 1987;10(suppl 9):S2–S6. [PubMed] [Google Scholar]
- 61.Hopkins GJ, Barter PJ. Role of triglyceride-rich lipoproteins and hepatic lipase in determining the particle size and composition of high density lipoproteins. J Lipid Res. 1986;27:1265–1277. [PubMed] [Google Scholar]
- 62.Musliner TA, McVicker KM, Iosefa JF, et al. Lipolysis products promote the formation of complexes of very-low-density and low-density lipoproteins. Biochim Biophys Acta. 1987;919:97–110. doi: 10.1016/0005-2760(87)90196-2. [DOI] [PubMed] [Google Scholar]
- 63.Pomeranze J, Beinfield WH. Serum lipids and fat tolerance studies in normal, obese and atherosclerotic subjects. Circulation. 1954;10:742–746. doi: 10.1161/01.cir.10.5.742. [DOI] [PubMed] [Google Scholar]
- 64.Williams PT, Krauss RM, Stefanick ML, et al. Effects of low-fat diet, calorie restriction and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism. 1994;43:655–663. doi: 10.1016/0026-0495(94)90210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruys T, Sturgess I, Shaikh M, et al. Effects of exercise and fat ingestion on high density lipoprotein production by peripheral tissues. Lancet. 1989;2:1119–1122. doi: 10.1016/s0140-6736(89)91488-8. [DOI] [PubMed] [Google Scholar]
- 66.Kiens B, Lithell H. Lipoprotein metabolism influenced by training-induced changes in human skeletal muscle. J Clin Invest. 1989;83:558–564. doi: 10.1172/JCI113918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slowinska-Srzednicka J, Zgliczynski S, Ciswicka-Sznajderman M, et al. Decreased plasma dehydroepiandrosterone sulfate and dihydrotestosterone concentrations in young men after myocardial infarction. Atherosclerosis. 1989;79:197–203. doi: 10.1016/0021-9150(89)90124-x. [DOI] [PubMed] [Google Scholar]
- 68.Kauppinen-Makelin R, Nikkila EA. Serum lipoproteins in patients with myocardial infarction. Atherosclerosis. 1988;74:65–74. doi: 10.1016/0021-9150(88)90192-x. [DOI] [PubMed] [Google Scholar]
- 69.Diehl AK, Fuller JH, Mattock MB, et al. The relationship of high density lipoprotein subfractions to alcohol consumption, other lifestyle factors and coronary heart disease. Atherosclerosis. 1988;69:145–153. doi: 10.1016/0021-9150(88)90008-1. [DOI] [PubMed] [Google Scholar]
- 70.Billingham MS, Milles JJ, Bailey CJ, et al. Lipoprotein subfraction composition in non-obese newly diagnosed non-insulin dependent diabetes after treatment with diet and glibenclarnide. Diabetes Res. 1989;11:13–20. [PubMed] [Google Scholar]