Patient-centered e-health interventions can and do work.1,2,3,4,5,6 But a key question remains: What are the causal psychological and social processes and intermediate outcomes that lead to their demonstrated successes? That is a call for theory to help researchers develop, implement and evaluate those interventions.7 Theory provides a framework guiding the selection of intervention components from a huge array of what might work, it guides the choice of study design and samples, and it helps select appropriate outcomes for measuring the effects of the intervention. In fact, as Kurt Lewin famously noted a half-century ago, “nothing is quite so practical as a good theory.8” Theory helps us (and forces us) to specify mechanisms explaining outcomes, a process that not only builds theory but that also improves research efficiency. For instance, an intervening mechanism may be much easier to measure than an ultimate outcome and may also be a purer measure of an important treatment effect than a distal, global outcome affected slowly and by multiple causes. And without measurement of putative mediators, one will not know whether treatments simply aren’t “working,” or whether the treatments are having their intended effects, but such effects are not sufficient to budge global and distal clinical endpoints. Finally, measures of purported or hypothesized mechanisms may uniquely tell us where treatments are failing. Thus, theory-based mediational analysis helps explain why treatments work, or don’t work better, and also points us in new directions for enhancing treatments.
Much of the above argument applies universally to all health intervention attempts, but has particular force for e-health. These interventions tend to be complicated and multifaceted, particularly patient-centered interventions that depend on the complex interplay among patients, clinicians, and the healthcare system.9 To adequately address this integrated model of care, interactive cancer communication systems offer many types of assistance, often informally crossed with diverse formats. In addition, such systems are meant to be used many times over months or even years to address patients’ changing needs as they react to their diagnoses, choose and receive treatment, deal with treatment sequelae, and rebuild their lives. Thus, what patients need from the system and the way they use it can change radically across the time span of the intervention. All of this means that theorizing about e-health needs to address this intervention complexity, variability of patient needs, and changes over repeated or long-term intervention use. Given the wide variety of purposes and techniques of e-health, there will probably never be a single general theory of e-health. But this does not argue for the opposite extreme of employing a host of lower-level theories to explain different facets of a single intervention. Where possible, researchers should strive to apply or develop theories large enough to cover the complexity of their interventions. Ideally, this work should be a starting point for e-health development: identify the outcome(s) to target, then some mechanisms known to causally affect them, and work backwards to design the e-health intervention to activate at least some of those mechanisms.
That sounds straightforward, but of course it’s not so simple. There are often many more causal steps than the above description implies, and complex interventions likely achieve their results by multiple causal paths, either happening simultaneously or different paths working situationally or for different people. This suggests a long-term, iterative research and development process. Alternatively, theory building can also largely occur after the fact. One can find oneself, as the current authors did, with a complex e-health intervention (CHESS) that was producing consistent changes in an important patient outcome (quality of life1,2,3), but without a clear theory of intervening mechanisms (to be clear: an atheoretic approach was not taken, but quite different theories were borrowed from to justify different intervention features10,11,12,13). In either case, however, what is needed is a serious attempt to fill in that causal model, and much of the remainder of this paper is used to illustrate the process from research by the current authors. First, a description is provided of the starting and ending points that had to be bridged. CHESS (the Comprehensive Health Enhancement Support System) will serve as a useful example, because it has been the object of considerable research and because its content comprises most elements of e-health interventions. CHESS helps patients understand their medical situation and treatment options, empowers them to become full participants in their healthcare, and provides opportunities to learn and practice communication and lifestyle skills that foster health and well being. To that end CHESS provides comprehensive accurate information, personal stories of similar others, peer and expert support, and interactive decision guides and skill building tools. These tools are bundled into a structure that provides multiple navigational options—casual browsing, indexed searching, and tailored navigation schemes that are guided by the CHESS system, or “prescribed” by a healthcare professional who is integrated into the CHESS system.2 Research suggests that coherent use of a range of CHESS’ information, support, and interactive tools resources to address a specific problem is associated with higher quality of life.14,15 CHESS also provides self-reflective learning tools to foster skills and emotional growth. Unlike the didactic and evidence-based features, these tools provide patients with a safe forum to construct their own knowledge about their cancer experience and what it means to them.16 These tools include the peer-led discussion group, open and guided journaling, and thought-provoking personal stories about other patients’ cancer experiences. (See www.chess.wisc.edu for more detail.)
Quality of life (QOL) was chosen as the target outcome, because it is the subjective self-appraisal of a person’s physical, emotional, functional, social, and perhaps spiritual status, all of which will be affected by life-threatening disease and by actual and perceived resources for coping with it. The word “appraisal” emphasizes a patient-centered perspective, since QOL assessments are typically less focused on the actual state-of-nature than on the person’s subjective evaluation. QOL is also a worthy target of investigation because it is multidimensional and can reflect the effects of multifactorial interventions as well as the various effects of disease and diagnosis over time (e.g., the shock of diagnosis and confronting the possibility of death, dealing with unfamiliar information and decisions, the physical impacts of treatment, and often the strains of long-term health self-management and anxiety about continued risk). Many cancer patients regain their initial quality of life after successful treatment, so that the goal of psychosocial e-health interventions is often to cushion the drop in quality of life and speed its recovery to baseline.17
The breadth and changing nature of challenges to quality of life, and the diversity of resources offered by CHESS and similar e-health systems call for a broad and fundamental theoretic approach to understand intervention effects. Self-determination theory18,19,20 (SDT) notes that humans need to influence things affecting their lives, and in particular that a person’s perceived quality of life is largely determined by the degree to which they experience three key needs as being sufficiently fulfilled.20 One of these, autonomy, is the sense that one’s actions and experiences are volitional rather than controlled by strong external or internal forces. Competence is a self-perception of efficacy,i while relatedness is the need to experience connection with others. Of course, individuals may vary in the relative importance of these needs,19,21 and a stressor such as cancer could affect different individuals in different ways. However, it seems clear that cancer diagnosis and treatment typically and substantially compromises the satisfaction of all three needs: “My life is out of control” (autonomy), “I can’t do anything about it” (competence), and “I feel all alone” (relatedness). Deficits in the satisfaction of these three basic needs would limit the ability to respond to a health crisis and would also provide motivation to repair the deficit. Adequate levels of autonomy, competence and relatedness seem intuitively supportive of QOL, and these relationships have been empirically supported by a range of field studies and RCTs of interventions.22,23 To first illustrate how these three SDT concepts might be worth pursuing as part of the bridge between an e-health system and quality of life, consider several examples. Receiving and understanding information—a generally assumed and frequently documented benefit of typical patient-centered e-health systems6—about disease, treatment and recovering should contribute to autonomy by making clear that choices and response options exist and can be pursued. Similarly, information can just as well lead to a sense of competence by providing the patient the particular facts needed to respond to the disease. Further, some e-health interventions explicitly offer skill training (e.g., guided decision aids), which could directly enhance competencies. In addition, an illness creates an unwelcome identity (e.g., ‘breast cancer patient’) that can create barriers between a woman and members of her current support systems, who at a minimum do not share that identity, are probably uncertain how to relate to it, and may even consider it stigmatizing. Thus, e-health interventions that provide new alternatives for social connections and the knowledge and skills to repair and maintain old ones should reduce deficits in relatedness. Furthermore, because interacting with clinicians is such an important part of illness experience, these social relationships, whether new or existing, take on vastly greater importance during the disease experience. Although satisfaction of these three needs can be conceived of as each independently supporting quality of life, they of course covary, and may have substantial dependencies.21 Deficits in one or two undermine strengths in the others: autonomy may be of little use without competence, and vice versa, and a deficit of either may inhibit relatedness with clinicians, limiting use of clinician advice.20
Further impetus to give these SDT concepts a central place in the attempt to understand and explain CHESS effects on Quality of Life came from analyses of one of the randomized-control studies within the Center of Excellence in Cancer Communication Research. After only 6 weeks of a 6-month intervention period, effects were found that confirmed the important role of autonomy, competence and relatedness in mediating CHESS effects on QOL.24
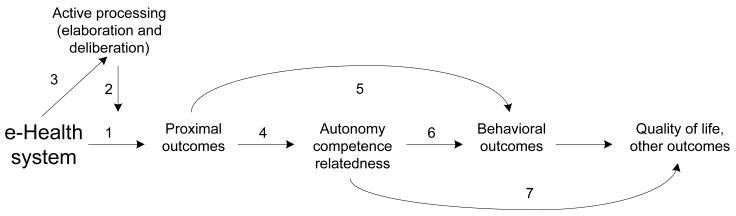
Figure 1 provides an overview of the model, laying out steps and relationships in how these effects are hypothesized to occur. Because of the complexity of CHESS and variability of the individual cancer experience, each element actually contains several specific concepts and their measures within it. Thus, each relationship among elements shown by a numbered arrow actually represents multiple potential relationships among particular concepts (insert Figure 1 here). Below is a discussion of the links in the causal order implied by the model, but it is worth noting that model development actually is easier and more productive by working backwards. For example, starting with autonomy, competence and relatedness, a list was first compiled of the immediate behaviors of cancer patients that might contribute to satisfying these needs, and then each item was considered to select those which might be affected by an e-health intervention with the structure and content of CHESS.ii Similarly, empirical results and theory were drawn on to also propose what sorts of behavioral outcomes might be responsible for quality of life, and which of these should be influenced by the SDT constructs.
Figure 1.
An e-health effects model
Below are the hypothesized relationships of the model:
-
Use of CHESS (and similar systems) causes proximal outcomes central to the aims of patient-centered care, such as knowledge (of cancer, treatment options), goal clarification, validation of experiences, social connections with peers, and skills for managing emotions and relationships, among others.
Although his discussion goes straight from e-health interventions to autonomy, competence and relatedness, effectively skipping proximal outcomes, Hesse proposes a useful and generalizable classification of which e-health tools affect each SDT construct.25 Briefly, he proposes that autonomy is supported by self-help tools, the personal health record, health portals, connective journalism, and what he calls ubiquitous health care. E-health features like these place control of health issues in the patient’s hands, reinforcing and boosting autonomy. Competence is supported by functional health literacy, information prescriptions, consumer involvement, and skill augmentation, all which provide the wherewithal for patient action. And relatedness is particularly supported when e-health contains health advocacy groups, networks of care, and shared communities of knowledge, which provide social connections allowing the patient to feel supported by others in the healthcare situation.
Research on CHESS1,2,3,14,15 has demonstrated effects on measures such as specific health-related competencies, self-reported cognitive functioning, perceived social support, anxiety, and relationships with physicians, all of which would seem to be likely proximal outcomes of specific content available in CHESS (or for that matter in many other patient-education materials as well). For example, the organized and detailed information about causes, treatment, side effects, and prognosis of breast cancer contained in CHESS allows the woman who uses it to understand her situation, her options, and what she is experiencing, which allow her to feel more competent dealing with both health information and healthcare situations. And users of the CHESS Discussion Group (an asynchronous bulletin board used repeatedly and for considerable time by cancer patients) provide each other considerable social support, both in emotional terms and through offering advice and alternative perspectives. Discussion Group participants also share experiences that give their readers real-world knowledge of what to expect,26 which for many people are more convincing knowledge than science-based generalizations.27 Other proximal outcomes of CHESS hypothesized but not yet documented include acquisition of skills (from the decision aid or from newly added explicit training in cognitive–behavioral therapy and relationship skills), and accurate disease perceptions.28
Although a number of these proximal outcomes have been demonstrated as effects of CHESS, the research does not so far allow us to clearly link use of the indicated portions of CHESS with those effects. Almost all CHESS research has employed randomized-control designs in which access to the entire system of services is contrasted with access to usual care or access to other materials or to the Internet generally. Individuals within the experimental groups do of course vary considerably in their use of the system portions of interest, but of course those differences are self-selected as well as covarying with many other characteristics.
However, although clear evidence linking particular content rather than a whole e-health system to proximal outcomes is difficult and awaits further research, that is not the main point of this step of the model. Instead, specifying proximal outcomes (themselves hypothesized to affect SDT concepts in Step 4) forces us to examine system design and content to ensure that the ‘active ingredients’ necessary to create proximal outcomes exist. Further, such reflective and detailed examination of system content helped us identify additional outcomes for future measurements, such as disease beliefs and skills knowledge.
Steps 2 and 3. Step 2 represents an interaction that often occurs as an individual difference in communication processes. More importantly, Step 3 illustrates the belief, held by the current authors, that effective e-health systems can feed this amplification, and that e-health designers can work to increase this effect.
-
Elaboration and deliberation strengthen stimulus effects. In mass communication, where passive and uninvolved reading and viewing are commonplace, readers or viewers who are more cognitively active are generally more affected by information or other beneficial effects and more able to defend themselves against persuasive messages.29,30,31,32 Even though there is probably much less individual variation in cognitive activity among cancer patients than with mass communication generally, there is some CHESS research14,15 indicating that more active, thoughtful use leading to greater quality-of-life benefits.
Previous studies by the current authors have not attempted any direct measurement of cognitive processing, at best inferring it from differential patterns of system use.14,15 However, the current study draws on a body of research on what are probably two key cognitive processes, elaboration and deliberation.33 Elaboration relates new information to existing knowledge, thus producing richer and connected meanings,34 while deliberation refers to evaluative processes, primarily weighing alternatives. CHESS evaluations now underway incorporate a set of survey items tapping elaboration and deliberation that pop up at scheduled points in CHESS use sessions. This is not quite the same as thought-listing interviews,35 but placing the items during naturally occurring use sessions should be less intrusive and artificial. It is hypothesized that greater elaboration and deliberation will be associated with increased effects on proximal outcomes, manifested as either or both of larger changes for those who elaborate and deliberate more, or by changes in correlations between amount of system use and proximal outcomes. In effect, the ‘active ingredients’ of CHESS discussed in Step 1 should become even more active as a user elaborates and deliberates on them.
-
CHESS encourages cognitively active communication reception and use. This has been a central tenet of CHESS design and of thinking about interactive media generally,36,37 starting with the relatively simple notion that the necessity of repeated selection and navigation leads to at least a minimal degree of cognitive activity by users of interactive media. But beyond this, interviews with CHESS users (and this is sure to be true of many other e-health websites) suggested that as different portions of CHESS are used over time (or the same portion is used repeatedly), explicit connections among parts of CHESS guide elaboration and encourage the user to find his/her own connections. Similarly, CHESS content continually emphasizes the importance of being actively engaged in one’s health care, and this responsibility requires both elaboration and deliberation, as well as autonomy, competence and relatedness.
This hypothesis has not previously been directly tested, but the measures of elaboration and deliberation will shortly allow us to perform such a test, by asking the questions during an initial CHESS use session and then again after the individual has had time to use the system and perhaps increase their level of cognitive elaboration and deliberation during use.
Proximal outcomes help people fulfill their needs for autonomy, competence and relatedness. Recent research on SDT-based interventions has emphasized the importance of internalizing autonomous self-regulation and feelings of competence.22 Most SDT intervention research so far has focused on autonomy support: “eliciting and acknowledging patients’ perspectives, supporting their initiatives, offering choice about treatment options, and providing information, while minimizing pressure and control.”38 The sorts of effects discussed under proximal outcomes (e.g., specific health competencies, perceived social support, skills, disease beliefs implying both personal responsibility and achievable goals) are each narrow and specific expressions of general autonomy, competence and relatedness. Thus, for example, attaining specific competencies reinforces one’s overall sense of competence, and probably favorably affects autonomy as well. However, these sorts of linkages have not yet been tested in e-health and are also largely untested in SDT research generally (see 23 for a partial exception).
Proximal outcomes directly affect behavioral outcomes, thus ‘stepping around’ the constructs of self-determination theory to have direct effects. Behavioral outcomes downstream from general need satisfaction but upstream from quality of life would include such things as treatment adherence, improved personal relationship behaviors, proactive use of the healthcare system, self-monitoring, caregiving, positive diet and exercise behaviors, and smoking cessation. As examples of such direct effects, skills acquisition and practice may allow a person to improve relationship quality, goals and values clarification can alter behavioral intent to engage in adherence or other self-management behaviors, and knowledge can lead to more efficient use of healthcare resources. Again, this aspect of the model has not been tested, but should be relatively straightforward to assess once relevant proximal outcomes, behavioral outcomes, and SDT concepts are separately measured in the same study.
Autonomy, competence and relatedness affect behavioral outcomes. As noted earlier, adequate levels of one or more of these self-determination theory constructs may be a necessary condition to carry out a particular behavior outcome, and this has been the focus of considerable SDT research.22,23,38 Some recent research also suggests that balance among them may be more important than higher levels of individual constructs in certain situations.21
Autonomy, competence and relatedness affect QOL. This is also a central result of research on SDT. Whereas deficits in autonomy, competence and relatedness undermine various dimensions of QOL, fulfilling these basic needs supports them. However, it is a valid question yet to be addressed whether this direct link exists or works only through the behavioral outcomes.
-
Behavioral outcomes affect QOL. The point of this link is that a wide variety of effective, positive behaviors, such as health self-management, more effective use of the healthcare system, treatment adherence, relationship building and maintenance, coping skills, and so on, themselves improve one’s actual quality of life and thus one’s perception of it as well.
Recursive links. Because use of CHESS or similar e-health systems occurs over considerable time involving potentially very large numbers of episodes of use, Figure 1 may be misleading in outlining a linear process. Quite likely many changes on the ‘right-hand’ side of the model have the potential to cause further change in elements to their left. But three relationship sets seem particularly likely to be important, and pursuing them is advocated. (1) Greater general competence and autonomy feed back to encourage even more active processing in further interactions with the e-health system. (2) Some behavioral outcomes may directly affect autonomy, competence and relatedness, a reciprocal of link #6. (3) Success at many of the behaviors instigated by CHESS – treatment adherence, self-monitoring, proactive use of the healthcare system – should itself help to build competence, and perhaps autonomy and relatedness as well.
This model is the result of induction after considerable research and experience on different aspects of e-health design, implementation, use by patients and their families, and analysis of quality of life and other outcomes. Put differently, it was developed as an attempt to understand and explain observed effects of CHESS on quality of life. As such, the model is intended to stimulate research testing it or variations of it with CHESS or other e-health systems.
But where this model should be important, and what it is particularly good for, is in improving e-health interventions, especially those designed to provide multiple inputs over time to support quality of life. The model invites developers and researchers to fill in any two or more of the boxes in Figure 1. Addressing e-health developers (including the current authors), questions are posed, such as: Just what are the active ingredients of your system? What proximal outcomes do you think each is affecting? Can you show, either experimentally or through post hoc analyses of different use patterns among the supposed active ingredients, which effects of linkage #1 exist and which do not? Once you have shown some proximal outcomes to directly result from your system, do those effects move on to enhance autonomy, competence and relatedness or in any other way affect quality of life? There are of course many more questions, but these are some important starting points for e-health, and answering them will allow us to produce e-health systems that are more effective by serving their users better.
It is believed that both the overall message and the causal analyses offered have general relevance to many e-health interventions. This is due to several factors: (1) the relevance of QOL to many health conditions and outcomes; (2) the general relevance of SDT mechanisms to a wide variety of health and behavioral health outcomes; and (3) the fact that CHESS comprises both representative e-health treatment elements and targets proximal outcomes of general relevance (information store, skills, support, and QOL). The current arguments for generalizability are supported by observations of specific relationships between self-efficacy and a variety of outcomes such as smoking cessation,39 perceived support, and diverse health outcomes of breast cancer,40 and autonomous motivation and multiple health outcomes.19
But of course this is not the only model possible. Others will want to (and should) amend or replace it to better apply to their own mix of outcomes, intervention tools and social and psychological mechanisms. For example, it is assumed that e-health interventions to support smoking cessation behaviors, although perhaps finding some utility in SDT, will utilize a quite different intervention ‘toolbox’ and mechanisms that include pharmacologic as well as psychosocial elements. Where prevention behaviors (immunizations, early-detection testing) are the goal, current practice suggests that the implicit models rely heavily on cognition and attitudes, requiring considerable theoretic elaboration around these elements. Including intervention elements such as feedback or other tailoring techniques to boost elaboration and deliberation may be particularly important for e-health prevention.41 E-health interventions targeting lifestyle prevention behaviors (e.g., diet, exercise) likewise probably share some elements with the current model and other prevention models, but probably need considerable elaboration of feedback and reward loops. Other goals may require quite different theory. CHESS and like systems are entirely patient-focused, but designing e-health to support patient-centered clinical functioning may draw on theories about individual perception and decision-making, but must also focus on issues of organizational structure and individual and group behavior within organizations, which will result in very different theoretic models.
Nonetheless, whether the above model proves widely useful or in the end only to the current authors and a few other researchers, it is hoped that the need is clear for a cohesive theory and for some such model to be a part of e-health research and development. Thinking theoretically guides the initial development process, forcing an explicitness and specificity necessary to all intervention research. Such initial thinking about just what should make up one’s intervention can avoid much wasted effort. This is probably particularly true for e-health, where so much of what is done is new and untested. Further, specifying the ‘innards’ of one’s theory (i.e., the intervening steps and processes) forces clarity and offers proximate outcomes that will likely be more useful initial measures than distal outcomes, effects on which are likely to be both delayed and contaminated by other causes. And assumptions about processes adopted from other contexts may serve as a good starting place, but deserve to be tested explicitly when moved to new contexts such as e-health.
As e-health development and testing proceeds, having an explicit theoretic model also provides a clear framework for correction and adaptation of one’s intervention. Seeing what proximal outcomes are and are not affected should direct developers to refocus their efforts: altering content, adding or replacing active ingredients, or even more drastically reshaping either the outcomes or the theory itself. The point is that both initial development and iterative improvement of e-health benefit enormously from explicit theoretic statements about what constitutes the intervention and how it is believed to work.
Acknowledgments
No financial disclosures were reported by the authors of this paper.
Footnotes
Competence and self-efficacy are nearly synonymous, although as a need in SDT, there is also an affective component as well as the cognitive expectation.
The authors are grateful to Willliam Rakowski of Brown University, who provided this insight and led initial discussions during the 2-day conference that led to initial development of the model.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gustafson DH, Hawkins R, Boberg E, Pingree S, Serlin RC, Graziano F, et al. Impact of a patient-centered, computer-based health information/support system. Am J Prev Med. 1999 Spring;16(1):1–9. doi: 10.1016/s0749-3797(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 2.Gustafson DH, Hawkins RP, Pingree S, McTavish F, Arora NK, Mendenhall J, et al. Effects of computer support on younger women with breast cancer. J Gen Intern Med. 2001;16:435–445. doi: 10.1046/j.1525-1497.2001.016007435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafson DH, Hawkins RP, McTavish F, Pingree S, Chen WC, Volrathongchai K, Stengle W, Stewart JA, Serlin RC, Shaw B. Internet-based interactive support for cancer patients: Are integrated systems better? J Comm. 2008;58(2):238–257. doi: 10.1111/j.1460-2466.2008.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gysels M, Higginson IJ. Interactive technologies and videotapes for patient education in cancer care: Systematic review and meta-analysis of randomised trials. Support Care Cancer. 2007;15:7–20. doi: 10.1007/s00520-006-0112-z. [DOI] [PubMed] [Google Scholar]
- 5.Murray E, Burns J, See Tai S, Lai R, Nazareth I. Interactive Health Communication Applications for people with chronic disease. Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD004274.pub4. Art. No.: CD004274. [DOI] [PubMed] [Google Scholar]
- 6.Strecher V. Internet methods for delivering behavioral and health-related interventions (e-health) Annu Rev Clin Psychol. 2007;(3):53–76. doi: 10.1146/annurev.clinpsy.3.022806.091428. [DOI] [PubMed] [Google Scholar]
- 7.Rimer B, Glanz K. National Cancer Institute; 2005. Theory at a glance: A guide for health promotion practice. NIH Publication No. 05-3896. [Google Scholar]
- 8.Lewin K. The research center for group dynamics at Massachusetts Institute of Technology. Sociometry. 1945;8(2):126–36. [Google Scholar]
- 9.Epstein R, Street R. Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. National Cancer Institute; 2007. NIH Publication No. 07-6225. [Google Scholar]
- 10.Folkman S, Lazarus RS. Coping as a mediator of emotion. J Pers Soc Psych. 1988;54(3):466–475. [PubMed] [Google Scholar]
- 11.Janz NK, Becker MH. The health belief model a decade later. Health Ed Behav. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein M, Triandis HC, Kanfer FH, et al. Factors Influencing Behavior and Behavior Change. In: Baum A, Revenson TR, Singer JE, editors. Handbook of health psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 3–17. [Google Scholar]
- 13.Bandura A. Health promotion from the perspective of social cognitive theory. In: Norman P, Abraham C, Conner M, editors. Understanding and changing health behaviour. Reading, England: Harwood; 2000. pp. 299–339. [Google Scholar]
- 14.Smaglik P, Hawkins R, Pingree S, Gustafson DH, Boberg EW, Bricker E. The quality of interactive computer use among HIV-infected individuals. J Hlth Comm. 1998;3(1):53–68. doi: 10.1080/108107398127508. [DOI] [PubMed] [Google Scholar]
- 15.Han JY. Examining effective use of an interactive health communication system (IHCS) University of Wisconsin; Madison: 2008. Unpublished doctoral dissertation. [Google Scholar]
- 16.Mezirow J. Transformative dimensions of adult learning. San Francisco: Jossey-Bass; 1991. [Google Scholar]
- 17.Hewitt M, Greenfeld S, Stovall E Committee on Cancer Survivorship: Improving Care and Cancer Survivorship. From Cancer Patient to Cancer Survivor: Lost in transition. IOM and National Research Council; 2005. [Google Scholar]
- 18.Deci E, Ryan E. Intrinsic motivation and self-determination in human behavior. New York: Plenum; [Google Scholar]
- 19.Deci E, Ryan E. Self-determination theory: A macrotheory of human motivation. Canadian Psychology/Psychologie canadienne. 2008 Aug;49(3):182–18. [Google Scholar]
- 20.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon KM, Niemiec CP. It’s not just the amount that counts: Balanced need satisfaction also affects well-being. J Pers Soc Psych. 2006;91(2):331–341. doi: 10.1037/0022-3514.91.2.331. [DOI] [PubMed] [Google Scholar]
- 22.Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating health behavior change and its maintenance: Interventions based on Self-Determination Theory. The European Hlth Psychologist. 2008 March 10;:2–5. [Google Scholar]
- 23.Wei M, Shaffer PA, Young SK, Zakalik RA. Adult attachment, shame, depression, and loneliness: The mediation role of basic psychological needs satisfaction. J Couns Psych. 2005;52(4):591–601. [Google Scholar]
- 24.Hawkins RP, Pingree S, Shaw B, Serlin RC, Swoboda C, Han J-Y, Carmack-Taylor C, Salner A. Mediating processes of effects of two communication interventions. Paper presented to the annual meeting of the International Communication Association; Montreal. May. 2008. [Google Scholar]
- 25.Hesse B. Enhancing consumer involvement in health care. In: Parker JC, Thorson E, editors. Healthcare communication in the new media. New York: Springer; 2008. [Google Scholar]
- 26.Shaw BR, McTavish F, Hawkins R, Gustafson DH, Pingree S. Experiences of women with breast cancer: exchanging social support over the CHESS computer network. J Hlth Comm. 2000;5:135–159. doi: 10.1080/108107300406866. [DOI] [PubMed] [Google Scholar]
- 27.Zillmann D. Exemplification theory of media influence. In: Bryant J, Zillmann D, editors. Media effects: Advances in theory and research. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 19–42. [Google Scholar]
- 28.Leventhal H, Nerenz D, Steele D. Illness representations and coping with health threats. In: Baum A, Singer J, editors. A Handbook of Psychology and Health. Vol. 4. Hillsdale, NJ: Erlbaum; 1984. pp. 219–252. [Google Scholar]
- 29.Rubin A. Comm Mono. Vol. 60. 1993. Audience activity and media; pp. 98–105. [Google Scholar]
- 30.Hawkins RP, Pingree S. Activity in the effects of television on children. In: Bryant J, Zillmann D, editors. Perspectives on Media Effects. Hillsdale, N.J: Lawrence Erlbaum Associates; 1986. [Google Scholar]
- 31.Hawkins RP, Pingree S, Hitchon J, Gorham BW, Kannaovakun P, Gilligan E, Radler B, Kolbens GH, Schmidt T. Predicting selection and activity in television genre viewing. Media Psychology. 2001;3(3):237–263. [Google Scholar]
- 32.Niederdeppe J, Hornik RC, Kelly B, Frosch DL, Romantan A, Stevens R, Barg F, Weiner JS, Schwartz JS. Examining the dimensions of cancer-related information seeking and scanning behavior. Hlth Comm. 2007;22(2):153–167. doi: 10.1080/10410230701454189. [DOI] [PubMed] [Google Scholar]
- 33.Eveland WP, Jr, Shah DV, Kwak N. Assessing causality in the cognitive mediation model: A panel study of motivations, information processing and learning during campaign 2000. Communication Research 2003. 2003;30:359–386. [Google Scholar]
- 34.Petty RE, Priester JR, Briñol P. Mass media attitude change: Implications of the elaboration likelihood model of persuasion. In: Bryant J, Zillmann D, editors. Media effects: Advances in theory and research. 2. Hillsdale, NJ: Erlbaum; 2002. pp. 155–198. 2002. [Google Scholar]
- 35.Cacioppo JT, von Hippel W, Ernst JM. Mapping cognitive structures and processes through verbal content: the thought-listing technique. J Consult Clin Psychol 1997. 1997;65(6):928–40. doi: 10.1037//0022-006x.65.6.928. [DOI] [PubMed] [Google Scholar]
- 36.Street RL, Jr, Rimal RN. Health promotion and interactive technology: A conceptual foundation. In: Street RL, Gold WR, Manning T, editors. Health promotion and interactive technology: Theoretical applications and future directions. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 1–18. [Google Scholar]
- 37.Rafaeli S. Interactivity: From new media to communication. In: Hawkins RP, Wiemann JM, Pingree S, editors. Advancing communication science: Merging mass and interpersonal. Newbury Park, CA: Sage; 1988. pp. 110–134. [Google Scholar]
- 38.Williams GC, McGregor HA, Sharp D. Testing a self-determination theory intervention for motivating tobacco cessation: Supporting autonomy and competence in a clinical trial. Hlth Psych. 2006;25(1):91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 39.Gwaltney DJ, Shiffman S, Balabanis MH, Paty JA. Dynamic Self-Efficacy and Outcome Expectancies: Prediction of Smoking Lapse and Relapse. J Abnorm Psychol. 2005 Nov;114(4):661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- 40.Shelby RA, Crespin TR, Wells-DiGregorio SM, Lamdan RM, Siegel JE, Taylor KL. Optimism, social support, and adjustment in African American women with breast cancer. J Behav Med. 2008 Oct;31(5):433–444. doi: 10.1007/s10865-008-9167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Hlth Ed Res 2008. 2008;23:454–466. doi: 10.1093/her/cyn004. [DOI] [PMC free article] [PubMed] [Google Scholar]