Abstract
Biological sex is an important determinant of stroke risk and outcome. Women are protected from cerebrovascular disease relative to men, an observation commonly attributed to the protective effect of female sex hormones, estrogen and progesterone. However, sex differences in brain injury persist well beyond the menopause and can be found in the pediatric population, suggesting that the effects of reproductive steroids may not completely explain sexual dimorphism in stroke. We review recent advances in our understanding of sex steroids (estradiol, progesterone and testosterone) in the context of ischemic cell death and neuroprotection. Understanding the molecular and cell-based mechanisms underlying sex differences in ischemic brain injury will lead to a better understanding of basic mechanisms of brain cell death and is an important step toward designing more effective therapeutic interventions in stroke.
Keywords: Gender, sex, sexual dimorphism, brain, stroke, ischemia, estrogen, progesterone, testosterone
1. Introduction
Clinical and epidemiological evidence suggests that stroke risk and outcome are sexually dimorphic, and emerging experimental data suggest that mechanisms of ischemic cell death may play differential roles in male vs. female cell death (Vagnerova et al., 2008). It is well established that women during their reproductive years enjoy protection from cardiovascular disease, including stroke, relative to age-matched men. This difference has been observed across cultures, ethnic groups and socio-economic status, suggesting that sex is an independent and strong predictor of stroke risk and outcome (Hurn & Macrae, 2000). Sex differences in ischemic brain injury has been historically attributed to the protective effect of female sex hormones, estrogen and progesterone (Hurn & Brass, 2003). However, sex differences in stroke risk persist well beyond the menopause (Giroud et al., 1991; Sacco et al., 1998), and female sex is associated with favorable outcome after brain injury in newborn children (Donders & Hoffman, 2002; Ingemarsson, 2003; Lauterbach et al., 2001), suggesting that sex differences in brain injury may not be entirely related to the influence of ovarian sex hormones.
To explore the basis of gender-linked ischemic brain injury, we have evaluated animal models that mimic the pathology of human stroke. We observed very early that the amount of brain tissue damage after experimental stroke is greater in male vs female rats (Alkayed et al., 1998), suggesting that sensitivity to cerebral ischemia, i.e. neural tissue damage once an ischemic event has occurred, is sex-specific (Fig. 1). We have replicated this initial observation in animal strains with pathological conditions known to be risk factors for stroke in humans, such as animals with a genetic form of insulin-dependent diabetes (Sieber et al., 2001; Toung et al., 2000), non-insulin dependent diabetes (Vannucci et al., 2001), and hypertension (Alkayed et al., 1998). In each genetic strain and despite deleterious complications from diabetes or hypertension, females were less sensitive to cerebral ischemia relative to their age-matched male counterparts (Fig. 2). This observation was also confirmed by multiple groups using a variety of animal models and experimental conditions (Hall et al., 1991; Li et al., 1996; Payan & Conrad, 1977; Wise et al., 2001; Zhang et al., 1998). In addition to studies showing that ischemic outcome is sexually dimorphic, the incidence of spontaneous strokes in a genetic strain of stroke-prone spontaneously hypertensive rats (SHRSP) is also lower in females than males (Yamori et al., 1976).
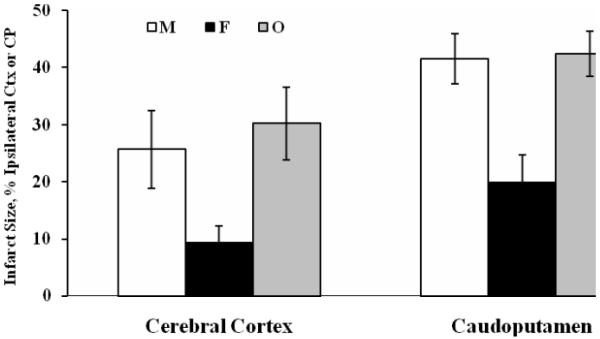
Fig. 1.
Sex Differences in Ischemic Brain Injury. Ischemia was induced by MCA occlusion for 2 hours in age-matched male (M), female (F) and ovariectomized (O) female rats. Infarct size was measured in brain sections by TTC staining at 22 hours of reperfusion and expressed as a percentage of ipsilateral cerebral cortex (CTX) or caudoputamen (CP). * Different from M and O (P < 0.05) (Alkayed et al., 1998).
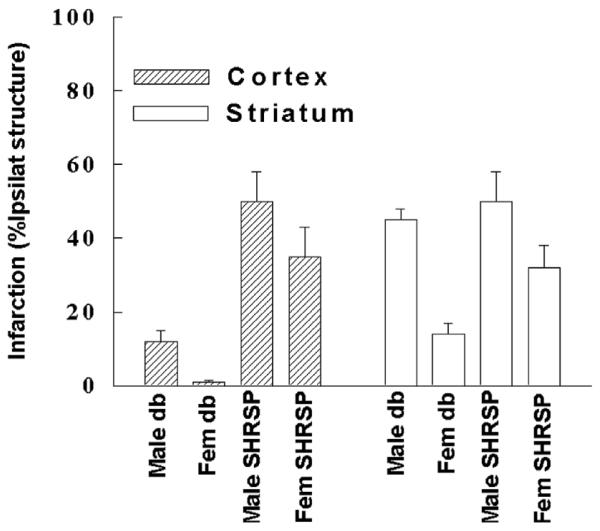
Fig. 2.
Sex differences in Ischemic Brain Injury are Unaffected by Diabetes and Hypertension. Hypoxia-ischemia (HI) was induced in male and female db/db mice, a genetic model of Type II diabetes, by combined common carotid artery ligation and hypoxia, and tissue damage assessed by hematoxylin and eosin staining. Focal cerebral ischemia was induced in age-matched male and female stroke prone spontaneously hypertensive rats (SHR-SP) rats by MCA occlusion for 2 hours, and infarct size was measured at 24 hours by triphenyl-tetrazolium chloride (TTC).
In summary, these studies suggest that male animals, unlike females, must cope with increased sensitivity to ischemic stress. Below we discuss the basis of this sex difference in sensitivity to ischemia and its potential mechanisms and cellular substrates.
2. Estrogen and the estrous cycle
In female animals, brain injury is influenced by the stage of the estrous cycle. Proestrus, the part of the cycle with high endogenous estrogen production, is associated with smaller infarct size after experimental stroke induced by middle cerebral artery occlusion (MCAO). In contrast, metestrus with low estrogen production is associated with large damage (Carswell et al., 2000). It is therefore apparent that both gender and stage in the estrous cycle influence the outcome of cerebral ischemia in the rat. These observations form the basis of the concept that female sex hormones influence stroke sensitivity and contribute to sex differences in outcome from cerebral ischemia.
To test the hypothesis that greater neuroprotection in females versus males is due to female sex hormones,we have evaluated low-hormone status by surgical ovariectomy in sexually mature young adult female animals. We found that ovariectomized females sustained larger ischemic damage relative to gonad-intact females, and infarct size in ovariectomized females was not different from males (Fig. 1) (Alkayed et al., 1998). We also examined sex-linked differences in experimental stroke outcomes in reproductively senescent animals, when ovarian function in females has naturally abated (Alkayed et al., 2000). We found that reproductive senescence in females was associated with larger infarct after MCAO, and that sex differences in infarct size observed in young animals disappeared in reproductively senescent rats, suggesting that female gonadal steroids are protective against ischemic brain injury.
To determine if hormone replacement would restore protection against cerebral ischemia in ovariectomized and reproductively senescent females, we implanted rats subcutaneously with pellets containing 25 μg 17β-estradiol (E2) for 7 days, which restores physiological plasma levels of E2. E2 is the principal and most potent mammalian estrogen and accordingly has been the best studied of the estrogens. Seven days after implantation, rats were subjected to 2 hours of MCAO, and infarct size was measured after 24 hours of reper-fusion. Infarct size was significantly reduced in both ovariectomized (Rusa et al., 1999) and reproductively senescent female rats (Alkayed et al., 2000) replaced with 17β-E2. The neuroprotective effect of acute and chronic exogenous 17β-E2 replacement has been extensively studied across different laboratories, injury models, animal breeders and genetic strains (Hurn & Macrae, 2000). The steroid is protective in both sexes in vivo and reduces neuronal cell death in vitro (Alkayed et al, 2001a). It should be noted, however, that in some studies where the MCA was permanently occluded, estrogen has been shown to exacerbate ischemic damage (Macrae & Carswell, 2006).
3. Role of estrogen receptors: Classical and non-classical
3.1. Classical estrogen receptors
E2 is a pleiotropic hormone with multiple effects and mechanisms of action. We demonstrated that pharmacological blockade of estrogen receptor (ER) using ICI182,780 exacerbates ischemic brain injury in female mice (Sawada et al., 2000), suggesting that the neuroprotective effect of E2 is mediated in part via its nuclear receptors (Fig. 3). At present, there are two known classical ER subtypes, α and β (Kuiper et al., 1996; White et al., 1987), which share homology in DNA-binding domains and potentially activate the same transcriptional elements. Anatomical distributions of both receptors overlap in some brain regions but are quite distinct in others. The highest concentrations of receptors are in areas involved in reproduction, such as the hypothalamus; however, mRNA encoding ERs have been identified in a wide variety of other brain regions, including the cerebral cortex. ERβ in neocortex is constitutively expressed and can be detected throughout life, while neocortical ERα expression is developmentally regulated. ERα is expressed in the cerebral cortex at high levels during developmental periods associated with cortical differentiation (Shughrue et al., 1997) and is inducible in adult cerebral cortex by cerebral ischemia (Dubal et al., 1999).
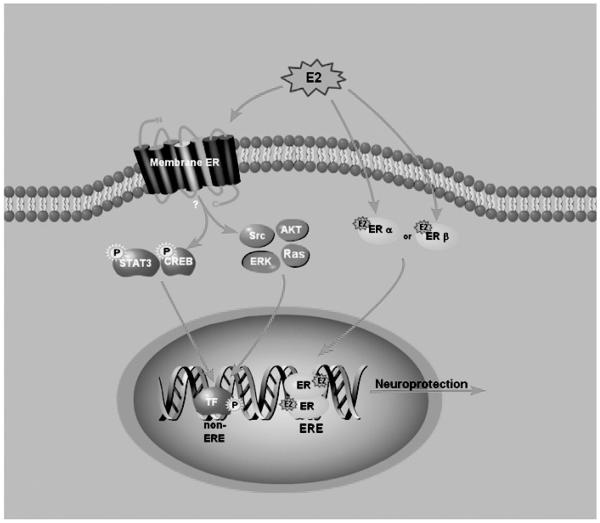
Fig. 3.
E2 induces neuroprotective genes after ischemia by classical and non-classical mechanisms. In the classical mechanism, E2 binds to either ERα or ERβ receptor and acts as a transcription factor that directly binds to estrogen response elements (EREs) in neuroprotective gene promoters. In the non-classical mechanism, E2 binds to a putative membrane estrogen receptor, resulting in rapid activation of signaling cascades (shown in pink). Activation of signaling cascades leads to the phosphorylation and activation of transcription factors, including STAT3 or Creb (shown in blue), which bind to their cognate response elements (non-ERE sites) in neuroprotective gene promoters.
To determine which receptor subtype may mediate the neuroprotective effect of E2, we subjected mice with ERα gene deletion (ERα Knockout, ERαKO) to transient MCAO and compared infarct size to mice with intact ERα (wild type, WT). We found that ERα gene deletion was not associated with increased cortical infarct size in either male or female mice. In female ERKO mice, ERα gene deletion was paradoxically associated with decreased infarct size, which may be related to enhanced brain tissue perfusion in ERαKO mice (Sampei et al., 2000a). In permanent cerebral ischemia, E2 reduced cortical infarct size in WT and ERβ KO mice, but not in ERαKO mice, suggesting that E2 may signal through ERα to reduce stroke damage in this model (Dubal et al., 2001). In a transient global ischemia model, a selective ERβ but not ERα agonist reduced hippocampal ischemic damage in ovariectomized mice (Carswell et al., 2004), suggesting that different receptor subtypes mediate protective effects in response to different types of injury as well as different brain regions.
However, 17β-E2 may exhibit neuroprotective effects without engaging its nuclear receptors. For example, non-feminizing estrogen-like compounds, with reduced or no binding to either ERα or ERβ, are protective against ischemia in vitro and in vivo, presumably via their antioxidant properties (Simpkins et al., 2004). This effect, however, is expected to play a role at high, supraphysiological levels of plasma E2. Micro-molar concentrations of 17β-E2 reduces reactive oxygen species (ROS) and the associated neuronal damage induced by FeSO4 in the presence of the ER antagonist, tamoxifen (Culmsee et al., 1999). Similarly, E2 prevents intracellular peroxide accumulation and lipid peroxidation by inactivating ROS that could otherwise oxidize lipoproteins (Keller et al., 1997; Vedder et al., 1999). Furthermore, there is evidence for a neuroprotective action of 17β- and 17α-E2 both in vitro (Behl & Manthey, 2000) and in vivo (Simpkins et al., 1997), suggesting that at these doses, E2 may exert neuroprotective effects independently of its receptors.
4. Non-classical estrogen receptors
Recent suggest that E2 may exert its neuroprotective action via so- called rapid effects, mediated through a currently unidentified, membrane-associated receptor (Fig. 3) (Kelly et al., 2003; Toran-Allerand et al., 1999; Toran-Allerand et al., 2002). Indeed, ultrastructural studies reveal ERα and ERβ localization to extra-nuclear regions (McEwen et al., 2001). In addition, a membrane estrogen receptor, GPR30, has now been identified. GPR30 is a G-protein coupled membrane-associated receptor that rapidly activates signal transduction cascades in response to E2 (Funakoshi et al., 2006). GPR30 is expressed in brain regions sensitive to ischemia including the hippocampus, cortex and striatum (Carmeci et al., 1997; Filardo & Thomas, 2005; O'Dowd et al., 1998). However the role of GPR30 in neuroprotection has yet to be determined.
The neuroprotective effect of E2 is associated with the rapid activation of ERK and AKT in vivo (Choi et al., 2004; Honda et al., 2001; Singh, 2001; Wang et al., 2006) and activation of src-family tyrosine kinases, p21(ras)-guanine nucleotide activating protein and ERK, in vitro. The protection afforded by E2 is dependent on ERK phosphorylation as E2-induced neuroprotection is abolished by the ERK (MEK) inhibititor, PD98059 (Singer et al., 1999; Wu et al., 2005). E2 is also associated with rapid phosphorylation of cAMP response element-binding protein (CREB) (Wade & Dorsa, 2003), which can be blocked by ER antagonist, ICI 182,780, and by the mitogen-activated protein kinase (MAPK) /ERK kinase-1 inhibitor PD98059. These data suggest that E2 activation of CREB requires ERK/MAPK signaling (Mize et al., 2003). Interestingly, signaling involving phosphorylation of CREB and MAPK can be elicited in the absence of either one of the classical receptors, but not in ER double-knockouts (Abraham et al., 2004).
In support of a membrane-associated receptor mediated mechanism, we demonstrated that transfected ERα localizes to neurites in cultured cortical neurons and that neurite-localized ERα activates MAPK in response to estradiol (Xu et al., 2003). We also determined that membrane associated receptors are capable of direct activation of transcription via estrogen response element (ERE) (Xu et al., 2004).
5. Mechanism of estradiol-mediated neuroprotection
E2 uses a variety of genomic and non-genomic mechanisms to exert its protective action. Non-genomic effects are exemplified by a variety of cytoplasmic signaling cascades, such as the activation of protein kinase C, phosphatidylinositol-3-OH kinase, protein kinase A/cAMP (Gu & Moss, 1996; Kelly et al., 1999) steroid receptor coactivator (src) and intracellular signaling that activates ion channels, neurotransmitter receptors, and enzymes. These mechanisms may be critical to E2's protection in experimental stroke (Segars & Driggers, 2002).
The genomic effects of E2 are brought about either via direct ER binding to a consensus DNA sequence, the ERE (Klinge, 2000), or indirectly by interacting with and influencing DNA binding affinity of other transcription factors, such as nuclear factor-kappa B (NF-κB) and activator protein 1 (AP1) (McKay & Cidlowski, 1999; Paech et al., 1997) SP1 (Castro-Rivera et al., 2001) and Jun/ATF-2 (Sabbah et al., 1999). Functional EREs have been identified in genes implicated in normal brain function and pathology (Garcia-Segura et al., 2001), including those encoding choline acetyltransferase (Miller et al., 1999), the GABA transporter GAT-1 (Herbison et al., 1995), α-adrenergic receptor (Lee et al., 1998), the opioid precursor preproenkephalin (PPE) (Zhu & Pfaff, 1995), the neuropeptides oxytocin and vasopressin (Harlan, 1988), somatostatin (Xu et al., 1998), galanin (Howard et al., 1997), glial fibrillary acidic protein (GFAP) (Stone et al., 1998b), brain-derived neurotrophic factor (BDNF) (Sohrabji et al., 1995), transforming growth factor-alpha (TGF-α, (El Ashry et al., 1996), cyclin D1 (Sabbah et al., 1999), vascular endothelial growth factor (VEGF) (Mueller et al., 2000) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG CoA) gene, which is regulated by estrogen via a variant of ERE with base pair mismatches (Croce et al., 1999).
Non-genomic and genomic effects of estradiol are not mutually exclusive. Supporting this idea, E2 induces many genes which do not contain EREs (O'Lone et al., 2004). Therefore E2 induces non-genomic or rapid activation of signaling cascades that result in the activation of other transcription factors, which may induce neuroprotective genes. For example, we have recently reported that E2 replacement increases the phosphorylation of the transcription factor, signal transducer and activator of transcription 3 (STAT3) after transient focal ischemia relative to ovariectomized female rats. The effect was observed in the cytosolic fractions where the total amount of STAT3 was unaltered, suggesting rapid signaling events after an ischemic event. The protective effect of E2 to reduce infarct volume appeared to be mediated via phospho-STAT3 because pharmacological inhibition of P-STAT3 abolished the protective effect of estradiol on infarct size (Dziennis et al., 2007). Furthermore, P-STAT3 was observed in cells that were also immunoreactive for bcl-2 or MAP-2 suggesting that P-STAT3 is expressed in surviving neurons.
To this end, we demonstrated that E2 upregulates two neuroprotective genes which lack promoter estrogen response elements; bcl-2 and CART. Bcl-2 is well established for protective effects in brain injury of all types (for review see (Graham et al., 2000; Mattson et al., 2000). E2 up-regulates bcl-2 expression in several brain regions of cycling female rats (Garcia-Segura et al., 1998). E2 increases the expression of bcl-2 mRNA and protein in the peri-infarct region after transient MCA occlusion in rats, accompanied by a decrease in infarct size (Alkayed et al., 1998; Alkayed et al., 2001b; Dubal et al., 1999). Moreover, bcl-2 overexpressing mice are protected from the exacerbation of cerebral ischemic injury ordinarily caused by ovariectomy in wild type female mice (Alkayed et al., 2001b). In the setting of ischemia, the ability of E2 to enhance bcl-2 expression may be through the multiple putative STAT binding sites and/or the CREB binding site in the absence of EREs (Teixeira et al., 1995). Interestingly Meller and colleagues found that CREB binding protein (CBP) rather than CREB, bound to the CRE in an in vitro model of ischemic preconditioning (Meller et al., 2005).
Using DNA microarrays, we identified a gene product, cocaine- and amphetamine-regulated transcript (CART), that is highly induced by E2 in the ischemic cerebral cortex and exhibits potent neuroprotective effects in models of neural injury both in vitro and in vivo. The cart gene encodes a neuropeptide that is implicated in food intake, drug addiction, and the neuroendocrine response to stress (Jaworski & Jones, 2006; Koylu et al., 2006; Kuhar et al., 2002; K. G. Murphy, 2005). We confirmed that E2 increased CART mRNA and protein in cortical neuronal cultures after OGD in the cortex after MCAO. CART co-localized with the neuronal marker NeuN but not the glial marker GFAP after MCAO, suggesting a role for CART in neuroprotection. To test the hypothesis that CART is neuroprotective, CART was administered to primary cortical cultures prior to OGD. CART administration significantly reduced OGD-induced DNA damage and cell death in primary cultured cortical neurons (Xu et al., 2006). CART peptide administration also significantly reduced cortical infarct size after MCAO, demonstrating a protective effect of CART against cerebral ischemia, in vivo. CART, like bcl-2, lacks an ERE, yet contains a STAT3 and a CREB binding site (Lakatos et al., 2002). We found CREB, but not STAT3 bound to the cart promoter after MCAO (Xu et al., 2006).
The mechanism of protection by E2 is multi-faceted and involves multiple brain cell types, including neurons, astrocytes, microglia and oligodendrocytes, as well as vascular and blood-borne cells (Azcoitia et al., 1999a; Gudino-Cabrera & Nieto-Sampedro, 1999; Mor et al., 1999). Depending on cell and injury type, E2's protection may involve any number of mechanisms triggered by E2. E2's reduction of tissue injury is in part related to the steroid's vasodilator actions and ability to promote optimal endothelial function (Mendelsohn & Karas, 1994; White et al., 1995). Cerebral blood flow is higher in premenopausal women than in men or in older women (Davis et al., 1983; Shaw et al., 1984), and estrogen influences blood flow during ischemic stress and reperfusion (Alkayed et al., 1998; Hurn et al., 1995; Pelligrino et al., 1998).
Sex-specific differences in vascular reactivity have been described in the periphery (Li & Duckles, 1994; Sakuma et al., 2002) and in the cerebral circulation (Geary et al., 1998). The effects of estrogen on vasodilation are mediated through changes in endothelium-dependent nitric oxide (NO), prostanoids and endothelial-derived hyperpolarizing factor (EDHF). In addition, estrogen deficiency, either as part of the estrus cycle or long-term after ovariectomy, alters endothelium-dependent nitric oxide (NO)-and prostacyclin-mediated responses of mesenteric arteries in female rats (Liu et al., 2001). Although the identity of EDHF is not known, we have demonstrated that estrogen suppresses soluble epoxide hydrolase, an enzyme involved in the metabolism of an endothelium-dependent hyperpolarizing factor (EDHF) candidate, namely epoxyeicosatrienoic acid (EET) (Koerner et al., 2008). Suppression of soluble epoxide hydrolase would increase EETs, leading to enhanced vasodilator capacity in response to vascular occlusion (Zhang et al., 2008), and may account for the increase in blood flow during MCAO in female rats (Alkayed et al., 1998).
Others have shown that in females, endothelium-dependent hyperpolarizing factor (EDHF)-mediated dilations of the middle cerebral artery (MCA) are significantly attenuated as compared with that of the male (Golding & Kepler, 2001). Dilations in ovariectomized females are enhanced to similar levels observed in the intact males and subsequently lost after chronic E2 replacement, suggesting that this effect is mediated by E2. In contrast to findings in cerebral vessels, estrogen appears to potentiate EDHF-mediated dilations in peripheral vessels (McCulloch & Randall, 1998; White et al., 2000), in agreement with other reports that the mechanism for the EDHF response is distinct in the periphery vs cerebrovasculature (Golding et al., 2002).
Other proposed mechanisms of protection by E2 include the suppression of excitotoxicity (Singer et al., 1996; Smith et al., 1987; Weaver et al., 1997), apoptosis (Alkayed et al., 2000; Alkayed et al., 2001), β amyloid toxicity (Goodman et al., 1996; Shi et al., 1998), edema formation (Roof et al., 1993; Roof et al., 1994), antioxidant production (Ayres et al., 1998; Bruce-Keller et al., 2000; Culmsee et al., 1999; Sawada et al., 1998) and regulation of growth factors (Gollapudi & Oblinger, 1999; Toran-Allerand, 1996). Finally, E2 is anti-inflammatory (Hunt et al., 1997; Ray et al., 1997; Salem et al., 2000; St Clair, 1997; Vegeto et al., 2001) in cerebral ischemia, reducing the number of active microglia (Lei et al., 2003), decreasing intravascular leukocyte adhesion and migration into brain (Santizo et al., 2000), and suppressing endothelial expression of adhesion molecules (Mori et al., 2004; Nathan et al., 1999). Moreover, E2 can suppress reactive gliosis and expression of the inflammation promoting transcription factor NF-kB after cerebral injury (Wen et al., 2004). Taken together, it appears that E2 can restrict cellular damage in the pro-inflammatory milieu of ischemia/reperfusion.
6. E2, neuroregeneration and recovery
E2 promotes recovery from CNS injury by stimulating neural cell proliferation and synaptogenesis and via modulation of synaptic connectivity, leading to axonal sprouting and regeneration (Garcia-Segura et al., 2001; Jones, 1993). These effects are, at least in part, mediated via E2's effects on expression of growth factors and neurotrophins, such as NGF, BDNF and NT-4 (Jezierski & Sohrabji, 2000; Wang et al., 2000), resulting in changes in expression of growth components, such as structural proteins and adhesion molecules. E2 may also affect cellular proliferation and neurite elongation and differentiation by directly regulating the transcription of genes required for growth; such as regulation by estrogen of the expression of tau microtubule-associated protein (Ferreira & Caceres, 1991), the growth-associated protein 43 (GAP-43), neuromodulin (Shughrue & Dorsa, 1993), neurofilament proteins (Scoville et al., 1997), synaptic proteins, such as synaptophysin, syntaxin, and spinophilin (Teter et al., 1999), and structural lipoproteins, such as apolipoprotein E (apoE) (Stone et al., 1998a). Finally, the signaling pathways for E2 and neurotrophins converge on the MAPK cascade, which includes activation of B-Raf and extracellular signal-regulated kinase (ERK) (Singh et al., 2000). This common pathway may explain the ability of E2 and neurotrophins to regulate the same broad array of cytoskeletal and growth-associated genes involved in neurite growth and differentiation. The interaction of E2 with growth factors may also prevent cell death by preventing initiation of apoptosis (Gollapudi & Oblinger, 1999). However, as mentioned above, E2 can also prevent neuronal cell death by directly regulating expression of effectors of cell death, such as members of the bcl-2 family of cell death-associated proteins (Alkayed et al., 2001b).
In adult brain as well as during brain development, ER s co-localize with, and reciprocally regulate expression of, neurotrophins and their cognate receptors, such as the high- (trkA) and low-affinity (p75) receptors for nerve growth factor (NGF). The net effect is to increase the sensitivity of these cells to neurotrophins (Sohrabji et al., 1994; Toran-Allerand et al., 1999). E2's effect on BDNF expression is region-specific, as E2 increases BDNF in cortex (Gibbs, 1999), while decreasing BDNF in hippocampus (Woolley, 1999). The gene encoding BDNF contains a sequence similar to the canonical ERE (Sohrabji et al., 1995).
E2's neuroregenerative effects may be mediated via interaction with other growth factors, such as insulin-like growth factor-1 (IGF-1) (Garcia-Segura et al., 2006). The trophic effects of IGF-I are mediated by IGF-I receptor, a member of the growth factor tyrosine kinase receptor family that signals through the phosphoinositol-3 kinase pathway and the MAPK cascade. E2 and IGF-I co-operate in hippocampus to protect neurons from kainic acid-induced cell death (Azcoitia et al., 1999b). IGF-I receptor blockade prevents E2-induced neuroprotection while IGF-I induced protection is blocked by ICI 182,780. E2 can also protect neurons via growth factors indirectly, via its interaction with growth factor-enhancing proteins, such as activin, a member of the transforming growth factor β (TGFβ) superfamily (Trudeau et al., 1996), which is important for the neuroprotective action of basic fibroblast growth factor (bFGF) (Tretter et al., 2000). Similarly, E2 increases IGF-1 levels by upregulating expression of IGF binding protein-2 (IGFBP-2) (Duenas et al., 1994).
7. Local estrogen production: Role of the P450 aromatase
Local brain E2 may also be relevant to neuroprotection. E2 levels in brain vary between males and females as early as the first day of life (Amateau et al., 2004). Mammalian E2 is naturally synthesized from testosterone by aromatization, a process that occurs not only in gonads, placenta and fat, but also in brain (Azcoitia et al., 2003; Naftolin, 1994). The role of local brain E2 formation on ischemic brain injury has been examined in mice with targeted deletion of P450 aromatase (CYP19A1), which are incapable of converting testosterone to E2. Mice with targeted disruption of exon 9 of the CYP 19A1 gene (aromatase knockout mice) sustained increased brain injury after experimental stroke compared to wild-type mice with intact P450 aromatase gene, suggesting an important role for P450 aromatase in protection from ischemic brain injury (Liu, et al., 2004b; McCullough et al., 2003). In addition to synthesis, local estrogen levels are also determined by metabolism. Estrogens are metabolized into catechol estrogens, 2- and 4-hydroxy estrogens, via the actions of P450 lA and lB. Both genes are expressed in brain (Muskhelishvili et al., 2001), and catechol estrogens have been detected in normal brain (Weisz & Crowley, 1986) and implicated in neuronal cell death (Desjardins et al., 1992).
Estradiol is not the sole hormone active in mediating outcome from ischemic brain injury. Progesterone and testosterone, as well as non-hormonal factors, have been shown to contribute importantly to sex differences in brain injury and recovery. Below, we discuss the contributions of these factors to the differences in ischemic brain injury between males and females.
8. Progesterone and cerebral ischemia
Progesterone has been less well studied in cerebral ischemia, but our studies suggest that the steroid is equipotent to E2 when administered in the correct therapeutic window (Fig. 4). We previously demonstrated that chronic progesterone administration reduces cortical infarct in middle-aged, reproductively senescent females, and protection is not related to effects of progesterone on cerebral blood flow (Azcoitia et al., 2003). Similarly, progesterone administration during reperfusion (Murphy et al., 2002), but not before the ischemia (Murphy et al., 2000), reduces ischemic injury in ovariectomized, young adult female rats. Finally, post-ischemic progesterone has also been shown to reduce lesion size and enhance functional recovery after transient MCAO in normotensive (Gibson & Murphy, 2004) and hypertensive male mice (Kumon et al., 2000) and rats (Chen et al., 1999; Jiang et al., 1996). While the neuroprotective mechanism is unclear, the steroid's membrane-stabilizing antioxidant actions may be relevant (Duenas et al., 1994). Progesterone is also known to modulate y-aminobutyric acid (GABA) receptor channel activity and expression (El Ashry et al., 1996) and attenuate excitatory neuronal responses (Eliasson et al., 1997), which may underlie progesterone's anxiolytic (Ferreira & Caceres, 1991) and antiepileptic (Frye & McCormick, 2000) properties.
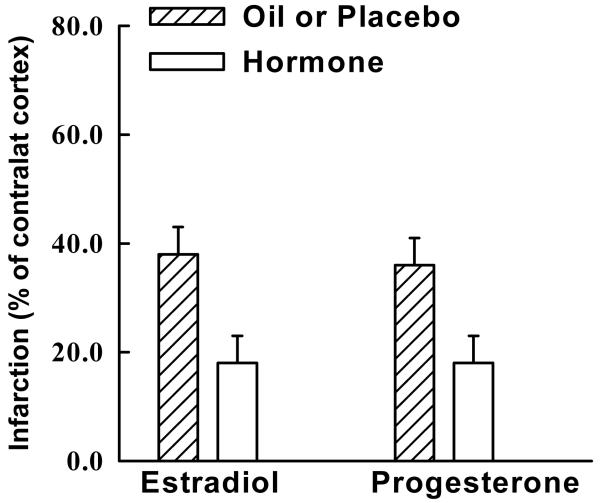
Fig. 4.
Female Sex Hormones 17β-Estradiol and Progesterone are Protective against Ischemic Brain Injury. Ischemia was induced in ovariectomized female rats with and without hormone replacement for two hours by MCA occlusion, and infarct size was measured in the cerebral cortex at 24 hours by triphenyl tetrazolium chloride (TTC) and expressed as a percentage of contralateral cortex to account for edema.
9. Role of male sex hormones: Testosterone
Male sex is an acknowledged risk factor for stroke and cerebrovascular disease. In men, normal circulating testosterone levels range from 10 to 30 nM (Winters, 1999), whereas much lower levels (0.6 to 2.5 nM) are found in women (Burger, 2002). Although the adrenal gland can synthesize small quantities of testosterone, testicular Leydig cells are the primary source of androgens in men (Winters, 1999). Ordinarily, plasma luteinizing hormone (LH) interacts with Leydig cell surface receptors to regulate pulsatile testosterone release in a diurnal pattern, with peak levels occurring in the morning. However, other hormones, including prolactin, cortisol, insulin, insulin-like growth factor and E2, are known to influence circulating testosterone (Winters, 1999). In women, peripheral sites such as liver, skin, and adipose tissue provide approximately half of the circulating testosterone, whereas adrenal (25%) and ovarian sources (25%) produce the remainder (Burger, 2002). As members of the steroid superfamily, androgens originate from the cholesterol-derived precursor, pregnenolone. Both E2 and testosterone are derived directly from pregnenolone's by-product, androstenedione. In addition, androstenedione can be formed indirectly from the progesterone degradation product 17β-hydroxyprogesterone. The enzyme 17β- hydroxysteroid dehydrogenase (17 β-HSD) catalyzes this metabolism to testosterone and is also requisite for 17 β-E2 production. Testosterone can be converted to 17 β-estradiol via the aromatase, as described above, or metabolized to dihydrotestosterone (DHT) via the enzyme 5α-reductase, a step that then does not permit aromatization to estradiol. Testosterone interacts with a single known androgen receptor (AR) to initiate gene transcription. However, testosterone has also been shown to produce rapid vascular effects that clearly do not involve genomic mechanisms.
Data in animal models of cerebral ischemia testing effects of androgens are surprisingly few and contradictory. In male rat, castration and subsequent low testosterone is associated with decreased or no difference in histological damage after MCAO (Hawk et al., 1998; Toung et al., 1998). Testosterone administration increases infarct size after MCAO in male rats (Hawk et al., 1998), in part via exacerbation of glutamate neurotoxicity (Yang et al., 2002). In accordance with a neurotoxic role, Yang et al demonstrated that brief anesthetic exposure increases ischemic tolerance by suppressing testosterone production (Yang et al., 2005). In contrast, testosterone or DHT treatment reduces dentate gyrus neuronal loss after adrenalectomy (Frye & McCormick, 2000). In neuronal culture, testosterone appears in some studies to protect cells from oxidative stress, β-amyloid toxicity, and serum deprivation through an AR-dependent mechanism (Ahlbom et al., 1999; Ahlbom et al., 2001; Hammond et al., 2001; Pike, 2001). Further study is required to understand how androgens impact on sex differences in ischemic injury.
10. Hormone-independent mechanisms of protection and injury
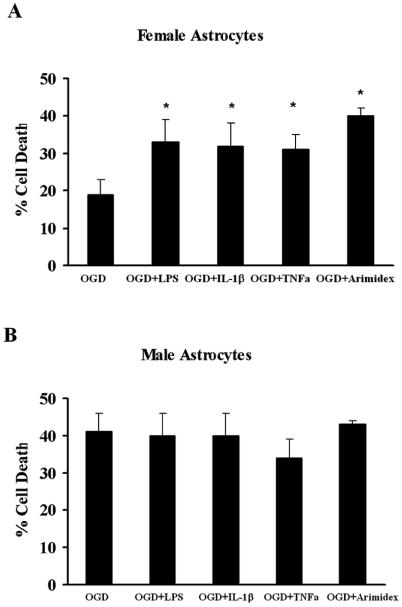
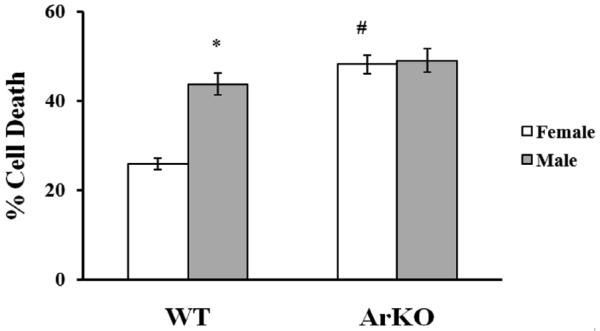
New data from cultured cells in which sex steroids are removed from the media support the concept that cell death mechanisms can be sex-specific. Some molecular pathways of cell death or survival diverge, depending on the genetic sex of the tissue (defined by chromosomal gene content as female XX or male XY). For example, female dopaminergic neurons cultured from 14 day old embryos (E14) tolerate exposure to toxic dopamine concentrations at the LD50 level and survive twofold relative to male cells (Lieb et al., 1995). Similarly, female neurons from cortical plate or ventricular zone have greater longevity in culture than do male cells, and differentially express higher levels of phosphorylated kinases such as Akt (Zhang et al., 2003). Sensitivity to glutamate, peroxynitrate (ONOO−) and staurosporine in neuronal culture (E17) is sex specific, with male neurons being more susceptible to glutamate and ONOO− than females. In contrast, response to oxidants such as H2O2 is not dependent on the sex of the cells (Du et al., 2004). We have recently found that sex specific sensitivity to neurotoxins is also evident in astrocytes. Astrocytes are important players in normal brain functioning (Harder et al., 1998), as well as the response to ischemic brain injury (Chen & Swanson, 2003; Swanson et al., 2004; Takuma et al., 2004). These cells are potentially important players in the regenerative and neuroprotective effects of sex steroids and in determining sex differences in synaptic connectivity and plasticity (Garcia-Segura et al., 1999). We have evaluated cell death resulting from oxygen-glucose deprivation (OGD) in sex-specific astrocyte cultures from rat pups and observed that female astrocytes are more resistant to OGD compared with male astrocytes, but sustain greater cell death when inflammatory mediators are combined with OGD compared to OGD alone. The aromatase inhibitor, Arimidex, abolishes sex differences in OGD-induced cell death (Fig. 5), suggesting that astrocytes isolated from neonatal cortex exhibit marked sex differences in sensitivity to OGD, in part due to enhanced aromatization and estradiol formation in female cells (Liu et al., 2004a; Liu et al., 2007). To verify sex-specific differences in P450 aromatase function in astrocyte cell death following OGD, we developed a novel method to establish sex-specific and genotype-specific single pup primary astrocyte cultures from wild-type (WT) and aromatase knockout (ArKO) mice (Liu et al., 2008). Consistent with data generated in sex-specific rat astrocytes (Liu et al., 2007), we found that female WT mouse astrocytes are more resistant to OGD than male WT mouse astrocytes. It is important to note that male sensitivity and female resistance to OGD are observed across species. Using the new technique developed for this study, we demonstrated that P450 aromatase gene deletion significantly increased cell death after OGD in female astrocytes and abolished the sex differences in sensitivity to OGD seen in WT female and male astrocytes (Fig. 6). These findings confirm that P450 aromatase is instrumental in mediating astrocyte survival following OGD and suggest that P450 aromatase is cytoprotective against ischemic brain injury. This is consistent with an earlier in vivo study of ischemia in which brain damage was greater in female ArKO mice compared to female WT mice (McCullough et al., 2003). These studies suggest that male and female brains may have inherently different susceptibility to ischemic stress. This is consistent with recent evidence suggesting that the brain begins to develop differently in males and females before sex hormones come into play, and that the brains of males and females differ not only in regions specialized for reproduction, but in other brain regions as well (Arnold et al., 2003).
Fig. 5.
Sex-specific astrocytic cell death. A. Female astrocytes are more resistant to cell death induced by oxygen-glucose deprivation. LPS, interleukin-1β (IL-1β), tissue necrosis factor (TNF)-1α and Arimidex exacerbate OGD-induced cell death in female astrocytes compared to OGD alone. * p < 0.05. B. Male astrocytes are more susceptible to cell death induced by oxygen-glucose deprivation. LPS, interleukin-1β (IL-1β), tissue necrosis factor (TNF)-1α and Arimidex did not alter OGD-induced cell death in male astrocytes. Adapted from Liu et al., 2007.
Fig. 6.
Sex differences in OGD-induced cell death in mouse astrocytes. WT female astrocytes are less susceptible to cell death compared to WT male astrocytes after OGD (#p < 0.05). Cell death markedly increased in ArKO female astrocytes compared to WT female cells (*p < 0.05), but there was no significant difference in cell death between ArKO male astrocytes compared to WT male cells. Sex-specific responses to OGD were abolished in ArKO astrocyte cultures. Adapted from Liu et al., 2008.
11. Sex-specific mechanisms of ischemic cell death
Data from genetically engineered mice also suggest that molecular mechanisms of cell injury are not necessarily identical in male and female brain. When both sexes are studied, ischemic outcome in transgenic mice can be overtly gender-dependent, even when the gene of interest is not linked to sexual development (Loihl et al., 1999; McCullough et al., 2005; Sampei et al., 2000b). For example, it is well known that neuronal nitric oxide synthase (nNOS) plays an important role in intiating ischemic cell death. Nitric oxide cytoxicity through its rapid reaction with superoxide anion results in peroxynitrite formation and protein nitration (Dawson & Dawson, 1998). Genetic deletion or pharmacological inhibition of nNOS is neuroprotection in male animals (Huang et al., 1994), presumably by reduction of NO. However, we have recently observed that loss of nNOS activity in female knockouts or through pharmacological enzyme inhibition paradoxically increases histological infarction after MCA occlusion (McCullough et al., 2005). Another example of a potentially sex-specific cell death mechanism is the activation of the DNA repair enzyme, poly-ADP ribose polymerase (PARP). PARP is hyper-activated in the presence of ischemic DNA damage and enhances neuronal death after exitotoxic or ischemic insults. However, these data all arise from male mice or from cell culture of mixed sex origin (Eliasson et al., 1997; Goto et al., 2002). In females, loss of PARP activity appears to be deleterious. Female PARP knockouts or females treated with PARP inhibitors sustain remarkable exacerbation of ischemic damage after MCA occlusion, unlike their male counterparts (McCullough et al., 2005).
12. Sex differences in response to therapy
The presence of gender specific mechanisms of ischemic brain injury suggests that responses to neuroprotective therapy could be different in males and females. For example, gender differences were observed regarding the efficacy of the anti-oxidant tirilizad in the treatment of subarachnoid hemorrhage (Kassell et al., 1996), with males displaying greater response to therapy than females. Similarly, posttraumatic hypothermia attenuates traumatic brain injury and subsequent neuronal loss in males, but not in females (Suzuki et al., 2003). This effect may be related to the observation that hypothermia reduces cerebral spinal fluid (CSF) markers of excitotoxicity and oxidative damage after brain injury to a greater degree in males than females, suggesting that hypothermia may be more beneficial in males than in females (Wagner et al., 2004). These findings underscore the importance of stratifying by sexing pre-clinical data, with the hope of predicting differing responses to therapeutic agents and intervention strategies in men and women.
13. Conclusion
Stroke is a sexually dimorphic disease. Sex differences in stroke risk and outcome have been observed in humans of all ages and are present in experimental models of cerebral ischemia at the whole animal and cellular levels. Male and female sex steroids, originating from gonads or locally produced within brain, account for some, but not necessarily all, of these differences. New observations suggest that fundamental mechanisms of ischemic cell death play differential roles in male vs. female brain. Stroke remains a major cause of death and disability in both men and women, however results from major clinical trials testing available neuroprotective compounds against stroke injury remain disappointing. Understanding sex-specific mechanisms of ischemic brain damage is warranted if we are to develop more effective therapies for human stroke.
Acknowledgments
This research was funded by American Heart Association grant 0535284N and National Institutes of Health, grant NS044313, NS33668, NS49210, NR03521 and Bugher Foundation of American Heart Association.
References
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11:1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Wang MM, Hurn PD. Clark R, editor. Reproductive hormones as neuroprotectants in brain injury. Brain injury molecular and cellular biology of critical care medicines series Kluwer-Lippincott. 2001a [Google Scholar]
- Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, et al. Estrogen and bcl-2: Gene induction and effect of transgene in experimental stroke. J Neurosci. 2001b;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: Sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Ayres S, Abplanalp W, Liu JH, Subbiah MTR. Mechanisms involved in the protective effect of estradiol-17 beta on lipid peroxidation and DNA damage. Am J Physi. 1998;274:E1002–E1008. doi: 10.1152/ajpendo.1998.274.6.E1002. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999a;26:260–267. [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: Interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999b;58:815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Behl C, Manthey D. Neuroprotective activities of estrogen: An update. J Neurocytol. 2000;29:351–358. doi: 10.1023/a:1007109222673. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):3–5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278:H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective oestrogen receptor {beta} agonist in a mouse model of global ischaemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E, Samudio I, Safe S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J Biol Chem. 2001;276:30853–30861. doi: 10.1074/jbc.M103339200. [DOI] [PubMed] [Google Scholar]
- Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1999;171:24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Choi YC, Lee JH, Hong KW, Lee KS. 17 beta-estradiol prevents focal cerebral ischemic damages via activation of akt and CREB in association with reduced PTEN phosphorylation in rats. Fundam Clin Pharmacol. 2004;18:547–557. doi: 10.1111/j.1472-8206.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, et al. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: Evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Davis SM, Ackerman RH, Correia JA, Alpert NM, Chang J, Buonanno F, et al. Cerebral blood flow and cerebrovascular CO2 reactivity in stroke-age normal controls. Neurology. 1983;33:391–399. doi: 10.1212/wnl.33.4.391. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Nitric oxide in neurode-generation. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- Desjardins GC, Beaudet A, Schipper HM, Brawer JR. Vitamin E protects hypothalamic beta-endorphin neurons from estradiol neurotoxicity. Endocrinology. 1992;131:2482–2484. doi: 10.1210/endo.131.5.1425446. [DOI] [PubMed] [Google Scholar]
- Di Croce L, Vicent GP, Pecci A, Bruscalupi G, Trentalance A, Beato M. The promoter of the rat 3-hydroxy-3-methylglutaryl coenzyme A reductase gene contains a tissue-specific estrogen-responsive region. Mol Endocrinol. 1999;13:1225–1236. doi: 10.1210/mend.13.8.0333. [DOI] [PubMed] [Google Scholar]
- Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16:491–499. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: A potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59:528–538. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Jia T, Ronnekleiv OK, Hurn PD, Alkayed NJ. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci. 2007;27:7268–7274. doi: 10.1523/JNEUROSCI.1558-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ashry D, Chrysogelos SA, Lippman ME, Kern FG. Estrogen induction of TGF-alpha is mediated by an estrogen response element composed of two imperfect palindromes. J Steroid Biochem Mol Biol. 1996;59:261–269. doi: 10.1016/s0960-0760(96)00118-5. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Caceres A. Estrogen-enhanced neurite growth: Evidence for a selective induction of tau and stable microtubules. J Neurosci. 1991;11:392–400. doi: 10.1523/JNEUROSCI.11-02-00392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: A seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM. Androgens are neuroprotective in the dentate gyrus of adrenalectomized female rats. Stress. 2000;3:185–194. doi: 10.3109/10253890009001122. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, Doncarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates bcl-2 expression in adult brain neurons. Neuroreport. 1998;9:593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 1999;40:574–584. [PubMed] [Google Scholar]
- Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-I and estradiol in the brain: Focus on neuroprotection. Neuroendocrinology. 2006;84:275–279. doi: 10.1159/000097485. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Giroud M, Milan C, Beuriat P, Gras P, Essayagh E, Arveux P, et al. Incidence and survival rates during a two-year period of intracerebral and subarachnoid haemorrhages, cortical infarcts, lacunes and transient ischaemic attacks. the stroke registry of dijon: 1985-1989. Int J Epidemiol. 1991;20:892–899. doi: 10.1093/ije/20.4.892. [DOI] [PubMed] [Google Scholar]
- Golding EM, Kepler TE. Role of estrogen in modulating EDHF-mediated dilations in the female rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2001;280:H2417–H2423. doi: 10.1152/ajpheart.2001.280.6.H2417. [DOI] [PubMed] [Google Scholar]
- Golding EM, Marrelli SP, You J, Bryan RM., Jr. Endothelium-derived hyperpolarizing factor in the brain: A new regulator of cerebral blood flow? Stroke. 2002;33:661–663. [PubMed] [Google Scholar]
- Gollapudi L, Oblinger MM. Estrogen and NGF synergistically protect terminally differentiated, ERalpha-transfected PC12 cells from apoptosis. J Neurosci Res. 1999;56:471–481. doi: 10.1002/(SICI)1097-4547(19990601)56:5<471::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Goto S, Xue R, Sugo N, Sawada M, Blizzard KK, Poitras MF, et al. Poly(ADP-ribose) polymerase impairs early and long-term experimental stroke recovery. Stroke. 2002;33:1101–1106. doi: 10.1161/01.str.0000014203.65693.1e. [DOI] [PubMed] [Google Scholar]
- Graham SH, Chen J, Clark RS. Bcl-2 family gene products in cerebral ischemia and traumatic brain injury. J Neurotrauma. 2000;17:831–841. doi: 10.1089/neu.2000.17.831. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17 beta-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudino-Cabrera G, Nieto-Sampedro M. Estrogen receptor immunoreactivity in schwann-like brain macroglia. J Neurobiol. 1999;40:458–470. doi: 10.1002/(sici)1097-4695(19990915)40:4<458::aid-neu4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain. hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;28:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- Harlan RE. Regulation of neuropeptide gene expression by steroid hormones. Mol Neurobiol. 1988;2:183–200. doi: 10.1007/BF02935345. [DOI] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Augood SJ, Simonian SX, Chapman C. Regulation of GABA transporter activity and mRNA expression by estrogen in rat preoptic area. J Neurosci. 1995;15:8302–8309. doi: 10.1523/JNEUROSCI.15-12-08302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, et al. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- Howard G, Peng L, Hyde JF. An estrogen receptor binding site within the human galanin gene. Endocrinology. 1997;138:4649–4656. doi: 10.1210/endo.138.11.5507. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Miller L, Roby KF, Huang J, Platt JS, DeBrot BL. Female steroid hormones regulate production of pro-inflammatory molecules in uterine leukocytes. J Reprod Immunol. 1997;35:87–99. doi: 10.1016/s0165-0378(97)00060-0. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Brass LM. Estrogen and stroke: A balanced analysis. Stroke. 2003;34:338–341. doi: 10.1161/01.str.0000054051.88378.25. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Littleton-Kearney MT, Kirsch JR, Dharmarajan AM, Traystman RJ. Postischemic cerebral blood flow recovery in the female: Effect of 17 beta-estradiol. J Cereb Blood Flow Metab. 1995;15:666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Ingemarsson I. Gender aspects of preterm birth. BJOG. 2003;110(Suppl 20):34–38. doi: 10.1016/s1470-0328(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Jones DC. The role of CART in the reward/reinforcing properties of psychostimulants. Peptides. 2006;27:1993–2004. doi: 10.1016/j.peptides.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Brain Res Mol Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Jones KJ. Gonadal steroids as promoting factors in axonal regeneration. Brain Res Bull. 1993;30:491–498. doi: 10.1016/0361-9230(93)90283-h. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Haley EC, Appersonhansen C, Stat M, Alves WM, Dorsch NW, et al. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage - a cooperative study in europe, australia, and new zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- Keller JN, Germeyer A, Begley JG, Mattson MP. 17Beta-estradiol attenuates oxidative impairment of synaptic Na+/K+-ATPase activity, glucose transport, and glutamate transport induced by amyloid beta-peptide and iron. J Neurosci Res. 1997;50:522–530. doi: 10.1002/(SICI)1097-4547(19971115)50:4<522::AID-JNR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: Regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front Biosci. 2008;13:2833–2841. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koylu EO, Balkan B, Kuhar MJ, Pogun S. Cocaine and amphetamine regulated transcript (CART) and the stress response. Peptides. 2006;27:1956–1969. doi: 10.1016/j.peptides.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Adams S, Dominguez G, Jaworski J, Balkan B. CART peptides. Neuropeptides. 2002;36:1–8. doi: 10.1054/npep.2002.0887. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg. 2000;92:848–852. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- Lakatos A, Dominguez G, Kuhar MJ. CART promoter CRE site binds phosphorylated CREB. Brain Res Mol Brain Res. 2002;104:81–85. doi: 10.1016/s0169-328x(02)00321-2. [DOI] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: The influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15:411–420. [PubMed] [Google Scholar]
- Lee K, Richardson CD, Razik MA, Kwatra MM, Schwinn DA. Multiple potential regulatory elements in the 5′ flanking region of the human alpha 1a-adrenergic receptor. DNA Seq. 1998;8:271–276. doi: 10.3109/10425179809008464. [DOI] [PubMed] [Google Scholar]
- Lei DL, Long JM, Hengemihle J, O'Neill J, Manaye KF, Ingram DK, et al. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003;121:659–666. doi: 10.1016/s0306-4522(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Li K, Futrell N, Tovar S, Wang LC, Wang DZ, Schultz LR. Gender influences the magnitude of the inflammatory response within embolic cerebral infarcts in young rats. Stroke. 1996;27:498–503. doi: 10.1161/01.str.27.3.498. [DOI] [PubMed] [Google Scholar]
- Li Z, Duckles SP. Influence of gender on vascular reactivity in the rat. J Pharmacol Exp Ther. 1994;268:1426–1431. [PubMed] [Google Scholar]
- Lieb K, Andrae J, Reisert I, Pilgrim C. Neurotoxicity of dopamine and protective effects of the NMDA receptor antagonist AP-5 differ between male and female dopaminergic neurons. Exp Neurol. 1995;134:222–229. doi: 10.1006/exnr.1995.1052. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Alkayed NJ. Sex-specific modulation of astrocyte cell death by inflammatory cytokines. In: Krieglestein J, editor. Pharmacology of cerebral ischemia 2004. Medpharm Scientific Publishers; Stuttgart, Germany: 2004a. [Google Scholar]
- Liu M, Hurn PD, Alkayed NJ. Cytochrome P450 in neurological disease. Curr Drug Metab. 2004b;5:225–234. doi: 10.2174/1389200043335540. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27:135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J Neurosci Methods. 2008;171:214–217. doi: 10.1016/j.jneumeth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br J Pharmacol. 2001;132:1035–1046. doi: 10.1038/sj.bjp.0703899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loihl AK, Asensio V, Campbell IL, Murphy S. Expression of nitric oxide synthase (NOS)-2 following permanent focal ischemia and the role of nitric oxide in infarct generation in male, female and NOS-2 gene-deficient mice. Brain Res. 1999;830:155–164. doi: 10.1016/s0006-8993(99)01388-8. [DOI] [PubMed] [Google Scholar]
- Macrae IM, Carswell HV. Oestrogen and stroke: The potential for harm as well as benefit. Biochem Soc Trans. 2006;34:1362–1365. doi: 10.1042/BST0341362. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–187. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Randall MD. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1998;123:1700–1706. doi: 10.1038/sj.bjp.0701781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, et al. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, et al. CREB-mediated bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall. Curr Opin Cardiol. 1994;9:619–626. doi: 10.1097/00001573-199409000-00018. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, et al. Estrogen and microglia: A regulatory system that affects the brain. J Neurobiol. 1999;40:484–496. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mori M, Tsukahara F, Yoshioka T, Irie K, Ohta H. Suppression by 17beta-estradiol of monocyte adhesion to vascular endothelial cells is mediated by estrogen receptors. Life Sci. 2004;75:599–609. doi: 10.1016/j.lfs.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci U S A. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic. 2005;4:95–111. doi: 10.1093/bfgp/4.2.95. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J Cereb Blood Flow Metab. 2002;22:1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Traystman RJ, Hurn PD, Duckles SP. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31:1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- Muskhelishvili L, Thompson PA, Kusewitt DF, Wang C, Kadlubar FF. In situ hybridization and immunohistochemical analysis of cytochrome P450 1B1 expression in human normal tissues. J Histochem Cytochem. 2001;49:229–236. doi: 10.1177/002215540104900210. [DOI] [PubMed] [Google Scholar]
- Naftolin F. Brain aromatization of androgens. J Reprod Med. 1994;39:257–261. [PubMed] [Google Scholar]
- Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo: Possible mechanisms for gender differences in atherosclerosis. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, et al. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GGJM, Nilsson S, Gustafsson JA, Kushner PJ, et al. Differential ligand activation of estrogen receptors ER alpha and ER beta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Payan HM, Conrad JR. Carotid ligation in gerbils. influence of age, sex, and gonads. Stroke. 1977;8:194–196. doi: 10.1161/01.str.8.2.194. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Santizo R, Baughman VL, Wang Q. Cerebral vasodilating capacity during forebrain ischemia: Effects of chronic estrogen depletion and repletion and the role of neuronal nitric oxide synthase. Neuroreport. 1998;9:3285–3291. doi: 10.1097/00001756-199810050-00026. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: Inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: Progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, et al. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sabbah M, Courilleau D, Mester J, Redeuilh G. Estrogen induction of the cyclin D1 promoter: Involvement of a cAMP response-like element. Proc Natl Acad Sci U S A. 1999;96:11217–11222. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and hispanic residents of an urban community: The northern manhattan stroke study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- Sakuma I, Liu MY, Sato A, Hayashi T, Iguchi A, Kitabatake A, et al. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: Influence of oestrogen. Br J Pharmacol. 2002;135:48–54. doi: 10.1038/sj.bjp.0704444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ML, Hossain MS, Nomoto K. Mediation of the immunomodulatory effect of beta-estradiol on inflammatory responses by inhibition of recruitment and activation of inflammatory cells and their gene expression of TNF-alpha and IFN-gamma. Int Arch Allergy Immunol. 2000;121:235–245. doi: 10.1159/000024323. [DOI] [PubMed] [Google Scholar]
- Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, et al. Stroke in estrogen receptor-alpha-deficient mice. Stroke. 2000a;31:738–43. doi: 10.1161/01.str.31.3.738. discussion 744. [DOI] [PubMed] [Google Scholar]
- Sampei K, Mandir AS, Asano Y, Wong PC, Traystman RJ, Dawson VL, et al. Stroke outcome in double-mutant antioxidant transgenic mice. Stroke. 2000b;31:2685–2691. doi: 10.1161/01.str.31.11.2685. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Anderson S, Ye S, Koenig HM, Pelligrino DA. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J Neurosci Res. 1998;54:707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, et al. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Scoville SA, Bufton SM, Liuzzi FJ. Estrogen regulates neurofilament gene expression in adult female rat dorsal root ganglion neurons. Exp Neurol. 1997;146:596–599. doi: 10.1006/exnr.1997.6565. [DOI] [PubMed] [Google Scholar]
- Segars JH, Driggers PH. Estrogen action and cytoplasmic signaling cascades. part I: Membrane-associated signaling complexes. Trends Endocrinol Metab. 2002;13:349–354. doi: 10.1016/s1043-2760(02)00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34:855–862. doi: 10.1212/wnl.34.7.855. [DOI] [PubMed] [Google Scholar]
- Shi J, Panickar KS, Yang SH, Rabbani O, Day AL, Simpkins JW. Estrogen attenuates over-expression of beta-amyloid precursor protein messager RNA in an animal model of focal ischemia. Brain Res. 1998;810:87–92. doi: 10.1016/s0006-8993(98)00888-9. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Dorsa DM. Estrogen modulates the growth-associated protein GAP-43 (neuromodulin) mRNA in the rat preoptic area and basal hypothalamus. Neuroendocrinology. 1993;57:439–447. doi: 10.1159/000126390. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sieber FE, Hurn P, Alkayed NJ, Traystman RJ. Gender-based differences in na+ -K+ adenosine triphosphatase activity occur in the microcirculation of the diabetic rat brain. Anesthesiology. 2001;94:372–375. doi: 10.1097/00000542-200102000-00037. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakamar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Liu R, Perez E, Cai ZY, Covey DF, et al. Estrogen-like compounds for ischemic neuroprotection. Stroke. 2004;35:2648–2651. doi: 10.1161/01.STR.0000143734.59507.88. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Strickland TM, Dorsa DM. Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: A possible mechanism for its anxiolytic action. Brain Res. 1987;400:353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair RW. Effects of estrogens on macrophage foam cells: A potential target for the protective effects of estrogens on atherosclerosis. Curr Opin Lipidol. 1997;8:281–286. doi: 10.1097/00041433-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: Implications for alzheimer's disease. J Neurosci. 1998a;18:3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I. Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology. 1998b;139:3202–3209. doi: 10.1210/endo.139.7.6084. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: Implications for neuroprotection. Prog Neurobiol. 2004;72:111–127. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Teixeira C, Reed JC, Pratt MA. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]
- Teter B, Harris-White ME, Frautschy SA, Cole GM. Role of apolipoprotein E and estrogen in mossy fiber sprouting in hippocampal slice cultures. Neuroscience. 1999;91:1009–1016. doi: 10.1016/s0306-4522(98)00630-7. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Mechanisms of estrogen action during neural development: Mediation by interactions with the neurotrophins and their receptors? J Steroid Biochem Mol Biol. 1996;56:169–178. doi: 10.1016/0960-0760(95)00234-0. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, et al. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G., Jr. Novel mechanisms of estrogen action in the brain: New players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke. 2000;31:2701–2706. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- Tretter YP, Hertel M, Munz B, ten Bruggencate G, Werner S, Alzheimer C. Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat Med. 2000;6:812–815. doi: 10.1038/77548. [DOI] [PubMed] [Google Scholar]
- Trudeau VL, Pope L, de Winter JP, Hache RJ, Renaud LP. Regulation of activin type-II receptor mRNA levels in rat hypothalamus by estradiol in vivo. J Neuroendocrinol. 1996;8:395–401. doi: 10.1046/j.1365-2826.1996.04618.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Vedder H, Anthes N, Stumm G, Wurz C, Behl C, Krieg JC. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J Neurochem. 1999;72:2531–2538. doi: 10.1046/j.1471-4159.1999.0722531.x. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: The impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Rabizadeh S, Tasinato A, Sperandio S, Ye X, Green M, et al. Dimerization-dependent block of the proapoptotic effect of p75(NTR) J Neurosci Res. 2000;60:587–593. doi: 10.1002/(SICI)1097-4547(20000601)60:5<587::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang QG, Han D, Xu J, Lu Q, Zhang GY. Inhibition of MLK3-MKK4/7-JNK1/2 pathway by Akt1 in exogenous estrogen-induced neuroprotection against transient global cerebral ischemia by a non-genomic mechanism in male rats. J Neurochem. 2006;99:1543–1554. doi: 10.1111/j.1471-4159.2006.04201.x. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Jr., Park-Chung M, Gibbs TT, Farb DH. 17beta-estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- Weisz J, Crowley WR. Catechol estrogen formation by the CNS: Regional distribution of estrogen-2/4-hydroxylase activity in rat brain. Neuroendocrinology. 1986;43:543–549. doi: 10.1159/000124580. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, et al. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- White MM, Zamudio S, Stevens T, Tyler R, Lindenfeld J, Leslie K, et al. Estrogen, progesterone, and vascular reactivity: Potential cellular mechanisms. Endocr Rev. 1995;16:739–751. doi: 10.1210/edrv-16-6-739. [DOI] [PubMed] [Google Scholar]
- White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther. 2000;292:375–380. [PubMed] [Google Scholar]
- Winters SJ. Androgens and antiandrogens. In: Brody TM, Lamer J, Minneman KP, editors. Human pharmacology molecular to clinical. Mosby Co.; St. Louis: 1999. pp. 519–531. [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL. Estradiol is a protective factor in the adult and aging brain: Understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Brain Res Rev. 2001;37:313–319. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Xu Y, Berelowitz M, Bruno JF. Characterization of the promoter region of the human somatostatin receptor subtype 2 gene and localization of sequences required for estrogen-responsiveness. Mol Cell Endocrinol. 1998;139:71–77. doi: 10.1016/s0303-7207(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Traystman RJ, Hurn PD, Wang MM. Neurite-localized estrogen receptor-alpha mediates rapid signaling by estrogen. J Neurosci Res. 2003;74:1–11. doi: 10.1002/jnr.10725. [DOI] [PubMed] [Google Scholar]
- Xu Y, Traystman RJ, Hurn PD, Wang MM. Membrane restraint of estrogen receptor alpha enhances estrogen-dependent nuclear localization and genomic function. Mol Endocrinol. 2004;18:86–96. doi: 10.1210/me.2003-0262. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, et al. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori Y, Horie R, Sato M, Ohta K. Proceedings: Prophylactic trials for stroke in stroke-prone SHR: Effect of sex hormones. Jpn Heart J. 1976;17:404–406. doi: 10.1536/ihj.17.404. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wen Y, Perez E, Cutright J, Brun-Zinkernagel AM, et al. Neuroendocrine mechanism for tolerance to cerebral ischemia-reperfusion injury in male rats. J Neurobiol. 2005;62:341–351. doi: 10.1002/neu.20103. [DOI] [PubMed] [Google Scholar]
- Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, et al. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li PP, Feng X, Barker JL, Smith SV, Rubinow DR. Sex-related differences in neuronal cell survival and signaling in rats. Neurosci Lett. 2003;337:65–68. doi: 10.1016/s0304-3940(02)01179-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- Zhu YS, Pfaff DW. DNA binding of hypothalamic nuclear proteins on estrogen response element and preproenkephalin promoter: Modification by estrogen. Neuroendocrinology. 1995;62:454–466. doi: 10.1159/000127035. [DOI] [PubMed] [Google Scholar]