Abstract
PURPOSE
To quantify accommodative and age-related changes in the anteroposterior position and thickness of the ciliary muscle in phakic and pseudophakic eyes.
SETTING
Department of Surgery/Bioengineering, UMDNJ–Robert Wood Johnson Medical School, Piscataway; Institute of Ophthalmology and Visual Science UMDNJ–New Jersey Medical School, Newark, New Jersey; MRI Research, Inc., Middleburg Heights, Ohio, USA.
METHODS
Magnetic resonance images were taken of phakic and pseudophakic eyes.
RESULTS
The cohort comprised 32 phakic volunteers and 8 volunteers with a monocular intraocular lens (IOL) aged 22 to 91 years. No anteroposterior accommodative movement of the ciliary muscle apex occurred in either group. The muscle moved closer to the cornea with advancing age in phakic eyes; IOL implantation returned the muscle to a youthful position. An age-dependent increase in ciliary muscle anteroposterior thickness occurred that was not mitigated by IOL implantation. Muscle thickness increased with accommodation only in phakic eyes.
CONCLUSIONS
Presbyopia-correction strategies cannot rely on accommodative anterior movement of the ciliary muscle. Forces on the uvea by crystalline lens–pupillary margin contact may increase with accommodation and lens growth, producing accommodative and age-dependent increases in muscle thickness and significant age-dependent anterior muscle displacement. Intraocular lens implantation removed these forces, allowing choroidal elasticity to restore the muscle to a youthful position; however, the increase in thickness was permanent and likely due to an age-dependent increase in connective tissue. This supports the geometric theory of presbyopia development and that the mechanical forces in human accommodation and presbyopia are very different from those in the rhesus monkey model.
In 1986, Thornton1 reported anterior optic movement in response to accommodative stimulus in patients with an intraocular lens (IOL) with specially designed haptics and the consequent restoration of accommodation in older patients. Many accommodating IOL models with haptics designed to promote anterior optic movement have since been introduced.2 Although one can envision several mechanisms that might produce anterior optic movement in response to ciliary muscle contraction, anterior accommodative ciliary muscle movement is arguably the most straightforward; however, this has never been observed in humans. Because the iris prevents its direct observation, the human ciliary muscle has generally been studied through indirect or in vitro methods or has been approximated by in vitro and in vivo animal models.3,4 Although a normally pigmented iris obscures visualization of the ciliary muscle in the intact human eye by in vivo optical imaging methods, optical coherence tomography (OCT) studies of albino patients showed no anterior accommodative movement of the ciliary muscle.5
Magnetic resonance imaging (MRI) is not impeded by the iris. This nonoptical noninvasive imaging method has unsurpassed soft tissue contrast that provides visualization of the entire anterior segment in any desired plane. It is thus uniquely suited to studying the ciliary muscle in the normal intact human eye. Although MRI is used clinically to diagnose several ocular diseases and disorders, because of its expense and the technical challenges of imaging small volumes, it remains a research tool when applied to the anterior segment. Other ocular research applications for MRI include high-resolution retinal imaging and the study of extraocular muscles.6–10 Strenk et al.11 developed high-resolution anterior segment MRI techniques and instrumentation that permit observation of the geometric relationship between the accommodative structures in the phakic eye and in the pseudophakic eye. These studies provide the only direct evidence in a group of subjects that the mechanism of human accommodation is primarily Helmholtzian; that is, in response to an accommodative stimulus, there is a decrease in the diameter of the ciliary muscle ring and of the lens equator, with a corresponding increase in lens thickness. In addition, these studies provide new information on human lens growth and accommodative mechanics12 and propose a new theory of presbyopia development; that is, the modified geometric theory.3
The original geometric theory of Koretz and Handelman13 attributes presbyopia to continually increasing zonular tension secondary to life-long increases in lens thickness, ultimately making ciliary muscle movement impossible. The modified geometric theory of Strenk et al.3 attributes presbyopia to continually decreasing zonular tension secondary to life-long increases in lens thickness, ultimately making accommodative ciliary muscle movement irrelevant. Although the original geometric theory may hold for the rhesus monkey because ciliary muscle excursion is reduced to zero with advancing age in this species,14 it became untenable in humans when MRI studies indicated that human ciliary muscle contraction is undiminished by age.4,11 Both geometric theories attribute presbyopia to a changing geometric relationship between the accommodative structures, describe the ciliary muscle as intrinsically capable, and suggest that any age-related mechanical changes in the lens material are a result, not a cause, of presbyopia. If this is the case, early interventions that return zonular tension to youthful levels might forestall presbyopia development in the phakic eye. We previously reported that strategies for the surgical correction of presbyopia can rely on ciliary muscle contraction (inward movement), which is undiminished by age in phakic eyes and pseudophakic eyes.4,11 Ultrasound biomicroscopy (UBM) studies of the ciliary body also suggest that the ciliary muscle remains active during life.15 We report our findings on the effects of age and accommodation on ciliary muscle anteroposterior position and thickness.
SUBJECTS AND METHODS
This study comprised eyes of emmetropic or mildly myopic volunteers; the ciliary muscle diameter findings in these eyes have been reported.4 A subset of the volunteers was monocularly pseudophakic. The research followed the tenets of the Declaration of Helsinki, and the Institutional Review Board, UMDNJ–Robert Wood Johnson Medical School, approved the research. After receiving an explanation of the nature and possible consequences of the study, all volunteers provided informed consent.
Surgical Technique
In the subset of monocularly pseudophakic patients, a standard single-plane clear corneal incision was made and a continuous curvilinear capsulorhexis created. Then, hydrodissection was performed to separate the cataract cortex from the underlying lens capsule. This was followed by hydrodelineation to separate the nucleus from surrounding cortex. The nucleus was split using the phaco-chop technique. Small pieces of cataract were then emulsified and removed, and the cortex was aspirated using the Infiniti phaco machine (Alcon, Inc.). A single-piece acrylic foldable IOL (AcrySof SA60AT, Alcon, Inc.) was implanted in the capsular bag.
Magnetic Resonance Imaging
Magnetic resonance imaging relies on the principle of nuclear magnetic resonance, a static magnetic field, magnetic field gradients, and the timed application of radiofrequency pulses. The technique produces images that are free of optical distortions and reflections. The image contrast is dependent on various intrinsic properties of soft tissue and can be optimized through the appropriate selection of the radiofrequency pulse-sequence timing parameters. Images can be collected in any desired anatomic plane. The high-resolution MRI method developed by Strenk et al.11 for in vivo imaging of the human anterior segment during accommodation provides complete visualization of the ciliary muscle and its geometric relationship to the iris and the crystalline lens (in the phakic eye) or IOL optic and haptics (in the pseudophakic eye). Moreover, the method does not obstruct binocular vision and uses physiologic accommodation. (Pharmacologically induced accommodation is reported to produce unnaturally high accommodation,16 probably as a result of the effect of the drug on iris contraction.17)
High-resolution magnetic resonance ocular images were acquired using a clinical magnetic resonance imager (1.5 Tesla, General Electric), a purpose-designed eye-imaging magnetic resonance receiver coil (MRI Research, Inc.), and a standard spin-echo radiofrequency pulse sequence (T1-weighted, single echo, multislice). A nonmagnetic stimulus apparatus was designed to present a visual target at the appropriate distance from the eyes to provide a strong (8.0 diopter [D]) or a minimum (0.1 D) binocular (accommodative and disparity) stimulus. The far target was viewed through a mirror to overcome the confines of the imager. The patient's head was restrained and the room darkened during scanning. The patient was first instructed to view the far visual target, which was a black cross hair on a white background illuminated by a fiber optic. The intensity varied between 50% and 100% at a rate of 2 Hz, providing a compelling visual stimulus and reducing the stabilized-image phenomenon. A previous study4 reported undiminished accommodative ciliary muscle contraction in these patients; thus, the stimulus used in the present study was sufficient to drive ciliary muscle activity.
An initial series of orthogonal localization scans was simultaneously acquired in 3 dimensions and used to plan the location of the final series of contiguous, 3.0 mm thick, multislice axial scans with a 4 cm field of view and a 256 pixel × 256 pixel matrix, resulting in a 0.156 mm pixel size with an absolute error of one half the pixel size, or 78 μm. The target was then repositioned for near viewing, after which the entire localization and scanning procedure was repeated. Additional details of the data acquisition and stimulus apparatus are reported elsewhere.11 Images were acquired during accommodation and with accommodation at rest from 1 eye of the phakic volunteers and both eyes of the patients with a monocular IOL. Figure 1, left, is a composite image of both eyes of a patient with a monocular IOL. The images were analyzed using the NIH Image software package (version 1.63, National Institutes of Health); the pixels corresponding to locations of the ciliary muscle apex, corneal apex, and iris root were obtained. Previous studies4,11 identified a pixel representing the medial ciliary muscle apex, which is hypointense to the surrounding ciliary body, and another pixel representing the lateral ciliary muscle apex; the distance between these points defines a line representing the ciliary muscle diameter. In this study, the anteroposterior distance from this line to the corneal apex was measured. An analogous method was used to identify pixels for the iris root (which is hyperintense to the adjacent ciliary muscle and sclera), and its anteroposterior distance from the cornea was calculated. Ciliary muscle thickness was calculated by subtracting the anteroposterior iris root position from the anteroposterior ciliary muscle position (Figure 1, right). The same scans were remeasured by 2 investigators, who were blind to each other's findings; the investigators identified the same pixels.
Figure 1.
Left: Composite MRI of each eye of a patient with a monocular IOL. Right: The ciliary muscle thickness is the distance between the ciliary muscle apex and the iris root.
In addition, studies were performed to determine the repeatability of the biometric measurements taken during different imaging sessions. Given the cost of imager time, it was not practical to perform a repeatability study for each subject. Nonetheless, images were acquired from 3 scanning occasions; the subject was removed from the imager after each of the 3 scanning sessions. This was done for accommodation and resting accommodation for 2 phakic subjects (ages 22 and 49 years) and 1 pseudophakic subject (age 65 years). Ciliary muscle ring diameter (apex to apex) results in the repeatability study have been reported.11 This paper reports the anteroposterior position and ciliary muscle thickness findings.
RESULTS
The mean age of the 40 volunteers (21 women, 19 men) was 46.15 years (range 22 to 91 years). The mean age of the 8 patients (5 women, 3 men) in the pseudophakic subset was 68.40 years (range 61 to 76 years). In the subset, the mean interval between surgery and MRI examination was 12.4 weeks (range 3.7 to 35.3 weeks).
Regarding the repeatability study, for the 22-year-old subject, the mean anteroposterior position was 4.610 mm ± 0.045 (SD) and the mean thickness was 0.651 ± 0.026 mm for resting accommodation; the means with accommodation were 4.609 ± 0.090 mm and 0.807 ± 0.026 mm, respectively. Although the accommodative difference in anteroposterior position was not statistically significant (P = .93), the accommodative difference in thickness was (P = .008). For the 49-year-old subject, the mean anteroposterior position was 4.321 ± 0.0002 mm and the mean thickness was 0.625 ± 0.0003 mm for resting accommodation; the means with accommodation were 4.271 ± 0.043 mm and 0.755 ± 0.011 mm, respectively. Although the accommodative difference in anteroposterior position was not statistically significant (P = .55), the accommodative difference in thickness was statistically different (P<.001). For the 65-year-old pseudophakic patient, the mean anteroposterior position was 4.209 ± 0.069 mm and the mean thickness was 0.902 ± 0.035 mm for resting accommodation; the means with accommodation were 4.197 ± 0.070 mm and 0.951 ± 0.014 mm, respectively. Neither the accommodative difference in the ciliary muscle anteroposterior position nor in thickness was statistically significant (P = .90 and P = .25, respectively).
Figure 2 shows the effects of age and accommodation on the anteroposterior position of the ciliary muscle apex with respect to the corneal apex in the phakic eye and the pseudophakic eye. The regression line for the phakic eye has a mean slope of −0.00898 mm per year ± 0.00283 (±SE) and a mean intercept of 4.705 ± 0.141 mm for the unaccommodated state and a mean slope of −0.00765 ± 0.00281 mm per year and mean intercept of 4.681 ± 0.140 mm for the accommodated state. The ciliary muscle apex in the phakic eye showed significant anterior movement with age for the accommodated state (r2 = 0.16358, P = .01) and the unaccommodated state (r2 = 0.20995, P = .003). However, neither the linear regression of the accommodative change in ciliary muscle location nor its mean value was statistically significant (not shown) (P = .55 and P = .915 respectively). Thus, although there was anterior movement of the ciliary muscle apex in the phakic eye with advancing age, there was no measurable anterior movement with accommodation.
Figure 2.
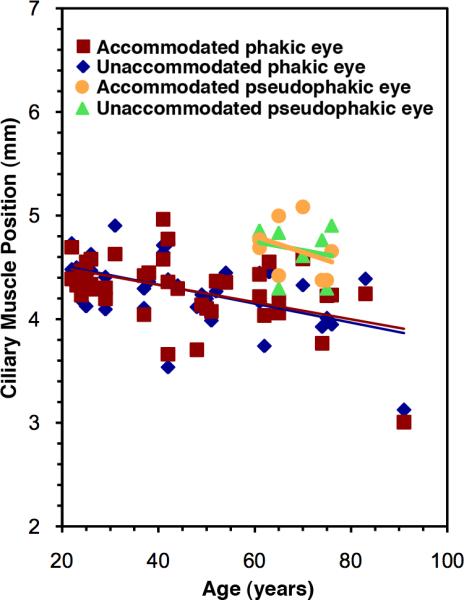
Ciliary muscle anteroposterior position versus age in the phakic eye and pseudophakic eye.
The regression line for the pseudophakic eye has a mean slope of −0.00850 ± ••• mm per year for the unaccommodated state and −0.0156 ± ••• mm per year for the accommodated state. The linear regression was not statistically significant for the accommodated state (r2 = 0.12462, P = .39) or the unaccommodated state (r2 = 0.04413, P = .62). Moreover, neither the regression line for the accommodative change with age in pseudophakic muscle anteroposterior position nor its mean value were statistically significant (not shown) (P = .703 and P = .72, respectively), indicating no accommodative anteroposterior movement of the ciliary muscle apex in the pseudophakic eye. The ciliary muscle apex location had a mean unaccommodated value of 4.675 ± 0.088 mm and a mean accommodated mean value of 4.669 ± 0.096 mm (both P<.000001). The mean value of pseudophakic ciliary muscle anteroposterior position (when normalized by dividing it by the anterior segment length of the contralateral phakic eye to minimize the effect of individual variation in globe size) corresponds to that of an approximately 29-year-old phakic eye; thus, IOL implantation appeared to return the ciliary muscle to a position of relative youth.
Figure 3 shows the ciliary muscle anteroposterior thickness in the phakic eye and pseudophakic eye as a function of age and accommodation. The regression line for the phakic eye has a mean slope of 0.0032 ± 0.0011 mm per year and a mean intercept of 0.427 ± 0.054 mm for the unaccommodated state and a mean slope of 0.0035 ± 0.0014 mm per year and a mean intercept of 0.497 ± 0.070 mm for the accommodated state. The ciliary muscle anteroposterior thickness increased with age in the phakic eye in the unaccommodated state (r2 = 0.18613, P = .005) and the accommodated state (r2 = 0.14407, P = .016). The regression line for the accommodative change in ciliary muscle anteroposterior thickness versus age (not shown) was not statistically significant (P = .83) yet had a statistically significant mean value of 0.085 ± 0.028 mm (P = .005), indicating that the accommodative change in ciliary muscle anteroposterior thickness in the phakic eye is statistically significant and essentially constant throughout life (probably indicating a maximum level of stretch in the uveal tissue at any given time, although connective tissue may build up over time). In the pseudophakic eye, the linear regression of the ciliary muscle anteroposterior thickness versus age for minimum accommodation was not statistically significant (P = .080), with a mean value of 0.768 ± 0.079 mm (P<.000001). Similarly, the linear regression of the ciliary muscle anteroposterior thickness versus age for the maximum accommodative state was not statistically significant (P = .613), with a mean value of 0.786 ± 0.060 mm (P<.000001). These means were statistically identical, indicating no change in ciliary muscle thickness occurs in the pseudophakic eye with accommodation because the iris is not in contact with the optic.
Figure 3.

Ciliary muscle anteroposterior thickness versus age in the phakic eye and pseudophakic eye.
DISCUSSION
A key finding is that in stark contrast to the in vivo rhesus monkey UBM data reported by Wasilewski et al.18 with accommodation produced by Edinger-Westphal mid-brain stimulation, we found that the human ciliary muscle apex does not move anteriorly with physiologic accommodation. However, these studies are not directly comparable. For example, it is not possible to perform an MRI study of nonpharmacologically stimulated rhesus accommodation because the electrodes used to stimulate the accommodation in rhesus monkeys are not MRI compatible. Similarly, studies of human accommodation involving electrode stimulation cannot be performed. Also, although MRI findings cannot be directly compared with histologic findings, we found that the human ciliary muscle apex position moved anteriorly with advancing age in the phakic eye; conversely, the rhesus muscle is reported to become fixed posteriorly with age.19 Other histologic studies20 show that the aging human ciliary muscle contains more connective tissue and in different regions than the rhesus model, further suggesting that species differences exist in the magnitude and the direction of forces applied to the ciliary muscle. Moreover, choroidal changes have been hypothesized to account for presbyopia in the rhesus monkey21; however, this cannot be the case in humans. We have previously reported that ciliary muscle contraction is undiminished by age or IOL implantation and therefore the restoring force supplied by the choroid is also undiminished.4,11 Thus, although both humans and rhesus monkeys have a loss of accommodation with age, the aging and behavior of the ciliary muscle, and thus the mechanics of accommodation and presbyopia, appear to be different between the species.3
The modified geometric theory states that as the crystalline lens continually thickens in the anteroposterior direction with advancing age, it places ever-increasing forces on the pupillary margin and causes anterior and inward (due to the constraint of scleral curvature) movement of the uvea with a concomitant reduction in the circumlental space and thus (anterior) zonular tension. Although the ciliary muscle still contracts, in the absence of zonular tension, it is unable to bring about a change in lens shape.3 We previously reported an age-dependent decrease in ciliary muscle diameter with advancing age,4,11 and here we report age-dependent anterior displacement in the same group of subjects. Although no measurable anteroposterior movement of the ciliary muscle apex occurs with accommodation, significant anterior displacement occurs with age, reflecting the cumulative effect of the forces applied by life-long lens growth. Moreover, ciliary muscle thickness increases significantly with age, likely due to the buildup of connective tissue or the creation of additional muscle fibers in response to the applied lenticular forces, which are opposed by the elasticity of the choroid. We found that the ciliary muscle anteroposterior thickness increased with accommodation in the phakic eye. The pupillary margin is in direct contact with the anterior surface of the crystalline lens, and accommodation and accommodative miosis will result in an anterior shift of the pupillary margin–lens contact zone and the application of increased anterior forces to the uvea. This accommodative increase in thickness is constant with age, probably indicating that the maximum possible stretch of the ciliary muscle exists at any given time, although connective tissue will build up over time.
The pseudophakic findings presented here are also consistent with the modified geometric theory. Extraction of the age-enlarged crystalline lens removes the anterior lenticular forces and thus returns the ciliary muscle apex to the anteroposterior position of a relatively youthful phakic eye. This postoperative posterior uveal shift may also explain the pressure-lowering effects observed by Poley et al.22 in hypertensive eyes after cataract surgery because crystalline lens growth displaces the uvea anteriorly, pinching off the trabecular meshwork. Removing the age-enlarged lens may restore aqueous outflow and eliminate stresses throughout the entire uveal tract. We hypothesize that the modified geometric theory may provide a mechanism for glaucoma development and/or progression in phakic eyes with inherent abnormalities in crystalline lens, zonular, and ciliary muscle biometry (in preparation) as well as in pseudophakic eyes with abundant Soemmerring ring, which also displaces the uvea anteriorly, as we observed in cadaver MRI images.
In the pseudophakic eye, no change in ciliary muscle anteroposterior thickness occurs with age or accommodation because in general, the optic is not in contact with the iris, nor does the optic thicken with age or accommodation; thus, it does not apply a force to the pupillary margin. Intraocular lens implantation does not reduce existing anteroposterior ciliary muscle thickness because removing the crystalline lens does not eliminate connective tissue in the ciliary muscle. We previously reported that the ciliary muscle ring diameter is significantly reduced with age in the phakic eye and remains so after IOL implantation, again probably due to an age-dependent increase in connective tissue throughout the ciliary muscle, which is not reversed by crystalline lens removal.4 Thus, strategies for presbyopia correction aimed at emulating the geometry of human phakic accommodation might require a dual surgical approach in cases of advanced presbyopia. One procedure would replace the age-enlarged crystalline lens with an IOL, thus restoring the ciliary muscle anteroposterior position. The other procedure would restore the circumlental space and thus zonular tension (if this is not sufficiently addressed by IOL haptic design and capsule contraction).
As in the phakic eye, we found no anteroposterior accommodative movement of the ciliary muscle apex in eyes implanted with a single-piece acrylic foldable monocular IOL. Nonetheless, anterior ciliary muscle movement is perhaps possible in a pseudophakic eye. Accommodative mechanics necessarily differ in a pseudophakic eye because the IOL has far less mass and volume than the crystalline lens and a different geometric relationship with the remaining accommodative structures; for example, the position of the zonulocapsular insertions. Any potential anterior movement of the ciliary muscle apex could also be confounded by oversized haptics that rest posterior to the ciliary body, although this position may be beneficial in increasing positional stability for nonaccommodating IOLs. Our ongoing cadaver studies indicate that the cadaver lens is larger than the in vivo lens; thus, IOL designs based on cadaver biometry may overestimate haptic size, which could impede forward accommodative movement (S.A. Strenk, MD, L.M. Strenk, MD, “In Vivo MRI…Visualizing the Haptics,” EyeWorld, September 2007, pages 49–52. Available at: http://www.eyeworld.org/article.php?sid=4004. Accessed October 29, 2009). Of course, anterior optic movement in an accommodating IOL might be accomplished without the need for anterior ciliary muscle apex movement. It is possible that inward ciliary muscle movement alone is sufficient to cause an anterior optic shift if the haptics are favorably designed and located. A recent UBM study of the monofocal IOL–capsular bag ciliary process–sulcus–zonular iris complex23 found accommodative variations in anterior chamber depth and a small (mean 0.028 ± 0.18 mm) accommodative decrease in the iris–anterior ciliary processes distance that appear to depend on haptic deformation by inward movement of the ciliary ring as well as the anteroposterior location of the haptic plane. In addition, it has been suggested that the vitreous plays a role during phakic accommodation24; however, at present, this theory cannot be validated in humans because of the invasive methods of measuring vitreous pressure. Nonetheless, it has been hypothesized that the vitreous might play a role in anterior optic movement in response to ciliary muscle contraction.1,25
In conclusion, our findings on the effect of age, accommodation, and IOL implantation on the ciliary muscle apex anteroposterior position and thickness support the modified geometric theory of presbyopia development and further suggest that very different mechanical forces are present in the aging rhesus accommodative structures. No anterior accommodative movement of the ciliary muscle apex was found in phakic eyes or in eyes with a single-piece acrylic foldable monocular IOL; however, haptic size and location relative to the ciliary body could be confounding factors. Pseudophakic accommodative mechanics necessarily differ from phakic mechanics and will vary with IOL haptic design, sizing, and location. Thus, multiple successful IOL designs (as well as other surgical strategies) for presbyopia correction can be envisioned.
*Synopsis.
An MRI study showed that the human ciliary muscle does not move anteriorly with accommodation. It thickens and moves anteriorly with age; these effects are partially reversed by cataract surgery.
Acknowledgments
Funded in part by National Eye Institute grants R43EY15655 (Dr. Strenk) and R01EY011529.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
REFERENCES
- 1.Thornton SP. Lens implantation with restored accommodation. Curr Canadian Ophthalmic Prac. 1986;4:60, 62, 82. [Google Scholar]
- 2.Findl O, Leydolt C. Meta-analysis of accommodating intraocular lenses. J Cataract Refract Surg. 2007;33:522–527. doi: 10.1016/j.jcrs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Strenk SA, Strenk LM, Koretz JF. The mechanism of presbyopia. Prog Retin Eye Res. 2005;24:379–393. doi: 10.1016/j.preteyeres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006;32:1792–1798. doi: 10.1016/j.jcrs.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baikoff G, Lutun E, Wei J, Ferraz C. Anterior chamber optical coherence tomography study of human natural accommodation in a 19-year-old albino. J Cataract Refract Surg. 2004;30:696–701. doi: 10.1016/j.jcrs.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. [October 29, 2009];Invest Ophthalmol Vis Sci. 1995 36:1125–1136. Available at: http://www.iovs.org/cgi/reprint/36/6/1125. [PubMed]
- 7.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872–878. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 8.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. [October 29, 2009];Invest Ophthalmol Vis Sci. 2002 43:2179–2188. Available at: http://www.iovs.org/cgi/reprint/43/7/2179. [PubMed]
- 9.Berkowitz BA, McDonald C, Ito Y, Tofts PS, Latif Z, Gross J. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: eliminating blinking artifacts and demonstrating proof of concept. Magn Reson Med. 2001;46:412–416. doi: 10.1002/mrm.1206. [DOI] [PubMed] [Google Scholar]
- 10.Trick GL, Berkowitz BA. Retinal oxygenation response and retinopathy. Prog Retin Eye Res. 2005;24:259–274. doi: 10.1016/j.preteyeres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. [October 29, 2009];Invest Ophthalmol Vis Sci. 1999 40:1162–1169. Available at: http://www.iovs.org/cgi/reprint/40/6/1162. [PubMed]
- 12.Strenk SA, Strenk LM, Semmlow JL, DeMarco JK. Magnetic resonance imaging study of the effects of age and accommodation on the human lens cross-sectional area. [October 29, 2009];Invest Ophthalmol Vis Sci. 2004 45:539–545. doi: 10.1167/iovs.03-0092. Available at: http://www.iovs.org/cgi/reprint/45/2/539. [DOI] [PubMed]
- 13.Koretz JF, Handelman GH. How the human eye focuses. [October 29, 2009];Sci Am. 1988 259:92–99. doi: 10.1038/scientificamerican0788-92. Available at: http://flash.lakeheadu.ca/~mwesner/S+Preadings/Accommodation/Scientific%20American%201-4.pdf. [DOI] [PubMed]
- 14.Lütjen-Drecoll E, Tamm E, Kaufman PL. Age changes in rhesus monkey ciliary muscle: light and electron microscopy. Exp Eye Res. 1988;47:885–899. doi: 10.1016/0014-4835(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 15.Stachs O, Martin H, Kirchhoff A, Stave J, Terwee T, Guthoff R. Monitoring accommodative ciliary muscle function using three-dimensional ultrasound. Graefes Arch Clin Exp Ophthalmol. 2002;240:906–912. doi: 10.1007/s00417-002-0551-2. [DOI] [PubMed] [Google Scholar]
- 16.Kriechbaum K, Findl O, Koeppl C, Menapace R, Drexler W. Stimulus-driven versus pilocarpine-induced biometric changes in pseudophakic eyes. Ophthalmology. 2005;112:453–459. doi: 10.1016/j.ophtha.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. [October 29, 2009];Invest Ophthalmol Vis Sci. 1990 31:2185–2190. Available at: http://www.iovs.org/cgi/reprint/31/10/2185.pdf. [PubMed]
- 18.Wasilewski R, McDonald JP, Heatley G, Lütjen-Drecoll E, Kaufman PL, Croft MA. Surgical intervention and accommodative responses, II: forward ciliary body accommodative movement is facilitated by zonular attachments to the lens capsule. Invest Ophthalmol Vis Sci. 2008;49:5495–5502. doi: 10.1167/iovs.08-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle. A quantitative morphometric study. Mech Ageing Dev. 1992;62:209–221. doi: 10.1016/0047-6374(92)90057-k. [DOI] [PubMed] [Google Scholar]
- 20.Pardue MT, Sivak JG. Age-related changes in human ciliary muscle. Optom Vis Sci. 2000;77:204–210. doi: 10.1097/00006324-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Tamm E, Croft MA, Jungkunz W, Lütjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey; role of the choroid. Arch Ophthalmol. 1992;110:871–876. doi: 10.1001/archopht.1992.01080180143043. [DOI] [PubMed] [Google Scholar]
- 22.Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34:735–742. doi: 10.1016/j.jcrs.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Marchini G, Pedrotti E, Modesti M, Visentin S, Tosi R. Anterior segment changes during accommodation in eyes with a monofocal intraocular lens: high-frequency ultrasound study. J Cataract Refract Surg. 2008;34:949–956. doi: 10.1016/j.jcrs.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Coleman DJ. Unified model for accommodative mechanism. Am J Ophthalmol. 1970;69:1063–1079. doi: 10.1016/0002-9394(70)91057-3. [DOI] [PubMed] [Google Scholar]
- 25.Cumming JS, Slade SG, Chayet A. Clinical evaluation of the model AT-45 silicone accommodating intraocular lens; results of feasibility and the initial phase of a Food and Drug Administration clinical trial; the AT-45 Study Group. Ophthalmology. 2001;108:2005–2009. doi: 10.1016/s0161-6420(01)00779-5. discussion by TP Werbin, 2010. [DOI] [PubMed] [Google Scholar]



