Abstract
We studied the effects of practice of an unusual two-hand finger force production task on electromyographic and force responses to transcranial magnetic stimulation (TMS). Prior to practice, force production by a hand resulted in decreased TMS-induced responses in the other hand. After practice, fingers that were explicitly required to produce force during practice showed a significant drop in these inhibitory effects, while other fingers did not. We conclude that interhemispheric inhibitory projections can show plastic changes that favor the execution of a practiced task.
Keywords: Hand, Force production, Coordination, Transcranial magnetic stimulation, Human
Transcranial magnetic stimulation (TMS) applied over the primary motor cortex area induces a sequence of motor effects in contralateral muscles typically involving a short-latency excitation followed by a silent period (reviewed in [13]). A number of studies have demonstrated changes in the short-latency response with practice [3,10,11]. In particular, a single practice session of a multi-finger force production task has been shown to lead to changes in the finger force responses induced by TMS applied unexpectedly during the task [6]. These changes were task-specific and involved a decrease in the TMS-induced perturbation of the total force and of the total pronation–supination moment.
Voluntary activation of a contralateral homologous muscle group changes motor responses to a standard TMS stimulus, probably via interhemispheric inihibitory pathways [9,15]. Reports on these changes, however, have been controversial including facilitation, inhibition, or both depending on the level of voluntary muscle activation and on the type of muscle contraction [7,9,15–17]. A recent study of musicians has suggested that their professional activity is associated with a drop in inhibitory interhemispheric effects [12].
There have been no studies on possible effects of practice on interhemispheric effects of voluntary muscle activation. Based on results of the previous study [6], we hypothesized that effects of voluntary muscle activation on TMS-induced responses in homologous contralateral muscles would show plastic changes with practice. Moreover, we expected these changes to be task-specific, i.e., show different effects on responses of effectors that are involved in the task explicitly versus those that are not.
A detailed description of the protocol and methods can be found in Kang et al. [5]. Twelve healthy subjects, six males and six females, took part in the experiment. Three male and three female subjects were right-handed, and the rest were left-handed, according to their preferential hand use during writing and eating. The average age of the subjects was 23.4 ± 1.1 years, the average weight was 62.4 ± 2 kg, and their average height was 167.3 ± 2.2 cm. The experiments were conducted in accordance with the Declaration of Helsinki; the subjects gave written consent according to protocols approved by the Office for Regulatory Compliance of the Penn State University.
During testing, the subject sat at the table in a comfortable position, with the upper arm at approximately 45° of abduction in the frontal plane and 45° of flexion in the sagittal plane, and the elbow at approximately 45° of flexion. The forearms rested on the board containing the force sensors, and were strapped to the board with sets of Velcro straps. The midline of the wooden board was aligned with the midline of the participant’s body, and the positions of the hands were symmetrical with respect to the body mid-line. Eight unidirectional piezoelectric force sensors (Model 208AO3; Piezotronic Inc.), with the diameter of 1.5 cm, were used to measure forces produced by each individual finger of both hands. Each sensor, covered with cotton pad, was mounted on an aluminum post. Four sensors were placed within an aluminum frame (140 mm × 90 mm each) in a groove on a wooden board. The sensors were mediolaterally distributed 30 mm apart within each frame. The position of the frames and sensors could be adjusted for differences in arm length, hand size and finger length. A custom-fitted support object was placed underneath each subject’s palm to assure a stable position of the hand with respect to the sensors.
The surface electromyogram (EMG) of the extrinsic finger flexor and extensor muscles was recorded with bipolar electrodes (10 mm diameter, silver–silver chloride, 20 mm apart center-to-center) that were placed over the muscle bellies. For the flexor muscles, electrodes were centered around the 50% point on the line joining the medial epicondyle to the styloid process of the ulna. These electrodes measured the summed activity of the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). For the extensor muscles, electrodes were centered around the 25% point on a line drawn from the lateral epicondyle to the styloid process of the ulna. These electrodes measured the activity of the extensor digitorum communis (EDC). The surface EMG signals were amplified (×3000) and band-pass filtered (10–500 Hz).
Analog output signals from the force sensors were connected to separate AC/DC signal conditioners (Model 484B06; Piezotronics Inc.), and fed into a Gateway 450 MHz microcomputer. The force and EMG data were collected at 1000 Hz with a 12-bit resolution using National Instruments A/D board and LabView-based data acquisition software.
The study involved a pre-test (day 1), two days of practice (days 2 and 3), and a post-test (day 4). In this paper, we present only the results of the TMS tests prior to practice and after practice. Practice involved performing a task of ramp force production that was purposefully made very unusual (25 trials on day 1, 50 trials on days 2 and 3, and 25 trials on day 4). During the ramp task, the screen located approximately 70 cm in front of the subject showed a thick red line. It was horizontal, corresponding to zero force for the first 5 s. Then, it became oblique going up over 3 s to 15% of the subject’s maximal voluntary contraction (MVC) force produced by all eight fingers. Then, the line stayed horizontal for 2 s. The signal shown on the screen corresponded to the sum of the four finger forces, two from each hand, from which the sum of the forces produced by the remaining four fingers was subtracted. We are going to address this variable as the task force (FTASK). Half of the subjects performed a task with FTASK = (FI,Lt + FR,Lt + FM,Rt + FL,Rt) − (FI,Rt + FR,Rt + FM,Lt + FL,Lt); the other half performed a task with FTASK = (FI,Rt + FR,Rt + FM,Lt + FL,Lt) − (FI,Lt + FR,Lt + FM,Rt + FL,Rt); I: index, M: middle, R: ring, and L: little fingers; Rt: right hand and Lt: left hand. After each trial, a numerical score was given to the subject reflecting the total deviation of the FTASK curve from the template. The score ranged from zero (worst) to 100 (perfect).
TMS tests were applied prior to the beginning of the practice on day 1 and at the end of day 4. During these tests, the TMS was applied over the contralateral primary motor area to produce finger flexion responses in the dominant hand. During each trial, the subject was required to sit relaxed and then to produce a constant level on the total force by pressing naturally with all four fingers of each hand. The dominant hand was always required to produce 5% of its MVC force. The non-dominant hand was required to produce different forces ranging from 5 to 10% of its MVC; the total of five force levels was used, each level was repeated twice. The monitor showed the subject the required levels of force for both hands and the actual forces using lines of different color. After the subject produced the required force levels, a single TMS stimulus was applied unexpectedly within a 5 s time window. After a TMS stimulus, the subject was required to return to the pre-stimulation force levels as quickly as possible and to keep them until the end of the 12 s trial.
Focal monophasic magnetic stimuli were delivered using a Magstim 200 stimulator (Magstim Corp., UK; peak magnetic field strength of 2.2 T). A tight elastic cap was placed on the subject’s head. A 4 cm ×4 cm grid was marked on the stimulation side, with its center 2 cm lateral to Cz. To determine an optimal position of the figure-of-eight coil, we proceeded as follows. The coil was A-side up, with its center placed over the central point of the grid. Stimuli at 60% of the stimulator output were applied at the nodes of the grid, while the subject was pressing with all four fingers at about 10% of MVC. The optimal location was assessed by looking for the largest increment in the total force. Keeping the coil at the optimal location, the intensity of the stimulation was slowly decreased until the stimulation induced visible muscle twitches or noticeable changes in the background EMG at the expected latency in less than 50% of trials. The output of the stimulator for this particular condition was taken as the threshold value. Then, throughout the experiment, the intensity of the stimulation was set at 130% of the threshold. The position of the coil and the intensity of the stimulus remained constant throughout the experiment. The coil position and orientation was ensured with double-sided adhesive tape; besides, at all times, the coil was handled by an experimenter.
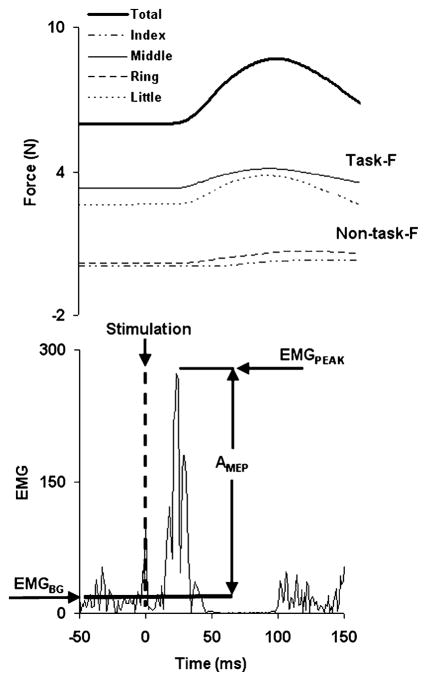
A typical response to TMS is illustrated in Fig. 1, with the total force in the top panel and the flexor EMG signal in the lower panel. A stimulus induced an increase (ΔF) in the background force (FBG) followed by a force drop slightly below FBG. For each finger i (where i = I, M, R, or L), FBG,i was defined as the mean force calculated over a period of 50 ms prior to the moment of TMS application. Individual finger forces (FPEAK,i) were measured at the time (tPEAK) of the total peak force. The difference between the two, ΔFi = FPEAK,i − FBG,i, was used to quantify the effect of the stimulation on individual finger forces. A similar procedure was used for the change in the total force (ΔFTOT = FPEAK − FBG).
Fig. 1.
A typical response to a TMS stimulus. The upper panel shows the force response, while the lower panel shows the EMG response of the extrinsic hand flexors.
EMG signals were rectified and low-pass filtered at 50 Hz using a second-order, zero lag Butterworth filter. Since reproducible responses were primarily seen in FDS EMG signals, we analyzed only this muscle’s responses. The background EMG (EMGBG) was defined as the mean EMG calculated over a 50 ms window prior to the moment of TMS application. The EMG peak (EMGPEAK) value was defined within a +10 to +70 ms window following a TMS stimulus. The size of the motor evoked potential (MEP) was defined as AMEP = EMGPEAK − EMGBG.
Data are presented in the text as means and S.D., while Figures show means with standard error bars. Linear regression analyses were run. Nonparametric Wilcoxson’s signed-rank tests were used to analyze practice related changes in the TMS responses.
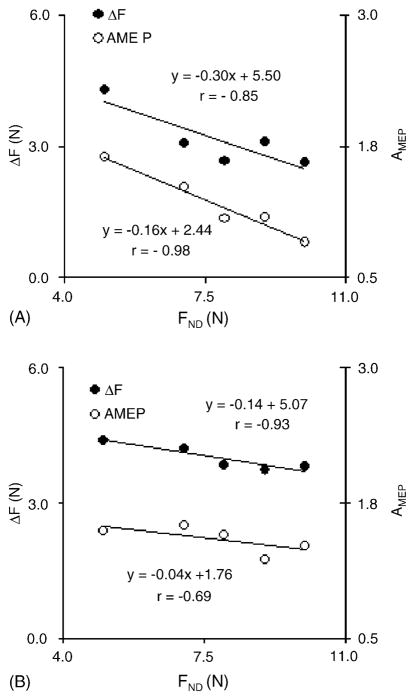
The background forces produced by the task and non-task fingers did not change after the practice; also, there was no interaction between the background forces produced by the dominant hand and the non-dominant hand target force. This was confirmed with a three-way repeated-measures ANOVA with the factors Test (two levels, pre- and post-practice), Finger (two levels: task and non-task), and Force-Level (five force levels produced by the non-dominant hand). The ANOVA failed to show significant main effect of Test and significant Finger × Test and Finger × Force-Level interactions. During voluntary force production by the non-dominant hand, the TMS-induced response in the dominant hand showed a general trend to decline. Prior to practice, 10 subjects showed significant negative linear relations between the magnitude of the force produced by the non-dominant hand (FND) and the magnitude of the response in the dominant hand (ΔF and AMEP). Fig. 2A illustrates the relations between the TMS-induced force and EMG responses and FND for a representative subject prior to practice; note that the dominant hand was always producing the same force level, 5% of its MVC force. Note the scaling of the responses with the magnitude of FND. Fig. 2B shows similar relations after practice; note the less steep regression lines.
Fig. 2.
The dependences between the force and EMG TMS-induced responses in the dominant hand and the level of force produced by the non-dominant hand. The dominant hand always produced the same background force. Note that both the force and EMG responses decreased with an increase in the non-dominant hand force. The slopes of the regression lines were larger prior to practice (A) as compared to the post-test (B). The data are for a representative subject.
To assess changes in the relations between FND and TMS-induced responses with practice, we computed the linear regression equations: ΔF = kF × FND + a; and AMEP = kMEP × FND + b, where a, b, kF, and kMEP are constant. The kF and kMEP coefficients were computed for each subject for the pre-test and post-test separately. Practice involved the production of a ramp profile of a signal (task force) that required force production by two fingers per hand (task fingers), while forces of the other fingers (non-task fingers) were subtracted. To test for possible differences in the effects of practice, linear regressions were run on responses of the task and non-task fingers separately: ΔFT = kF,T × FND + aT, and ΔFNT = kF,NT × FND + aNT, where subscripts T and NT refer to task and non-task fingers, respectively.
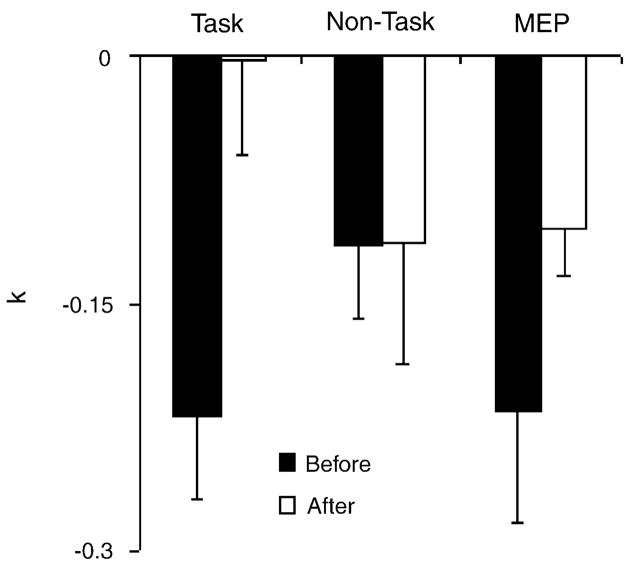
Fig. 3 shows the average values of the slope coefficients (kF,T, kF,NT, and kMEP) from the linear regression equations prior to and after practice. On average, all coefficients were negative. Prior to practice, kF,T absolute magnitude tended to be larger than kF,NT (non-significant). Practice induced a significant decrease in the magnitude of both kF,T and kMEP (p < 0.05, Wilcoxson’s signed-rank test) and no significant changes in kF,NT or kF. No difference associated with the hand preference was found in kF,T, kF,NT, and kMEP. Practice did not lead to significant changes in the intercepts of the regression lines. The average magnitude of the total force responses to TMS increased by about 10% after practice (non-significant), while there were no changes in the MEPs.
Fig. 3.
Practice-related changes in the slope coefficients of the linear regression equations between the TMS-induced responses of the dominant hand and force produced by the non-dominant hand. Force responses in the task fingers (kF,T) and in the non-task fingers (kF,NT) were analyzed separately. The EMG responses are reflected by the kMEP index. Note a significant drop with practice of kMEP and kF,T, but not of kF,NT. Averaged across subjects data are shown with standard error bars.
Changes in TMS-induced responses with practice have been documented in many studies (reviewed in [2,10]). Most of these studies, however, describe rather general changes in the responses such as their facilitation or inhibition. In a few studies, changes in TMS-induced responses were task-specific. Liepert et al. [7,8] have concluded that plastic changes of inhibitory and facilitatory loops within the hand representation vary according to the selective requirements of the motor program. Classen et al. [3] observed that practice of a voluntary thumb motion led to a change in the direction of a thumb kinematic response to TMS closer to that of the practiced movement. Latash et al. [6] saw a drop in the total force and total pronation–supination moment response of fingers to TMS with practice of a task that combined accurate force production and keeping the pronation–supination moment within a narrow range. Our current study extends these observations and shows that effects of voluntary muscle activation on TMS-induced responses of contralateral homologous muscles also show task-specific plastic changes with practice. A recent study by Butefisch et al. [1] has demonstrated that the direction of movement induced by TMS can change with practice. These authors have shown that practicing a thumb movement in a particular direction lead to a change in the direction of a thumb movement induced by a stimulus applied over the contralateral motor cortex; after practice, the directions of the two movements nearly coincided. Along similar lines, our results show that practice leads to differential changes in the excitability of motoneuronal pools serving different hand muscles.
The main task practiced by the subjects was novel and unusual. It involved a non-trivial coordination of control signals sent to the two hands as well as of signals sent to fingers within each hand. The subjects were able to learn the task and showed a dramatic improvement in indices of performance [5]. Analysis of covariation of signals to individual digits has shown the emergence of within-a-hand synergies that stabilized each hand’s contribution to the task force. Between-hand synergies were present at the pre-test and persisted over practice [4]. Note that the contributions of the two hands to the task force were summed up. This favored parallel force production by the task fingers of the two hands while avoiding force production by the non-task fingers. Our finding of a decrease in the inhibitory effects of voluntary force production by the non-dominant hand on TMS-induced responses of the dominant task fingers but not of the non-task fingers suggests selective alleviation of the inhibition in a task-specific manner.
Most of the earlier studies have concluded that changes in the TMS-induced responses in a muscle group with activation of contralateral homologous muscles were due to in-terhemispheric transcallosal pathways [12,14,15]. Based on these reports, we would like to conclude that interhemispheric inhibitory projections can show plastic changes that favor the execution of a practiced task. This conclusion suggests a cooperative action of the two hemispheres in the bimanual task (in contrast to a conclusion reached by Foltys et al. [4]).
Acknowledgments
We are grateful to Dr. Minoru Shinohara for his help in running the experiments. The study was in part supported by NIH Grants NS-035032 and AG-018751.
References
- 1.Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 2.Carroll TJ, Riek S, Carson RG. Corticospinal responses to motor training revealed by transcranial magnetic stimulation. Exerc Spotr Sci Rev. 2001;29:54–59. doi: 10.1097/00003677-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 4.Foltys H, Sparing R, Boroojerdi B, Krings T, Meister IG, Mottaghy FM, Topper R. Motor control in simple bimanual movements: a transcranial magnetic stimulation and reaction time study. Clin Neurophysiol. 2001;112:265–274. doi: 10.1016/s1388-2457(00)00539-3. [DOI] [PubMed] [Google Scholar]
- 5.Kang N, Shinohara M, Zatsiorsky VM, Latash ML. Learning multi-finger synergies: An uncontrolled manifold analysis. Exp Brain Res. 2004;157:336–350. doi: 10.1007/s00221-004-1850-0. [DOI] [PubMed] [Google Scholar]
- 6.Latash ML, Yarrow K, Rothwell JC. Changes in finger coordination and responses to single pulse TMS of motor cortex during practice of a multi-finger force production task. Exp Brain Res. 2003;151:60–71. doi: 10.1007/s00221-003-1480-y. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- 8.Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- 9.Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Leone A. The brain that plays music and is changed by it. Ann NY Acad Sci. 2001;930:315–329. doi: 10.1111/j.1749-6632.2001.tb05741.x. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Leone A, Dang N, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- 12.Ridding MC, Brouwer B, Nordstrom MA. Reduced interhemispheric inhibition in musicians. Exp Brain Res. 2000;133:249–253. doi: 10.1007/s002210000428. [DOI] [PubMed] [Google Scholar]
- 13.Sohn YH, Hallett M. Motor evoked potentials. Phys Med Rehabil Clin N Am. 2004;15:117–131. doi: 10.1016/s1047-9651(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 14.Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- 15.Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve. 1998;21:1033–1039. doi: 10.1002/(sici)1097-4598(199808)21:8<1033::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Stinear CM, Walker KS, Byblow WD. Symmetric facilitation between motor cortices during contraction of ipsilateral hand muscles. Exp Brain Res. 2001;139:101–105. doi: 10.1007/s002210100758. [DOI] [PubMed] [Google Scholar]
- 17.Weiss AC, Weiller C, Liepert J. Pre-movement motor excitability is reduced ipsilateral to low force pinch grips. J Neural Transm. 2003;110:201–208. doi: 10.1007/s00702-002-0780-x. [DOI] [PubMed] [Google Scholar]