Abstract
Resistance to antiviral therapy is the limiting factor in the successful management of HIV. In general, the K65R mutation is rarely selected (1.7–4%) with tenofovir disoproxil fumarate (TDF), abacavir (ABC), didanosine (ddI), and stavudine (d4T), as compared with the high incidence (>40%) of thymidine analog mutations associated with zidovudine and d4T. The high barrier to the development of K65R may reflect a combination of factors, including the high potency of K65R-selecting drugs, including recommended TDF/emtricitabine and ABC/lamivudine (ABC/3TC) combinations; the partial (low–intermediate level) profile of cross-resistance conferred by K65R to TDF, ABC and 3TC; the favorable viral fitness constraint imposed by K65R and the 3TC/emtricitabine-associated M184V mutations; the bidirectional antagonism between the K65R and thymidine analog mutation pathways; and unique RNA structural considerations in the region surrounding codon 65. Nevertheless, surprisingly high levels of treatment failures and K65R resistance may be associated with triple nucleoside analog regimens. The use of TDF + ABC, TDF + ddI and ABC + d4T in combination with 3TC or emtricitabine should be avoided. This selection of K65R may be reduced by the inclusion of zidovudine in two–four nucleoside reverse-transcriptase regimens. Clinical studies have demonstrated an increased frequency of K65R in association with suboptimal d4T and ddI regimens, as well as nevirapine and its resistance mutations Y181C and G190A. The potential for the development of the K65R mutation in subtype C is particularly problematic wherein a signature KKK nucleotide motif, at codons 64, 65 and 66 in reverse transcriptase, appear to lead to template pausing, facilitating the selection of K65R. Optimizing regimens may attenuate the emergence of K65R, leading to better long-term treatment management in different geographic settings. TDF-based regimens are the leading candidates for first- and second-line therapy, microbicides and chemoprophylaxis strategies.
Keywords: HIV-1 drug resistance, K65R, nucleoside analogs, subtype C, tenofovir
Combination antiretroviral therapy (ART) has led to marked decreases in mortality and morbidity in both western and developing world settings. The failure to suppress viral replication during therapy leads to the selection and expansion of drug-resistant viruses. Control of the emergence of drug resistance has become an integral part of the successful management of HIV infection.
Nucleoside reverse-transcriptase inhibitors (NRTIs) remain the most commonly utilized components of HIV antiretroviral combinations. Most dual–dual NRTI combinations consist of a primary NRTI with lamivudine (3TC) or emtricitabine (FTC) [1,101]. The M184V mutation, conferring high-level resistance to 3TC and FTC, develops rapidly in approximately 50% of treated persons but remains a clinical benefit [2–5]. This mutation impairs the enzymatic function of HIV reverse transcriptase (RT), reduces viral replicative capacity, delays the appearance of thymidine analog mutations (TAMs), and improves drug susceptibility to zidovudine (AZT), stavudine (d4T) and tenofovir [2,3]. The M184V mutation can confer 0.7–1.0 log-fold diminution in viral load in the presence of other NRTIs [2,3].
The earliest antiviral drugs used in clinical practice were the thymidine analogs, AZT and d4T used in combination with 3TC [4]. Two distinct TAM (TAM-1 and -2) pathways lead to the stepwise accumulation of major (M41L, K70R and T215Y/F), minor/secondary (D67N and L210W) and compensatory (E44D, V118I and H208Y) mutations that confer a 5–500-fold reduced susceptibility to AZT and broad cross-resistance between NRTIs (Figure 1) [4–6]. In addition, AZT and d4T may also select for the more rarely observed Q151M nucleoside analog mutational (NAM) pathway (Q151M, A62V, V75I, F77L and F116Y), which confers broad-spectra NRTI resistance [6].
Figure 1. Interactive pathways of resistance to the nucleoside reverse-transcriptase inhibitors, including TDF, ddI, ABC, d4T, AZT, 3TC and FTC.
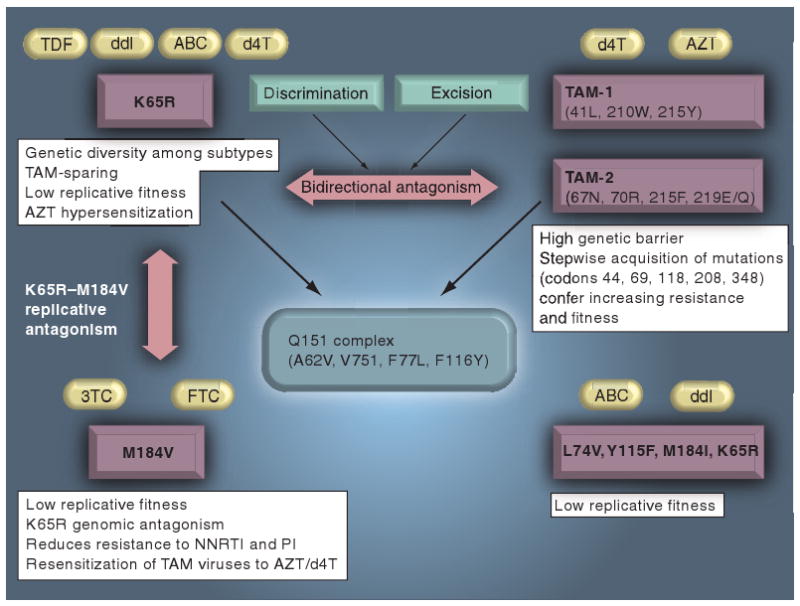
3TC: Lamivudine; ABC: Abacavir; AZT: Zidovudine; d4T: Stavudine; ddI: Didanosine; FTC: Emtricitabine; NNRTI: Non-nucleoside reverse-transcriptase inhibitor; PI: Protease inhibitor; TAM: Thymidine analog mutation; TDF: Tenofovir.
While AZT and d4T were of considerable importance in early stages of the epidemic, their use has declined in western-world settings owing to drug toxicities, clinical complications and the introduction of newer TAM-sparing NRTIs [1,7,101]. AZT is associated with anemia and lipoatrophy, while d4T regimens show significant toxicity, including mitochondrial-associated complications, peripheral neuropathy, lipoatrophy, triglyceride elevations and pancreatitis [8,101]. In resource-poor settings, lower doses may reduce toxicity without adversely affecting antiviral activity or increasing the emergence of resistant viruses. The optimum doses of AZT and d4T are being assessed in South Africa and other parts of the world, in well-controlled clinical trials [4,8].
Tenofovir disoproxil fumarate (TDF) was first introduced into clinical practice in 2001, with proven antiviral activity against HIV and HBV [7]. This second-generation NRTI showed improved antiviral activity against infections harboring TAMs (D67N, K70R and T215Y) and NAMs (Q151M) [7,9,10]. Unlike other NRTIs, TDF requires one, rather than two, phosphorylation steps, resulting in high intracellular concentrations, allowing for antiviral activity in resting and activated CD4+ cells [7,9]. TDF regimens combined with 3TC or FTC are the dual NRTI combination of choice for the management of HIV-1 infection [101]. This is based on its once-daily formulation, its prolonged intracellular half-life and more favorable lipid profiles than AZT/d4T [7,9].
Resistance to TDF is rarely selected through the point mutation K65R (AAA→AGA) in reverse transcriptase (RT) [7,9–12]. While the K65R mutation is often considered the TDF resistance mutation, it also arises with other nucleoside analogs, including abacavir (ABC), didanosine (ddI), d4T and amdoxovir [7,11–13]. The K65R resistance pathway has a favorable resistance profile conferring low- to intermediate-level phenotypic resistance to most NRTIs while hypersensitizing viruses to AZT [3–7]. The K65R pathway may be associated with the NAM pathway, which is associated with the Q151M, A62V, V75I, F77L and F116Y mutations [7].
Clinical studies and large genotypic databases show a low incidence of K65R resistance in drug-naive and treatment-experienced patients [7,9,14,102]. The K65R mutation is rarely selected with an overall incidence in 2–5% in genotyped patients, despite the increasing use of TDF and ABC since 2001 [14,102]. This compares to the frequency of TAMs in 40–60% in genotyped patients failing earlier AZT- and d4T-based regimens.
The profiles for resistance to different NRTIs and interactions amongst resistance pathways are summarized in Figure 1. TDF/FTC (Truvada™; Gilead, CA, USA) and TDF/FTC/efavirenz (EFV) (Atripla™; Bristol-Myers Squibb, NY, USA and Gilead) are the NRTIs of choice for first-line therapy according to the latest Department of Health and Human Services (DHHS) guidelines [101]. This is based on viral regimen potency, intracellular half-life, single-drug dosing, the low risk of K65R development and a more favorable phenotypic resistance profile. Cumulative findings from a number of clinical trials demonstrate a benefit of TDF-based regimens in treatment-naive patients, in switch studies replacing AZT and d4T regimens and in treatment failures harboring NRTI-resistant infections [7,9]. Intensified virological response can be observed with K65R in the presence of the M184V mutation [7]. Drug-related toxicities include decreased bone mineral density (osteopenia/osteoporosis) and potential renal toxicity [101]. Data on TDF safety during pregnancy and for children are yet to be established [101].
Abacavir/3TC (Epzicom™; GlaxoSmithKline, NC, USA) with lopinavir has been used extensively as an alternate NRTI regimen with a favorable resistance profile that includes K65R. ABC requires HLA-B*5701 testing to avoid a rash and is not recommended for individuals with pre-existing cardiac risk factors [101]. AZT/3TC remains the preferred treatment for pregnant women and newborns [101]. While ddI + 3TC/FTC remains the recommended treatment option, ddI is associated with an increased risk of pancreatitis and peripheral neuropathy [101].
Barrier to K65R selection is dependent on NRTI & NNRTI regimens
The low incidence of the K65R mutation in drug-naive and treatment-experienced patients cannot be directly attributed to its' high genetic barrier. Indeed, the barrier to K65R selection is very low, requiring a single transition of AAG→AGG in subtype C and, AAA→AGA in subtype B and other non-C subtypes [15]. Moreover, such transitions (replacements of a purine by another purine: A–G) are, for steric reasons, 2.5-times more frequent than transversions (the replacement of a purine by a pyrimidine) [15].
The low incidence and high genetic barrier to K65R selection in drug-naive and treatment-experienced patients may be attributed to multiple factors including: impaired K65R viral replicative capacity, viral fitness constraints imposed by K65R in the presence of M184V – conferring resistance to 3TC and FTC, counter-selection of K65R- and TAM-resistance pathways, regimen potency and viral subtype (Figures 1 & 2).
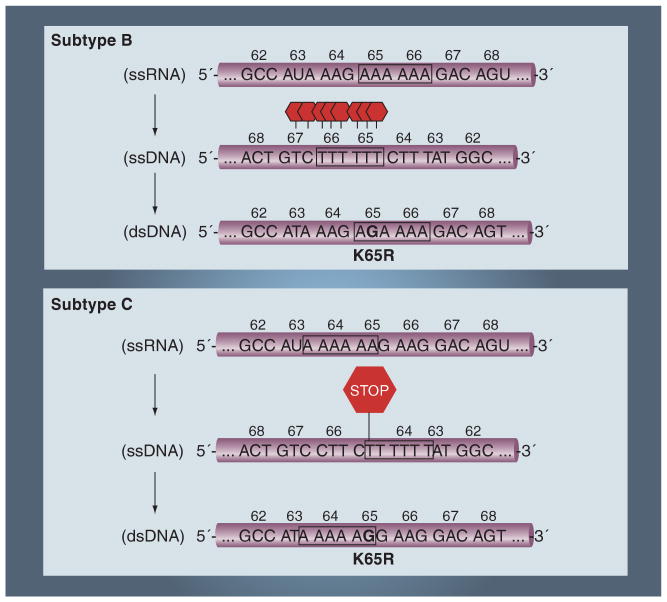
Figure 2. Facilitated selection of K65R in subtype C.
The lower genetic barrier in subtype C may be attributed to enzymatic pausing arising at the end of poly-adenine stretches. A strong pausing is observed at codon 65 in subtype C, which may favor the evolution of K65R. By contrast, the homopolymeric region in subtype B (and other subtypes) begins later and stretches beyond codon 65, to include codon 66, and end at codon 67. Selection of K65R in subtype C is facilitated by a poly-A stretch between codons 63 and 65.
The K65R mutation is associated with reduced viral replication capacity and fitness, similar to the 3TC/FTC-associated M184V mutation – hallmarks that can be demonstrated at the enzymatic level [16–19]. Viruses harboring the K65R mutation show:
Decreased incorporation rate (kpol) of dNTPs
Decreased excision of NRTIs
Increased fidelity
Decreased viral replication capacity
The severe compromise in replicative fitness imposed by K65R contributes to the bidirectional phenotypic antagonism between K65R and TAM pathways [16,17]. K65R reduces enzymatic excision of chain-terminating thymidine analogs. TAMs counterbalance K65R by decreasing enzymatic discrimination of d-nucleotide analogs and by increasing rates of nucleoside excision. Single-genome sequence analysis demonstrates a negative association of K65R with TAMs (T215Y/F + ≥2 TAMs) on the same genome, except when facilitated with Q151M complex mutations [17]. In addition, there is a strong negative association of K65R with the M184V, L74V and K70E mutations, conferring resistance to 3TC/FTC, ddI and TDF, respectively. These four mutations severely compromise viral fitness, wherein double mutants impose a marked diminution in replicative capacity and may be mutually deleterious [2,3,18–21]. While M184V and K65R may prevail in patients, these mutations markedly compromise viral replicative capacity [2,3,18].
The accumulation of secondary/compensatory mutations following acquisition of K65R is rare and quite distinct from the observed step-wise accumulation of TAMs. The mutation S68G appears to partially compensate for the replication defect associated with K65R [22]. The most common coselected mutation is Q151M and the Q151M–NAM resistance pathway [23–26], which confers a reduced susceptibility to all NRTIs, with the exception of 3TC and TDF. The presence of K65R and Q151M counter-selects M184V and confers more resistance than either of the single mutations alone [23–26].
The replicative compromise conferred by K65R is reflected by the strong selective pressure driving its rate of disappearance/reversion upon treatment interruption – K65R (1 month) > M184I/V (3 months) > TAMs (4–6 months) and Q151-NAMs (5.6 months) [27]. The low incidence and rapid disappearance of K65R is important with respect to second-line and salvage treatment options.
Surprisingly, the development of K65R is strongly NRTI regimen-dependent. The use of triple nucleoside analog combinations facilitates the selection of K65R. Several clinical studies were designed to test the clinical benefit of Trizivir® (GlaxoSmithKline; AZT/3TC/ABC), TDF/ABC/3TC, TDF/ddI/ABC and TDF/ddI/3TC as TAM-sparing regimens in treatment-naive subjects [28–31]. These studies were terminated after unexpectedly early failure rates (30–60%) occurred in association with K65R resistance.
The mechanism underlying the increased emergence of K65R and the unanticipated interaction between TDF + ABC and TDF + ddI, remain unclear [28–33]. Poor virological suppression could not be directly associated with either circulating drug levels or intracellular NRTI triphosphate levels [28]. Virological failure was initially associated with the rapid emergence of M184V and K65R on separate viral genomes [28–33].
Early virological failure occurred despite apparent susceptibilities to two or three drugs in regimens, according to genotypic algorithms. The rapid selection of K65K/R and M184M/I/V as minority species emerging on separate clones underestimated resistance to TDF, ABC and ddI in phenotypic assays [28–33]. This caveat of phenotypic assays should be recognized for K65R-selecting drugs, including TDF, ABC, d4T and ddI [33]. Testing for minority K65R and M184V species using ultrasensitive allele-specific PCR may be important in ascribing the role of resistance in virological failure to K65R-selecting drugs [34–36]. Changing to AZT-containing four-drug regimens has been demonstrated to be successful in regaining virological suppression [28,37,38].
Indeed, combining AZT with K65R-selecting drugs may be useful in preventing the evolution of K65R resistance [31,37,38]. A composite retrospective analysis of data from patients receiving a TDF + ABC regimen revealed a success rate of 86% when the regimen contained AZT, compared with 62% when it did not, and no K65R mutations were observed in subjects on regimens containing AZT [31]. Similarly, a reduced selection of the K65R mutation was observed when AZT was added to an ABC-containing regimen [37]. There was a lower incidence of the K65R and L74V in ABC regimens containing AZT than in those without [39]. In several clinical studies involving a triple-NRTI regimen without AZT, 24–92% of patients identified as treatment failures had a virus with the K65R mutation [32,40–44]. Only one out of 90 patients who failed therapy in clinical studies using three or four NRTIs, which included AZT, had the K65R mutation and this person had received only once-daily AZT [45,46]. Moreover, recent findings in the COL40263 study looking at a ABC/3TC/ZDV + TDF-combined regimen, found that therapeutic failure was more likely to occur via the TAM pathway rather than K65R [38].
The rising incidence of K65R from 0.4 to 3.6% between 1998 and 2003 was linked to ddI therapy [47,48]. There was also a strong association for K65R selection with nevirapine (NVP) and the Y181C/G190A/S mutations associated with non-NRTI (NNRTI) resistance [49–51]. A synergistic fitness interaction has been observed for K65R and Y181C mutations [49–51]. The selection of K65R is negatively associated with K103N [48].
Altogether, ddI, NVP and triple NRTI therapy may be associated with higher frequencies of selection of K65R. Careful avoidance of coadministration of TDF with ABC or ddI in first-line treatment regimens and genotypic resistance testing for NNRTIs has led to decreasing trends in K65R appearance (<2% of the treated population) in the years following 2005 [47–52]. A total of four NRTI-containing regimens, including TDF coformulated Trizivir have been recently proposed as a more stable regimen that may offset the development of K65R, while representing TAM-sparing regimens [53–55].
The aforementioned considerations provide clinicians with reasonable clinical approaches in preventing and managing HIV drug resistance. Highly potent TDF/FTC and ABC/3TC coformulated regimens with EFV or protease inhibitors prevent the advent of K65R resistance and are the drug combinations of choice in resource-rich settings.
Accelerated risk for the development of K65R in the developing world
The growing genetic diversification of HIV-1 represents one of the most serious challenges of the ongoing pandemic. ARTs and emergent drug-resistance profiles have been established on paradigms of subtype B infections present in western-world settings, with little comparative information available for non-B subtypes in developing-world settings. The epicenters of the HIV-1 pandemic are concentrated in the resource-constrained nations, which have limited access to healthcare services and treatment. As access to antiviral therapy in developing-world settings increases, it remains imperative to establish appropriate treatment strategies for long-term clinical benefit and limit the emergence of drug resistance. Subtype differences, suboptimal therapies and deficiencies in healthcare delivery systems can create conditions for the accelerated development of resistance [56–59].
In resource-poor settings, patients at multiple disease stages with significant comorbidities and harboring numerous subtypes are treated with diverse antiretroviral regimens. While the conclusions of early studies suggest significant virological, immunological and clinical benefits of antiviral therapy, aggregating data from numerous clinical studies may mask intersubtype and drug regimen differences [56–60].
Drug regimens provided to resource-poor nations are often suboptimal and may be associated with significant toxicity that affects drug adherence. Whereas TDF and ABC are the backbone NRTIs used in resource-rich settings, d4T, ddI, AZT and NVP represent the major drugs used in resource-limited settings [8,60,101]. These regimens may be suboptimal and fail to adequately achieve viral suppression, leading to a more rapid emergence of K65R resistance in non-B subtypes [52,56].
Genetic diversity amongst subtypes may facilitate the development of resistance [52,56,58–60]. The fastest growing epidemics are subtype C and subtype A variants (A1, A2, CRF01_AE and CRF02_AG) representing approximately 50 and 30% of global infections, respectively [56–58]. These epidemics represent 30–50% of new infections in Europe and 10–20% of new infections in the Americas [56,58,59]. The 10–15% sequence diversity and polymorphisms amongst non-B subtypes may contribute to differences in resistance profiles to NRTIs, as well as interactions between NRTI and NNRTI resistance. Differential replicative fitness and pathogenesis may also affect the duration to develop resistance.
Facilitated development of K65R in non-B subtype infections
The impact of HIV-1 subtypes on drug resistance is most noteworthy in viral resistance to NNRTIs. NVP has become widely used in developing countries as an effective and inexpensive drug for prophylaxis to prevent mother–child transmission [56,59]. In this regard, the acquisition of resistance to NNRTIs is somewhat unique, in that single point mutations, including K103N, Y181C and V106M, arise within days or weeks and confer more than 100–1000-fold resistance. Since NNRTIs are noncompetitive inhibitors, they do not significantly impose fitness constraints on the viral RT enzyme. The slow rates of NVP clearance lead to a rapid appearance and retention of NNRTI mutations, which can facilitate the emergence of K65R resistance in developing countries where NVP is extensively used [49–51]. In this regard, retrospective analysis has revealed significant intersubtype differences in the acquisition of NVP resistance in mothers and infected children, involving K103N or Y181C in 69, 36, 19 and 21% of women with subtype C, D, A and CRF02_AG infections, respectively [56]. Ultrasensitive PCR detection procedures identified minority NNRTI species (K103N or Y181C) in 70–87% of persons with subtype C, as compared with 42% of persons with subtype A/AE infections [56].
In addition, high rates of virological failure have been observed for once-daily TDF/3TC/NVP regimens with K65R in subtype B and non-B subtype infections [50,51]. Virological failure was linked to the synergistic selection of K65R, Y181C and/or G190A resistance.
The development of K65R and Q151M may be facilitated for HIV-2 variants originating from West Africa (Senegal and Portugal), harboring NNRTI mutations (K101A, V106I, V179I, Y181I, Y188L and G190A) and TAMs/NAMs (T69N, V75I, V118I, L210N, T215S and K219E) as natural polymorphisms [61–63]. K65R has been reported to occur in 20% of patients receiving TDF at some point during their treatment. In addition, three single point mutations, K65R, Q151M and M184V, can confer high-level cross-class resistance to all commercially available NRTIs for HIV-2 infections [62].
K65R development in subtype C HIV-1
There is clinical and tissue culture evidence to indicate an accelerated risk in developing the K65R mutation in subtype C infections [64–69]. The first reported study demonstrated that 30% of Botswanan patients failing ddI- or d4T-based regimens developed K65R within 8 months of treatment [64]. While one report disputed a facilitated selection of K65R in subtype C in early TDF trials, recent clinical studies demonstrates a higher incidence of K65R in subtype A and C infections receiving d4T- and ddI-based regimens [64,66–69]. The incidence of K65R in genotyped persons failing treatment was 7, 9, 14, 23 and 30% in clinical studies in Thailand, Senegal, South Africa, Malawi and Botswana, respectively [63,64,66–68]. Although no direct head-to-head comparisons of subtype C versus B have been conducted clinically, the available data suggest that an HIV-1 subtype plays an associative role in the accelerated development of K65R resistance.
Tissue culture selections of virus isolates from Botswana and Ethiopia confirmed the facilitated development of K65R in subtype C relative to subtype B [65,70–72]. Development of K65R in subtype C occurred regardless of regimen, including TDF, d4T, ddI, ABC, TDF + 3TC and d4T + ddI [70–72].
Cell culture studies, site-directed mutagenesis and enzymatic studies have begun to unravel the novel molecular mechanisms that may account for the more rapid appearance of K65R in subtype C [70–73]. Subtype C has a unique KKK nucleotide motif at codons 64, 65 and 66 (AAA–AAG–AAG) when compared with subtype B and other non-B subtypes (AAG–AAA–AAA). The rapid selection of K65R in subtype C strains (AAG→AGG), as compared with the slow evolution of K65R in subtype B (AAA→AGA), cannot be explained by codon usage [70–72]. Enzymatic analyses of subtype B- and subtype C-derived RT enzymes reveal that, mechanistically, the biochemical profiles of both enzymes are very similar [70,74].
The development of K65R between subtypes may be explained by the nucleotide sequence dissimilarities that exist in subtype-specific templates (Figure 2). Introduction of the 64/65 nucleotide polymorphisms of subtype C into subtype B HIV-1 accelerates the selection of the K65R mutation in subtype B to levels observed for subtype C [71]. Preferential selection of K65R in subtype C HIV-1 may be attenuated by silent mutations at codons 70, 210 and 219, implicated in the TAM-resistance pathway [72].
The higher propensity for K65R selection in subtype C, relative to other subtypes, may be related to differences in the poly-adenine stretches within the RT template spanning codons 63–68 (Figure 2) [70]. The RT enzyme is known to exhibit characteristic pausing at the end of such sequences – these pausing events, in turn, contribute to mutagenesis [70,73–80]. In the case of the subtype C sequence, a homopolymeric stretch of adenine bases is present and ends at the exact nucleotide that is responsible for the AAG to AGG transition that gives rise to K65R. By contrast, the subtype B sequence homopolymeric region begins later and stretches beyond codon 65, to include codon 66, and end at codon 67 (Figure 2).
When subtype C RT was used to synthesize DNA on the subtype C template containing the K65 homopolymeric region, strong pausing was seen at the exact nucleotide position responsible for K65R development. When subtype B RT was used to synthesize DNA on the subtype B template containing the K65 homopolymeric region, a ladder of pausing events was observed that started at codon 65 and ended at codon 67, which may be important for the selection of D67N associated with the TAM-1 pathway (Figure 2). No matter what subtype RT enzyme was used to synthesize DNA, the pausing patterns were determined exclusively by the subtype of the template [70,79].
This facilitated selection of K65R in subtype C is a grave concern, given that this subtype accounts for 50% of the worldwide pandemic. It is clear that this subtype may be ‘less forgiving’ for suboptimal regimens. The 23% incidence of K65R in the Malawi study was associated with a d4T/3TC/NVP first-line regimen [68]. This drug combination led to rapid treatment failure by either K65R or TAM pathways. This consequently led to limited treatment options. The long duration of this study (36 months), as compared with the Thai, Kenyan and South African studies, clearly demonstrate the dangers of d4T-based regimens in accelerating the development of resistance, including the K65R pathway [8,64–69,81]. This provides a strong argument for replacing d4T in first-line regimens for TDF-based substitutes [8]. Similarly, AZT/3TC/NVP remains a cost-effective, viable treatment alternative to d4T/3TC/NVP in resource-poor settings until access to TDF is addressed. At least this way, K65R is not likely to be selected, even in the presence of Y181C and/or G190A, and AZT will remain active against M184V viruses.
The issues of costs and access to TDF in resource-poor settings needs to be addressed [8]. The DART studies in Uganda and Zimbabwe using TDF/3TC/AZT regimens show low K65R development [66]. Such regimens are well tolerated with low renal toxicity [82].
Future perspective
Over the past decade, advances in HAART have led to significant improvements of survival rates in resource-poor settings, as well as in developed-world settings. In general, emergent resistance to K65R is rare. The K65R mutation results in diminutions in viral replicative capacity similar to that observed for the M184V mutation [2,3,7,9]. These mutations confer severe fitness constraints and are rapidly lost upon treatment interruption. The high barrier to K65R development and the benefit of K65R and M184V in viral suppression, have led to the hope that TDF and TDF/FTC regimens may be used in microbicide- and chemoprophylaxis-prevention strategies [83–85].
The bidirectional antagonism between K65R and TAM pathways hold the promise of developing NRTI resistance-sparing regimens that combine AZT/TDF/ABC with FTC or 3TC. While clinical trials with triple-drug combinations had to be discontinued because of early treatment failure, quadruple-drug combinations appear to lead to more favorable outcomes in treatment-naive patients and treatment-experienced patients harboring multi-drug-resistant infections [53–55]. In a 96-week prospective trial, ZDV/3TC/ABC + TDF in treatment-naive patients provided a well-tolerated NRTI/NNRTI/protease inhibitor-sparing regimen, even for patients with a high viral load [54]. TDF–AZT combined regimens may block the emergence of NRTI resistance, as well as offer combination regimens towards salvage therapies in individuals harboring multidrug resistance.
The facilitated development of K65R, M184V and/or L74V as a minority species leads to early treatment failure with suboptimal treatment regimens, in particular d4T, ddI, NVP and triple-NRTI drug combinations [21,28–36]. This may be accelerated in non-B subtype infections, notably HIV-2 and subtype C [61–69]. The recent report of transmitted K65R resistance in four out of four breast-feeding infants from mothers harboring K65R illustrates the potential public health concern of transmitted drug resistance [86].
Most importantly, the use of ddI/d4T and d4T/3TC/NVP regimens in resource-poor settings needs to be questioned [8,64–69,81]. The combined dangers of TAM and K65R resistance, facilitated by synergistic NVP resistance (Y181C/G190A), may lead to situations where the choice of second-line regimens is limited [68]. The reasons for replacing d4T–NVP and ddI–NVP regimens with TDF-based regimens in resource-limited settings are compelling and therefore must be addressed [8]. AZT/3TC/NVP is a viable treatment alternative to d4T/3TC/NVP in resource-limited settings and is cost effective until access to TDF occurs.
Currently, AZT is the only licensed antiretroviral for use in pregnancy [87]. In macaque models, perinatal exposure to a very high dose of TDF results in bone toxicity in some offspring [87]. Single-dose (SD) NVP is the only antiretroviral option in many resource-poor settings worldwide. Recent clinical trials in Africa demonstrate that SD TDF used with SD NVP may be of benefit in further reducing mother–child transmission of HIV while preventing emergent NNRTI resistance [87].
In summary, the rarity of K65R development with TDF-based regimens has enormous clinical potential towards the development of cost-effective HIV therapeutic and prevention strategies.
Executive summary.
K65R resistance pathway
K65R is selected by tenofovir disoproxil fumarate (TDF), abacavir, didanosine (ddI), stavudine (d4T) and amdoxovir.
K65R confers partial resistance to most nucleoside analogs while remaining susceptible to zidovudine (AZT).
TDF exhibits improved antiviral activity against isolates harboring some thymidine analog mutations (TAMs).
Barrier to K65R is regimen associated
The overall incidence of K65R is in 1–4% of the genotyped population.
The infrequent development of K65R in drug-naive and treatment-experienced patients is due to impaired K65R replicative capacity and fitness constraints imposed by K65R and M184V – associated with resistance to lamivudine (3TC) and emtricitabine (FTC) and regimen potency (e.g., TDF–FTC).
Bidirectional antagonism of TAMs and K65R pathways lead to a counter selection of K65R in AZT/d4T-experienced patients.
Clinical trials with triple nucleoside reverse-transcriptase inhibitors (NRTIs) regimens were discontinued due to virological failure with high rates of K65R. TDF + abacavir, TDF + ddI and d4T + ddI in combination with 3TC or FTC should be avoided.
Inclusion of AZT may offset virological failure and K65R resistance in two–four NRTI regimens containing K65-selecting drugs.
Higher frequencies of K65R are linked to suboptimal ddI and d4T regimens.
Increased selection of K65R with nevirapine (NVP) is associated with the Y181C and/or G190A mutations.
Facilitated development of K65R in non-B subtype infections
Elevated K65R selection in resource-limited settings may be related to suboptimal ddI, d4T and NVP regimens.
K65R is selected in 9–30% of subtype C patients failing therapy in Africa.
A signature mutational motif in subtype C accelerates K65R selection.
AZT/3TC/NVP is a viable treatment alternative in resource-poor settings and is cost effective until access to TDF is addressed.
Conclusion
Optimizing NRTI and NRTI/non-NRTI regimens deter the emergence of K65R.
The favorable barrier to K65R development makes TDF-based regimens leading candidates for microbicides and pre- and postexposure prophylaxis.
Recent clinical trials are evaluating the efficacy of quadruple-NRTI analog combinations as TAM/K65R-sparing regimens.
Footnotes
Financial & competing interests disclosure: Bluma Brenner is Assistant Professor in the Department of Surgery/Medicine and the McGill AIDS Centre, McGill University, Canada. She is presently directrice de L'axe de résistance, Réseau SIDA et Malades Infectieuses, Fonds de la recherche en santé du Québec (FRSQ), and co-coordinator of Quebec Genotyping Program under the Institut nation de sansté publique du Québec (INSQ) and has received collaborative research funding with Mark Wainberg from the Canadian Institutes for Health Research (CIHR), the Fonds de la Recherche en Santé du Québec-Réseau SIDA (FRSQ-SIDA) and the NIH. Dimitrios Coutsinos has received an MD/PhD CIHR fellowship award and is affiliated with the Departments of Medicine, Microbiology and Immunology, McGill University. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Riddler SA, Haubrich R, DiRienzo AG. Class-sparing regimens for initial treatment of HIV-1 infection. N Eng J Med. 2008;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrella M, Oliveira M, Moisi D, Detorio M, Brenner BG, Wainberg MA. Differential maintenance of the M184V substitution in the reverse transcriptase of human immunodeficiency virus type 1 by various nucleoside antiretroviral agents in tissue culture. Antimicrob Agents Chemother. 2004;48(11):4189–4194. doi: 10.1128/AAC.48.11.4189-4194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner D, Brenner BG, Routy JP, Petrella M, Wainberg MA. Rationale for maintenance of the M184V resistance mutation in human immunodeficiency virus type 1 reverse transcriptase in treatment experienced patients. New Microbiol. 2004;27(2 Suppl 1):31–39. [PubMed] [Google Scholar]
- 4.Cozzi-Lepri A, Ruiz L, Loveday C, et al. Thymidine analogue mutation profiles: factors associated with acquiring specific profiles and their impact on the virological response to therapy. Antivir Ther. 2005;10(7):791–802. [PubMed] [Google Scholar]
- 5.Marcelin AG, Delaugerre C, Wirden M, et al. Thymidine analogue reverse transcriptase inhibitors resistance mutations profiles and association to other nucleoside reverse transcriptase inhibitors resistance mutations observed in the context of virological failure. J Med Virol. 2004;72(1):162–165. doi: 10.1002/jmv.10550. [DOI] [PubMed] [Google Scholar]
- 6.Johnson VA, Brun-Vézinet F, Clotet B, et al. Update of drug resistance mutations in HIV-1: December 2008. Topics HIV Med. 2008;16(5):138–145. [PubMed] [Google Scholar]; ▪ The International AIDS Society panel meets biannually to discuss resistance mutations associated resistance to the five major drug classes.
- 7.Miller MD. K65R, TAMs and tenofovir. AIDS Rev. 2004;6(1):22–33. [PubMed] [Google Scholar]; ▪▪ Important review detailing the distinct features of thymidine analog mutation (TAM) and K65R resistance pathways.
- 8.Bartlett JA, Maro VP. Stavudine in first-line antiviral regimens in resource-limited settings; time for a better solution. HIV Ther. 2009;3(2):109–111. [Google Scholar]; ▪▪ Compelling editorial raising concerns regarding the use of suboptimal stavudine (d4T) regimens and issues regarding tenofovir disoproxil fumarate (TDF) costs and access in resource-poor settings.
- 9.Pozniak A. Tenofovir: what have over 1 million years of patient experience taught us? Int J Clin Practice. 2008;62(8):1285–1293. doi: 10.1111/j.1742-1241.2008.01817.x. [DOI] [PubMed] [Google Scholar]; ▪▪ Summarizes the clinical trials demonstrating the clinical benefit of TDF-based regimens in treatment-naive and treatment-experienced patients.
- 10.Antinori A, Trotta MP, Lorenzini P, et al. Virological response to salvage therapy in HIV-infected persons carrying the reverse transcriptase K65R mutation. Antiviral Ther. 2007;12(8):1175–1183. [PubMed] [Google Scholar]
- 11.Wainberg MA, Miller MD, Quan Y, et al. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir Ther. 1999;4:87–94. doi: 10.1177/135965359900400205. [DOI] [PubMed] [Google Scholar]; ▪ First in vitro demonstration of K65R selection with TDF (PMPA).
- 12.Gu Z, Gao Q, Fang H, et al. Identification of a mutation at codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1994;38:275–281. doi: 10.1128/aac.38.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ First identification of K65R resistance in cell-culture selections with didanosine (ddI) and dideoxycytidine.
- 13.Bazmi HZ, Hammond JL, Cavalcanti SCH, et al. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (-)-β-d-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob Agents Chemother. 2000;44:1783–1788. doi: 10.1128/aac.44.7.1783-1788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First study to indicate the potential value of zidovudine to reduce or prevent the selection of K65R.
- 14.McColl DJ, Chappey C, Parkin NT, Miller MD. Prevalence, genotypic associations and phenotypic characterization of K65R, L74V and other HIV-1 RT resistance mutations in a commercial database. Antivir Ther. 2008;13(2):189–197. [PubMed] [Google Scholar]
- 15.van de Vijver DA, Wensing AM, Angarano G, et al. The calculated genetic barrier for antiretroviral drug resistance substitutions is largely similar for different HIV-1 subtypes. J Acquir Immune Defic Syndr. 2006;41(3):352–360. doi: 10.1097/01.qai.0000209899.05126.e4. [DOI] [PubMed] [Google Scholar]
- 16.Parikh UM, Zalina S, Sluis-Cramer N, Mellors JW. Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS. 2007;21:1405–1407. doi: 10.1097/QAD.0b013e3281ac229b. [DOI] [PubMed] [Google Scholar]; ▪▪ This landmark study used molecular analysis to show the bidirectional phenotypic antagonism between the K65R and TAM resistance pathways. K65R reduces enzymatic excision of chain-terminating nucleoside reverse-transcriptase inhibitor (NRTI) monophosphates (e.g., zidovudine). TAMs counterbalance K65R by decreasing its enzymatic discrimination of d-nucleotide analogs.
- 17.Parikh UM, Barnas DC, Faruki H, Mellors JW. Antagonism between the HIV-1 reverse-transcriptase mutation K65R and thymidine-analogue mutations at the genomic level. J Infect Dis. 2006;194:651–660. doi: 10.1086/505711. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates genomic counter selection of K65R and TAMs.
- 18.Frankel FA, Invernizzi CF, Oliveira M, Wainberg MA. Diminished efficiency of HIV-1 reverse transcriptase containing the K65R and M184V drug resistance mutations. AIDS. 2007;21:665–675. doi: 10.1097/QAD.0b013e3280187505. [DOI] [PubMed] [Google Scholar]
- 19.Ly JK, Margot NA, MacArthur HL, Hung M, Miller MD, White KL. The balance between NRTI discrimination and excision drives the susceptibility of HIV-1 RT mutants K65R, M184V and K65R + M184V. Antivir Chem Chemother. 2007;18(6):307–316. doi: 10.1177/095632020701800603. [DOI] [PubMed] [Google Scholar]
- 20.Kagan RM, Lee TS, Ross É, Lloyd RM, Lewinski MA, Potts SJ. Molecular basis of antagonism between K70E and K65R tenofovir associated mutations in HIV-1 reverse transcriptase. Antivir Res. 2007;75:210–218. doi: 10.1016/j.antiviral.2007.03.006. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates that the bidirectional antagonism between K65R and K70E is associated with the profound replicative disadvantage of K65R (2.4%) and K65R + K70E (0.01%) versus K70E (97%) and with virus.
- 21.Waters É, Nelson M, Mandalia S, et al. The risks and incidence of K65R and L74V mutations and subsequent virologic response. Clin Infect Dis. 2008;46(1):96–100. doi: 10.1086/523001. [DOI] [PubMed] [Google Scholar]
- 22.Svarovskaia ES, Feng J, Margot NA, et al. The A62V and S68G mutations in HIV-1 reverse transcriptase partially restore the replication defect associated with the K65R mutation. J Acquir Immune Def Syndr. 2008;48(1):428–436. doi: 10.1097/QAI.0b013e31817bbe93. [DOI] [PubMed] [Google Scholar]; ▪ S68G is the only mutation that appears to offset the replicative disadvantage of K65R. Many current genotypic algorithms do not report this compensatory mutation.
- 23.Van Laethem K, Pannecouque C, Vandamme AM. Mutations at 65 and 70 within the context of a Q151M cluster in human immunodeficiency virus type 1 reverse transcriptase impact the susceptibility to the different nucleoside reverse transcriptase inhibitors in dzistinct ways. Infect Genet Evol. 2007;7(5):600–603. doi: 10.1016/j.meegid.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Boucher S, Recordon-Pinson P, Ragnaud JM, Dupon M, Fleury H, Masquelier B. HIV-1 reverse transcriptase (RT) genotypic patterns and treatment characteristics associated with the K65R RT mutation. HIV Med. 2006;7(5):294–298. doi: 10.1111/j.1468-1293.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 25.Trotta MP, Bonfigli S, Ceccherini-Silberstein F. Clinical and genotypic correlates of mutation K65R in HIV-infected patients failing regimens not including tenofovir. J Med Virol. 2006;78(5):535–541. doi: 10.1002/jmv.20573. [DOI] [PubMed] [Google Scholar]
- 26.Feng JY, Myrick FT, Margot NA, et al. Virologic and enzymatic studies revealing the mechanism of K65R- and Q151M-associated HIV-1 drug resistance towards emtricitabine and lamivudine. Nucleosides Nucleotides Nucleic Acids. 2006;25(1):89–107. doi: 10.1080/15257770500379157. [DOI] [PubMed] [Google Scholar]
- 27.Trignetti M, Sing T, Svicher V, et al. Dynamics of NRTI resistance mutations during therapy interruption. AIDS Res Hum Retroviruses. 2009;25(1):57–64. doi: 10.1089/aid.2008.0159. [DOI] [PubMed] [Google Scholar]; ▪▪ The rapid disappearance of K65R relative to other NRTI mutations upon treatment interruption is a clinical indication of its impaired replicative capacity.
- 28.Saag MS. Perspective. Initiation of antiretroviral therapy: implications of recent findings. Topics HIV Med. 2004;12(3):83–88. [PubMed] [Google Scholar]
- 29.Gallant JE, Rodriguez AE, Weinberg W, et al. Early virologic non-response to tenofovir, abacavir and lamivudine in HIV-infected antiretroviral-naive subjects. J Infect Dis. 2005;192:1921–1930. doi: 10.1086/498069. [DOI] [PubMed] [Google Scholar]; ▪ First study to report the rapid selection of K65R with triple nucleoside analogs. This strategy has been discontinued.
- 30.Martin-Carboner É, Gil P, Garcia-Benayas TG, et al. Rate of virologic failure and selection of drug resistance mutations using different triple nucleos(t)ide analogue combinations in HIV-infected patients. AIDS Res Hum Retroviruses. 2006;22(12):1231–1235. doi: 10.1089/aid.2006.22.1231. [DOI] [PubMed] [Google Scholar]
- 31.Gillam BL, Sajadi MM, Amoroso A, Davis CE, Cleghorn FR, Redfield RR. Tenofovir and abacavir combination therapy: lessons learned from an urban clinic population. AIDS Patient Care STDs. 2007;21(4):240–245. doi: 10.1089/apc.2006.0070. [DOI] [PubMed] [Google Scholar]
- 32.Delaunay C, Brun-Vézinat F, Landman R, et al. Comparative selection of the K65R and M184V/I mutations in human immunodeficiency virus type 1-infected patients enrolled in a trial of first-line triple-nucleoside analog therapy (Tonus IMEA 021) J Virol. 2005;79(15):9572–9578. doi: 10.1128/JVI.79.15.9572-9578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Demonstrates genomic antagonism of K65R and M184V leading to treatment failure.
- 33.Underwood MR, Ross LI, Irlbeck DM, et al. Sensitivity of phenotypic susceptibility analyses for non-thymidine nucleoside analogs conferred by K65R or M184V in mixtures with wild type HIV-1. J Infect Dis. 2009;199:84–88. doi: 10.1086/595296. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates how K65K/R and M184M/I/V mixtures can underestimate phenotypic resistance to NRTIs.
- 34.Ntemgwa ML, Toni T, Brenner BG, et al. Nucleoside and nucleotide analogs select in culture for different patterns of drug resistance in human immunodeficiency virus types 1 and 2. Antimicrob Agents Chemother. 2009;53(2):708–715. doi: 10.1128/AAC.01109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzner KJ, Giulieri SG, Knoerfel SA, et al. Minority quasispecies of drug resistant HIV-1 that lead to early therapy failure in treatment-naive and -adherent patients. Clin Infect Dis. 2009;48:239–247. doi: 10.1086/595703. [DOI] [PubMed] [Google Scholar]
- 36.Svarovskaia ES, Margot NA, Bae AS, et al. Low-level K65R mutation in HIV-1 reverse transcriptase of treatment-experienced patients exposed to abacavir or didanosine. J Acquir Immune Defic Syndr. 2007;46(2):174–180. doi: 10.1097/QAI.0b013e31814258c0. [DOI] [PubMed] [Google Scholar]
- 37.Lanier ER, Givens N, Stone C, et al. Effect of concurrent zidovudine use on the resistance pathway selected by abacavir-containing regimens. HIV Med. 2004;5(6):394–399. doi: 10.1111/j.1468-1293.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 38.Ross L, Elion R, Lanier R, et al. Modulation of K65R selection by zidovudine inclusion: analysis of HIV resistance selection in subjects with virologic failure receiving once-daily abacavir/lamivudine/zidovudine and tenofovir DF (Study COL40263) AIDS Res Hum Retroviruses. 2009;25(7):665–672. doi: 10.1089/aid.2008.0302. [DOI] [PubMed] [Google Scholar]
- 39.Ait-Khaled M, Rakik A, Griffin P, et al. Mutations in HIV-1 reverse transcriptase during therapy with abacavir, lamivudine and zidovudine in HIV-1-infected adults with no prior antiretroviral therapy. Antivir Ther. 2002;7(1):43–51. [PubMed] [Google Scholar]
- 40.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 41.Khanlou H, Yeh V, Guyer B, Farthing C. AIDS Patient Care STDS. 2005;9(3):135–140. doi: 10.1089/apc.2005.19.135. [DOI] [PubMed] [Google Scholar]
- 42.Miller MD, Margot NA, McColl DJ, et al. Characteristics of resistance mutation patterns emerging over 2 years during first-line antiretroviral treatment with tenofovir DF or stavudine in combination with lamivudine and efavirenz. Antivir Ther. 2003;8:S151. [Google Scholar]
- 43.Roge BT, Katzenstein TL, Obel N, et al. K65R with and without S68: a new resistance profile in vivo detected in most patients failing abacavir, didanosine and stavudine. Antivir Ther. 2003;8:173–182. [PubMed] [Google Scholar]
- 44.Jemsek J, Hutcherson P, Harper E. Poor virologic responses and early emergence of resistance in treatment naive, HIV-infected patients receiving a once daily triple nucleoside regimen of didanosine, lamivudine, and tenofovir DF. Presented at: 11th Conference on Retroviruses and Opportunisistic Infections; San Francisco, CA, USA. 8–11 February 2004; Abstract 42. [Google Scholar]
- 45.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 46.Elion R, Cohen C, DeJesus E, et al. COL40263: resistance and efficacy of once -daily trizivir and tenofovir DF in antiretroviral-naive subjects. Presented at: 11th Conference on Retroviruses and Opportunisistic Infections; San Francisco, CA, USA. 8–11 February 2004; Abstract 53. [Google Scholar]
- 47.Theys K, Vercauteren J, Abecasis AA, et al. The rise and fall of K65R in a Portugese HIV-1 drug resistance database, despite continuing use of tenofovir. Infect Genet Evol. 2009;4(9):683–688. doi: 10.1016/j.meegid.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 48.de Mendoza C, Jimenez-Nacher I, Garrido C, et al. Changing patterns in HIV reverse transcripase mutations after the availability of tenofovir. Clin Infect Dis. 2008;46:1782–1785. doi: 10.1086/588045. [DOI] [PubMed] [Google Scholar]
- 49.von Wyl V, Yerly S, Boni J, et al. Factors associated with the emergence of K65R in patients with HIV-1 infection treated with combination antiretroviral therapy containing tenofovir. Clin Infect Dis. 2008;46:1299–1309. doi: 10.1086/528863. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates that emergence of K65R and virologic failure is linked to ddI, non-NRTIs (G190A/S or Y181C) and baseline CD4+ cell count.
- 50.Rey D, Hoen B, Chavanet P, et al. High rate of early virological failure with the once daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother. 2009;63:380–388. doi: 10.1093/jac/dkn471. [DOI] [PubMed] [Google Scholar]
- 51.Clotet B. Once daily dosing of nevirapine in HAART. J Antimicrob Chemother. 2008;61:13–16. doi: 10.1093/jac/dkm432. [DOI] [PubMed] [Google Scholar]; ▪ The DAUFIN trial showed unexpected high rate of K65R with once-daily TDF/lamivudine (3TC)/nevirapine (NVP).
- 52.Deforche K, Camacho RJ, Grossman Z, et al. Bayesian network analysis of resistance analysis of resistance pathways against efavirenz and nevirapine. AIDS. 2008;22:2107–2115. doi: 10.1097/QAD.0b013e32830fe940. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ The non-B HIV Working Group is involved in ascertaining the role of subtype diversity in HIV resistance. There is an association between non-B subtype and K65R resistance. Pooled analysis of all non-B subtypes and restrictions to amino acid, rather than nucleotide diversity may, however, fail to adequately define the role of subtype diversity in the selection of resistance mutations.
- 53.Llibre JM, Bonjoch A, Iribarren J, et al. Targeting only reverse transcriptase with zidovudine/lamivudine/abacavir plus tenofovir in HIV-1-infected patients with multidrug-resistant virus: a multicentre pilot study. HIV Med. 2008;9:508–513. doi: 10.1111/j.1468-1293.2008.00581.x. [DOI] [PubMed] [Google Scholar]; ▪ A promising salvage strategy to bridge K65R and TAM resistance in patients harboring multidrug resistance.
- 54.Ferrer E, Gatell J, Sanchez P, et al. Zidovudine/lamivudine/abacavir plus tenofovir in HIV-infected naive patients: a 96 week prospective one-arm pilot study. AIDS Res Hum Retroviruses. 2008;24(7):931–934. doi: 10.1089/aid.2007.0271. [DOI] [PubMed] [Google Scholar]; ▪ An intriguing future strategy for NRTI/non-NRTI/protease inhibitor-sparing regimens for long-term HIV-1 management, based on the bidirectional antagonism between K65R and TAM pathways.
- 55.Hurwitz SJ, Asif G, Kivel NM, Schinazi RF. Development of an optimized dose for coformulation of zidovudine with drugs that select for the K65R mutation using a population pharmacokinetic and enzyme kinetic simulation model. Antimicrob Agents Chemother. 2008;52:4241–4250. doi: 10.1128/AAC.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenner BG. Resistance and viral subtypes. How important are the differences and why do they occur? Curr Opin HIV AIDS. 2007;2:94–102. doi: 10.1097/COH.0b013e32801682e2. [DOI] [PubMed] [Google Scholar]
- 57.Kantor R, Katzenstein DA, Efron B, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2(e112):325–337. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ This international collaborative team has been established to compile non-B subtypes sequences from different geographic regions.
- 58.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2009;358(15):1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geretti AM. HIV subtypes: epidemiology and significance for HIV management. Curr Opin Infect Dis. 2006;19:1–7. doi: 10.1097/01.qco.0000200293.45532.68. [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Cajas JL, Pant-Pai N, Klein M, Wainberg MA. Role of genetic diversity amongst non-B subtypes in drug resistance: a systematic review of virologic and biochemical evidence. AIDS Rev. 2008;10:12–23. [PubMed] [Google Scholar]; ▪ Summarizes clinical and cell culture data of subtype differences in emergent resistance to antiretroviral therapy.
- 61.Ntemgwa ML, Toni TD, Brenner BG, Camacho RJ, Wainberg MA. Antiretroviral drug resistance in human immunodeficiency virus type-2 (HIV-2) Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.00154-09. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith RA, Anderson DJ, Pyrak CL, Preston BD, Gottlieb GS. Antiretroviral drug resistance in HIV-2: three amino acid changes are sufficient for classwide nucleoside analogue resistance. J Infect Dis. 2009;199:1323–1326. doi: 10.1086/597802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gottlieb GS, Badiane NM, Hawkes S, et al. Emergence of multiclass drug-resistance in HIV-2 in antiretroviral-treated individuals in Senegal: implications for HIV-2 treatment in resource limited West Africa. Clin Infect Dis. 2009;48:476–483. doi: 10.1086/596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doualla-Bell F, Avalos A, Brenner B, et al. High prevalence of the K65R mutation in human immunodeficiency virus type 1 subtype C isolates from infected patients in Botswana treated with didanosine-based regimens. Antimicrob Agents Chemother. 2006;50(12):4182–4185. doi: 10.1128/AAC.00714-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Among the earliest studies to demonstrate the accelerated development of K65R in subtype C infections. It led to studies characterizing the molecular basis for the facilitated development of K65R in subtype C.
- 65.Brenner BG, Oliveira M, Doualla-Bell F, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS. 2006;20(9):F9–F13. doi: 10.1097/01.aids.0000232228.88511.0b. [DOI] [PubMed] [Google Scholar]; ▪▪ Landmark study using a cell-culture selection approach to demonstrate the accelerated development of K65R in subtype C. Follow-up studies have combined site-directed mutagenesis and enzymatic studies to mechanistically define the molecular basis for facilitated development of K65R in subtype C [72–74]. This has important clinical implications in K65R development using ‘less forgiving’ suboptimal d4T and ddI regimens in third-world settings.
- 66.Miller MD, Margot N, McColl D, Cheng AK. K65R development among subtype C HIV-1 patients in tenofovir DF clinical trials. AIDS. 2007;21:265–266. doi: 10.1097/QAD.0b013e32801199ee. [DOI] [PubMed] [Google Scholar]; ▪ While these authors argued against the findings of a previous reference [65], clinical studies clearly demonstrate the facilitated development of K65R in resource-poor settings with d4T–NVP and d4T–ddI. However, the potency of TDF/emtricitabine regimens may offset the development of K65R in subtype C.
- 67.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, Saekang N, Pairoj W, Chantratita W. Prevalence and risk factors for developing K65R mutations among HIV-1 infected patients who fail an initial regimen of fixed-dose combination of stavudine, lamivudine, and nevirapine. J Clin Virol. 2008;41(4):310–313. doi: 10.1016/j.jcv.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Hosseinipour M, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Important study demonstrating clinical correlates for the disparate high rates of K65R in subtype C viruses failing d4T/3TC/NVP regimens in Malawi.
- 69.Pillay V, Pillay C, Kantor R, Venter F, Levin É, Morris L. HIV type 1 subtype C drug resistance among pediatric and adult South African patients failing antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24(11):1449–1454. doi: 10.1089/aid.2008.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coutsinos D, Invernizzi CF, Xu H, et al. Template usage is responsible for the preferential acquisition of the K65R reverse transcriptase mutation in subtype C variants of human immunodeficiency virus type 1. J Virol. 2009;83(4):2029–2033. doi: 10.1128/JVI.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Important enzymatic study, summarized in Figure 2, which illustrates the mechanism for accelerated development of K65R in subtype C.
- 71.Invernizzi CF, Coutsinos D, Moisi D, et al. Introduction of the 64/65 nucleotide polymorphisms of subtype C into subtype B HIV-1 selects for the K65R mutational pathway in cell culture. Presented at: 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 3–6 February 2008; Poster 849. [Google Scholar]; ▪▪ An important paper using site-directed mutagenesis of two nucleotides in codons 64 and 65 of subtype B to demonstrate the template-based mechanism for the facilitated selection of K65R in subtype C infections.
- 72.Invernizzi C, Oliveira M, Moisi D, Brenner B, Wainberg M. Preferred K65R pathway in subtype C HIV-1 can be altered by silent polymorphisms at thymidine analogue mutation sites in cell culture drug selections. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 16–20 February 2009; Poster 628. [Google Scholar]
- 73.Xu HT, Martinez-Cajas JL, Ntemgwa ML, et al. Effects of the K65R and K65R/M184V reverse transcriptase mutations in subtype C HIV on enzyme function and drug resistance. Retrovirology. 2009;6:14–25. doi: 10.1186/1742-4690-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobard C, Lipscomb J, Johnson JA, Garcia-Lerma JG, Heneine W. Recombinant viruses expressing subtype B or subtype C reverse transcriptase reveal no difference in the rate of K65R resistance to tenofovir in cell culture. Antivir Ther. 2009;14(Suppl 1):A23. [Google Scholar]; ▪ Recombinant viruses expressing subtype B or subtype C reverse transcriptase revealed no differences in subtype B and C enzymatic activity. This study failed to observe differences in the emergence times of K65R resistance with TDF in MT-4 cells. The rapid selection times for HXB2 viruses in MT-4 cell lines may have masked subtype B and C differences. It should be noted that subtype C clinical isolates are primarily R5-tropic and do not grow in MT-2 and MT-4 cells.
- 75.Bebenek K, Abbotts J, Wilson SH, Kunkel TA. Error-prone polymerization by HIV-1 reverse transcriptase. Contribution of template-primer misalignment, miscoding, and termination probability to mutational hot spots. J Biol Chem. 1993;268(14):10324–10334. [PubMed] [Google Scholar]
- 76.Buiser RG, Bambara RA, Fay PJ. Pausing by retroviral DNA polymerases promotes strand transfer from internal regions of RNA donor templates to homopolymeric acceptor templates. Biochim Biophys Acta. 1993;1216(1):20–30. doi: 10.1016/0167-4781(93)90033-a. [DOI] [PubMed] [Google Scholar]
- 77.Hu WS, Temin HM. Retroviral recombination and reverse transcription. Science. 1990;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- 78.Huber HE, McCoy JM, Seehra JS, Richardson CC. Human immunodeficiency virus 1 reverse transcriptase. Template binding, processivity, strand displacement synthesis, and template switching. J Biol Chem. 1989;264(8):4669–4678. [PubMed] [Google Scholar]
- 79.Klarmann GJ, Schauber CA, Preston BD. Template-directed pausing of DNA synthesis by HIV-1 reverse transcriptase during polymerization of HIV-1 sequences in vitro. J Biol Chem. 1993;268(13):9793–9802. [PubMed] [Google Scholar]
- 80.Schinazi RF, Lloyd RM, Ramanathan CS, Taylor EW. Antiviral drug resistance mutations in HIV-1 reverse transcriptase occur in specific RNA structural regions. Antimicrob Agents Chemother. 1994;38:268–274. doi: 10.1128/aac.38.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Frst paper to show that antiviral drug-resistance mutations in HIV-1 reverse transcriptase occur in specific RNA structural regions. This has implications in the facilitated selection of K65R in subtype C.
- 81.Steegen K, Luchters S, Dauwe K, et al. Effectiveness of antiretroviral therapy and development of drug resistance in HIV-infected patients in Mombasa, Kenya. AIDS Res Ther. 2009;6:12. doi: 10.1186/1742-6405-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andrew R, Stöhr W, Walker S, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-Infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46:1271–1281. doi: 10.1086/533468. [DOI] [PubMed] [Google Scholar]
- 83.García-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLos Med. 2008;5(2):e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paltiel AD, Freedberg KA, Scott CA, et al. HIV pre-exposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost–effectiveness. Clin Infect Dis. 2009;48(6):806–815. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okwundu CI, Okoromah CA. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. Cochrane Database Syst Rev. 2009;21(1):1–11. doi: 10.1002/14651858.CD007189.pub2. [DOI] [PubMed] [Google Scholar]
- 86.Lidstrom J, Kumwenda N, Kafulafula G, et al. Antiretroviral treatment of HIV-infected women can induce multiclass drug resistance in their breastfeeding infants. Antivir Ther. 2009;14(Suppl 1):A158. [Google Scholar]; ▪ Demonstrates the transmission of K65R to four out of four infants through breastfeeding.
- 87.Foster C, Lyall H, Olmscheiid B, et al. Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: is it time to move on from zidovudine? HIV Med. 2009;10(7):397–406. doi: 10.1111/j.1468-1293.2009.00709.x. [DOI] [PubMed] [Google Scholar]; ▪▪ Important article with respect to the potential benedit of TDF and TDF/3TC in preventing mother–child transmission.
Websites
- 101.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; Nov 3, 2008. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; pp. 1–139. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]; ▪ The Department of Health and Human Services guidelines for HIV-1 treatment are updated regularly. TDF/FTC and TDF/3TC are the NRTI regimens of choice to be used in treatment-naive and experienced patients. ABC/3TC were also recommended until recent students of increased risk of cardiac implications.
- 102.Stanford University HIV drug resistance database. http://hivdb.stanford.edu.; ▪▪ Openly available and frequently updated database allows researchers and clinicians alike to determine the incidence of drug resistance mutations and genotypic–phenotypic correlations for resistance, treatments and subtypes.