Abstract
Objective
To estimate and interpret time trends in vertical transmission rates for HIV using data from national obstetric and paediatric surveillance registers.
Design
Prospective study of HIV infected women reported through obstetric surveillance. HIV infection status of the child and onset of AIDS were reported through paediatric surveillance. Rates of vertical transmission and progression to AIDS rate were estimated by methods that take account of incomplete follow up of children with indeterminate infection status and delay in AIDS reporting.
Setting
British Isles.
Subjects
Pregnant women infected with HIV whose infection was diagnosed before delivery, and their babies.
Main outcome measures
Mother to child transmission of infection and progression to AIDS in children.
Results
By January 1999, 800 children born to diagnosed HIV infected women who had not breast fed had been reported. Vertical transmission rates rose to 19.6% (95% confidence interval 8.0% to 32.5%) in 1993 before falling to 2.2% (0% to 7.8%) in 1998. Between 1995 and 1998 use of antiretroviral treatment increased significantly each year, reaching 97% of live births in 1998. The rate of elective caesarean section remained constant, at around 40%, up to 1997 but increased to 62% in 1998. Caesarean section and antiretroviral treatment together were estimated to reduce risk of transmission from 31.6% (13.6% to 52.2%) to 4.2% (0.8% to 8.5%). The proportion of infected children developing AIDS in the first 6 months fell from 17.7% (6.8% to 30.8%) before 1994 to 7.2% (0% to 15.7%) after, coinciding with increased use of prophylaxis against Pneumocystis carinii pneumonia.
Conclusions
In the British Isles both HIV related morbidity and vertical transmission are being reduced through increased use of interventions.
Key messages
Reliable estimates of HIV vertical transmission rates can be derived from surveillance data
Infected pregnant women are increasingly taking up elective caesarean section and antiretroviral treatment to reduce the risk of transmitting HIV to their babies
Vertical transmission rates have fallen greatly over the past four years and progression to AIDS among infected children may also have slowed
These benefits can occur only if infected women are diagnosed before or during pregnancy
Introduction
Randomised controlled trials have established that zidovudine can significantly reduce the risk of vertical transmission of HIV.1 The protective effect of elective caesarean section has been confirmed by a recent meta-analysis of cohort studies2 and a randomised controlled trial.3 The combined effect of these interventions is reported to reduce transmission to 2% or less in some cohort studies.2 4
However, the general population of infected women may differ from those recruited into trials or cohort studies in terms of adherence to antiretroviral treatment, previous exposure to antiretroviral treatment, and uptake of elective caesarean section. It is therefore essential to monitor uptake of interventions and vertical transmission rates in the wider population. Here we present estimates of HIV vertical transmission rates in the British Isles.5
Methods
Population studied
Since 1989 pregnant women in the British Isles known to be infected with HIV have been notified to the Royal College of Obstetricians and Gynaecologists. Obstetricians are asked to report on outcome of pregnancy and, since 1995, on mode of delivery and uptake of antiretroviral treatment. After initial notification of children born to infected mothers to the British paediatric surveillance unit, paediatricians are asked for diagnostic and clinical data. Subsequently, children of indeterminate infection status are followed until infection status is known.5 All reporting is voluntary and confidential. This analysis is confined to prospectively ascertained children whose mothers' HIV infection was reported to have been diagnosed before delivery.
Statistical methods
Statistical methods to avoid biased estimation of vertical transmission rates due to incomplete follow up of indeterminates6 were adapted and extended. These involve estimating the probability of infection for each indeterminate child based on when they were last known to be seropositive and the fact that they have not yet been reported to have AIDS. The method takes account of AIDS incubation time, delay in AIDS reporting, and time to antibody loss in uninfected children. It was assumed that progression to AIDS in the first six months would have been reduced since 1994 because prophylaxis against Pneumocystis carinii pneumonia became routine at that time following UK and US guidelines.7–8
The estimation-maximisation algorithm was used to estimate yearly vertical transmission rates since 1984, and the effect of mode of delivery and antiretroviral treatment during 1995 to 1998. Confidence intervals were estimated by bootstrap sampling from the data (n=999).9 Assumptions on date when P carinii prophylaxis became routine and on distribution of delay in AIDS reporting were subjected to sensitivity analysis.
Results
By the end of January 1999, a total of 800 prospectively ascertained non-breast fed children born to HIV infected women between 1984 and 1998 had been reported (table 1).
Table 1.
Infection status of infants born to diagnosed, HIV infected women between 1984 and 1998 who were not reported to have been breast fed, and vertical transmission rates estimated with estimation-maximisation algorithm
| Year of birth | Total live births | Infection status
|
Estimated vertical transmission rate (95% CI) |
||
|---|---|---|---|---|---|
| Negative | Positive (AIDS) | Indeterminate | |||
| 1984 | 3 | 1 | 1 (0) | 1 | |
| 1985 | 17 | 10 | 3 (2) | 4 | 9.6* (2.1 to 18.7) |
| 1986 | 36 | 31 | 1 (0) | 4 | |
| 1987 | 38 | 30 | 2 (2) | 6 | 5.7 (0 to 14.2) |
| 1988 | 38 | 29 | 6 (4) | 3 | 16.5 (5.5 to 29.7) |
| 1989 | 41 | 32 | 3 (1) | 6 | 8.0 (0 to 17.6) |
| 1990 | 41 | 27 | 6 (4) | 8 | 16.6 (5.4 to 29.3) |
| 1991 | 53 | 36 | 4 (2) | 13 | 8.9 (0 to 18.1) |
| 1992 | 55 | 38 | 5 (1) | 12 | 10.6 (2.2 to 19.8) |
| 1993 | 52 | 28 | 8 (2) | 16 | 19.6 (8.0 to 32.5) |
| 1994 | 69 | 38 | 10 (1) | 21 | 19.3 (9.7 to 30.5) |
| 1995 | 61 | 36 | 9 (1) | 16 | 18.9 (8.4 to 30.8) |
| 1996 | 91 | 58 | 10 (3) | 23 | 14.1 (6.4 to 22.5) |
| 1997 | 85 | 52 | 4 (0) | 29 | 6.8 (1.6 to 14.7) |
| 1998 | 120 | 42 | 1 (0) | 77 | 2.2 (0 to 7.8) |
| Total | 800 | 488 | 73 (23) | 239 | 12.1 (9.7 to 14.7) |
1984-6 estimate pooled, due to small numbers.
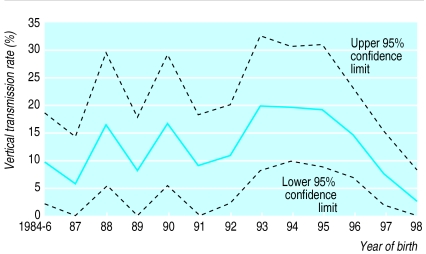
The overall estimation-maximisation algorithm estimate for vertical transmission, which removes the bias caused by indeterminate children, is 12.1% (9.7% to 14.7%). The transmission rate rose to 19.6% (8.0% to 32.5%) in 1993 and from 1995 decreased steeply to 2.2% (0% to 7.8%) in 1998 (figure). The decline in transmission rate from 1995 onwards was significant (odds ratio of 0.51 a year; 95% confidence interval 0.27 to 0.77, χ22=8.8, P=0.003).
Infected children progressed to AIDS within the first 6 months of life at an estimated baseline rate of 17.7% (6.8% to 30.8%) before 1994 and 7.2% (0% to 15.7%) from 1994 onwards. The difference did not reach significance (χ2 =2.51, P=0.11). After 6 months of age, an estimated 5.0% (2.4% to 8.5%) of children who had not yet developed AIDS would do so each year.
Uptake of antiretroviral treatment increased significantly between 1995 and 1998, as did the proportion taking more than one antiretroviral drug (table 2). The proportion of mother-baby pairs receiving all three components of treatment1 (antenatal, during delivery, and post partum to the baby) also increased significantly.
Table 2.
Trends in mode of delivery and use of antiretroviral treatment 1995-8
| Year of birth | % (No) of elective caesarean sections | % (No) receiving antiretroviral drugs | % (No) receiving >1 antiretroviral drug | % (No) receiving all 3 components of treatment* |
|---|---|---|---|---|
| 1995 | 42 (24/58) | 66 (40/61) | 0 (0/39) | 54 (15/28) |
| 1996 | 38 (32/85) | 77 (66/86) | 3 (1/36) | 85 (50/59) |
| 1997 | 42 (35/84) | 84 (70/83) | 26 (18/70) | 86 (54/63) |
| 1998 | 62 (74/119) | 97 (115/119) | 57 (66/116) | 89 (93/104) |
Antenatal, during labour, and post partum to baby.
Table 3 shows that the risk of HIV transmission was significantly lower in infants with exposure to antiretroviral treatment than in those without. The effect of caesarean section was not significant after antiretroviral treatment was adjusted for. The baseline transmission rate among infants delivered vaginally or by emergency caesarean section in the absence of antiretroviral treatment was estimated to be 31.6%. Elective caesarean section or antiretroviral treatment alone reduced the transmission rate to 15.3% and 10.1%, respectively, and when combined reduced transmission to 4.2%.
Table 3.
Effects of elective caesarean section and antiretroviral treatment on vertical transmission rates of HIV, 1995-8 from logistic regression
| Vaginal/emergency caesarean section | Elective caesarean section | Adjusted odds ratio (95% CI) | |
|---|---|---|---|
| No antiretroviral treatment | 31.6 (13.6 to 52.2) | 15.3 (3.4 to 32.7) | 1 |
| Antiretroviral treatment | 10.1 (4.6 to 16.4) | 4.2 (0.8 to 8.5) | 0.24 (0.08 to 0.76)* |
| Adjusted odds ratio (95% CI) | 1 | 0.39 (0.08 to 1.04)† |
χ21=6.9, P=0.009.
χ21=3.3, P=0.07.
Discussion
HIV vertical transmission rates rose gradually from 1984 to 1994 and then fell rapidly between 1995 and 1998. The earlier rise could be accounted for by changes in the epidemiology of HIV in infected women.5
In the four years ending 1998, during which uptake of elective caesarean section and antiretroviral treatment was increasing, the estimated vertical transmission rate fell steeply to 2.2% (0% to 7.8%) in 1998. This is comparable with a 2.0% estimate based on a meta-analysis,2 and 0.8% (1/133) in the French cohort4 among women taking antiretroviral treatment (mainly zidovudine only) and having an elective caesarean section. These findings are consistent with previously published data.10
Progression to AIDS (70% of which was P carinii pneumonia) within the first 6 months of life was reduced by nearly 60% in infants born after the beginning of 1994 compared with those born before. The effect was not significant, but a similar reduction of 66% in AIDS in infancy since 1993 was recently reported in the United States.11 This change is likely to be due to prophylactic co-trimoxazole given to most children born to infected women from 6 weeks of age.7,8
Prospective cohort studies tend to be set in specialist centres, whereas randomised trials may use exclusion criteria. National surveillance data have the advantage of being population based but are more likely to suffer from missing data and less frequent follow up. However, as long as bias caused by children of indeterminate status is removed, surveillance data should accurately reflect vertical transmission rates in clinical practice. Trials may, in any case, become increasingly difficult to carry out. Antiretroviral treatment is becoming increasingly diverse, more women will have received antiretroviral treatment before pregnancy, and the transmission rates now being reported are very low.12–15 If the possibility of teratogenic effects becomes a major concern,16 compliance with treatment regimens may fluctuate. Surveillance data may eventually become the most reliable way to monitor uptake of interventions and HIV vertical transmission rates.
Supplementary Material
Figure.
Year on year estimates of vertical transmission rates and 95% confidence intervals for HIV among children born to mothers known to be infected with HIV and who did not breast feed
Acknowledgments
We thank obstetric and paediatric respondents to the surveillance schemes of the Royal College of Obstetricians and Gynaecologists and the British paediatric surveillance unit of the Royal College of Paediatrics and Child Health, as well as colleagues at the Communicable Disease Surveillance Centre, Colindale, and the Scottish Centre for Infection Environment and Health, Glasgow. We also thank David Dunn for help in developing the statistical methods.
Editorial by Nicoll
Footnotes
Funding: TD, PAT, and JM were supported by grants from AVERT (Aids Education and Research Trust) and the Department of Health.
Competing interests: None declared.
website extra: A longer version of this paper is available on the BMJ's website www.bmj.com
References
- 1.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.International Perinatal HIV Group. Mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1. A meta-analysis of fifteen prospective cohort studies. N Engl J Med. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 3.European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 4.Mandelbrot L, Le Chenadec J, Berrebi A, Bongain A, Benifla JL, Delfraissy JF, et al. Perinatal HIV-1 transmission. Interaction between zidovudine prophylaxis and mode of delivery in the French perinatal cohort. JAMA. 1998;280:55–60. doi: 10.1001/jama.280.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Nicoll A, McGarrigle C, Brady T, Ades AE, Tookey PA, Duong T, et al. Epidemiology and detection of HIV-1 among pregnant women in the United Kingdom: results from national surveillance 1988-1996. BMJ. 1998;316:253–258. doi: 10.1136/bmj.316.7127.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn DT, Ades AE. Estimating the HIV vertical transmission rate and the pediatric AIDS incubation period from prospective data. J Am Stat Assoc. 1997;91:935–943. [Google Scholar]
- 7.Gibb DM, Walters S. Guidelines for management of children with HIV infection. Horsham: AVERT; 1993. pp. 1–29. [Google Scholar]
- 8.US Public Health Service recommendations for human immunodeficiency virus counselling and voluntary testing for pregnant women. MMWR. 1995;44:1–14. [PubMed] [Google Scholar]
- 9.Efron B, Tibshirani J. Bootstrap measures for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 10.Kind C, Rudin C, Siegrist CA, Wyler CA, Biedermann K, Lauper U, et al. Prevention of vertical HIV transmission: additive protective effect of elective cesarean section and zidovudine prophylaxis. Swiss Neonatal HIV Study Group. AIDS. 1998;12:205–210. doi: 10.1097/00002030-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Lindegren ML, Byers B, Roger M, Wortley P, Fleming P. On the verge of elimination of perinatally acquired HIV/AIDS? Updated trends in the United States. 6th conference on retroviruses and opportunistic infections Chicago 1999; Abstract 228.
- 12.Fiscus S, Adimora AA, Schoenbach VJ, Wilfert C, Johnson VA. Can zidovudine monotherapy continue to reduce perinatal HIV transmission? The North Carolina experience 1993-1997. XII World AIDS Conference Geneva June 1998; Abstract 33162.
- 13.Ciria LM. Impact of zidovudine on perinatal HIV transmission in Mallorca Island, Spain. XII World AIDS Conference Geneva June 1998; Abstract 23314.
- 14.Galvaao NO, Silva CL, Naud PSV,Chaves EBM, Zachia SA, Larangeira M, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine (ZDV) treatment. XII World AIDS Conference Geneva June 1998; Abstract 12155.
- 15.Rakusan TA, Temple V, Hart L, Loechelt B, Rana S, Young M, et al. Use of zidovudine to reduce the risk of perinatal transmission of HIV infection in the Washington metropolitan area. XII World AIDS Conference Geneva June 1998; Abstract 12157.
- 16.Lorenzi P, Spicher VM, Laubereau B, Hirschel B, Kind C, Rudin C, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. AIDS. 1998;12:F241–F247. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.