Abstract
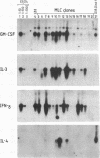
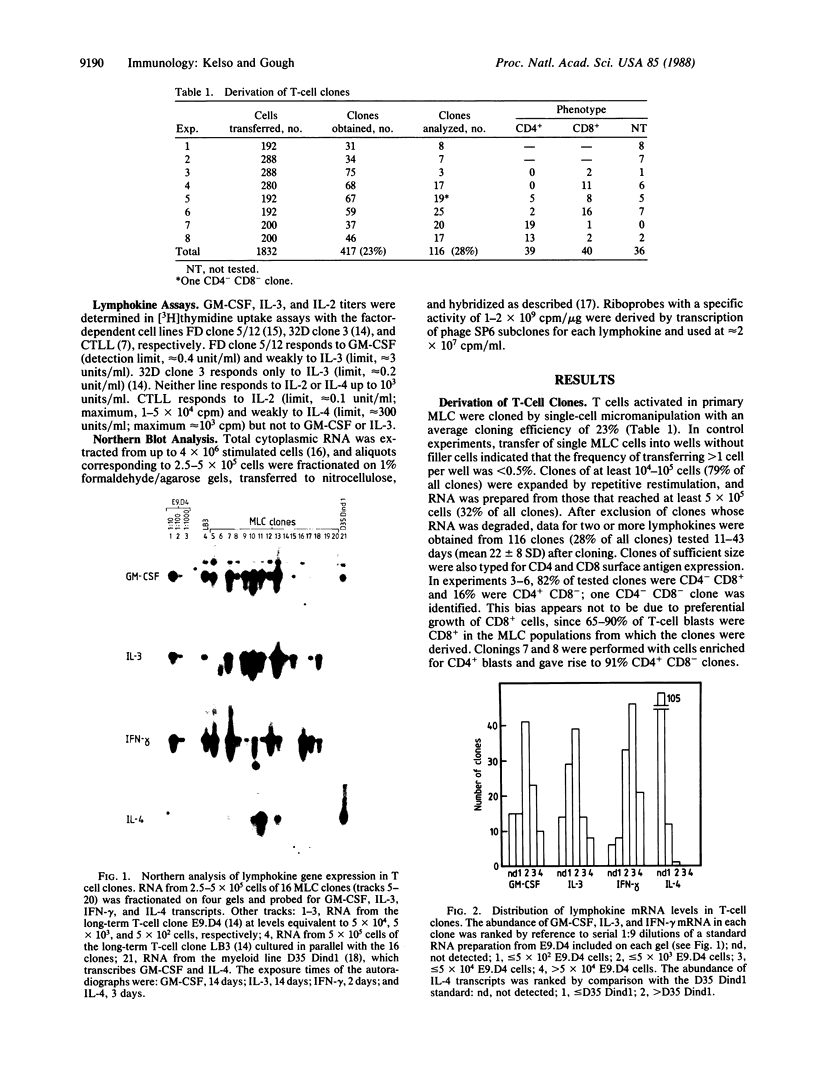
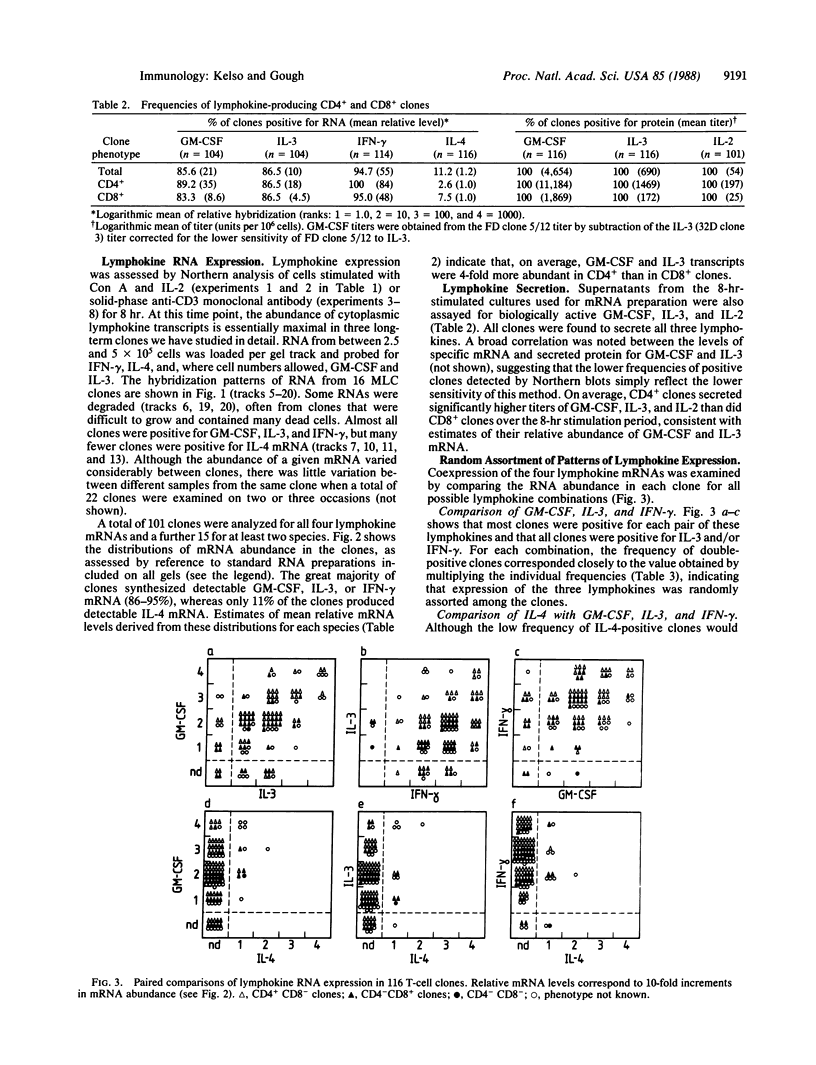
Lymphokine gene expression was examined in a panel of 116 short-term murine T-lymphocyte clones derived by single-cell micromanipulation from allogeneic mixed leukocyte cultures. About 30% of clonable T cells, including both CD4+ CD8- and CD4- CD8+ cells, could be expanded for assay at an average of 22 days after cloning. By RNA blot-hybridization analysis, most clones (85-96%) expressed detectable granulocyte-macrophage colony-stimulating factor, interleukin 3, and gamma interferon mRNAs, and 11% expressed interleukin 4 mRNA. Although no differences were noted between CD4+ and CD8+ clones in the combinations of lymphokines produced, CD4+ clones on average transcribed and secreted higher levels. When the frequencies of coexpression of any pair of lymphokine mRNAs were determined, all were found to correspond to the values predicted for random assortment of the individual frequencies. For example, among 13 interleukin 4-positive clones, 11 also transcribed gamma interferon, giving the frequency of double-positive clones expected for random association (9.6% versus 10.8%). Therefore, expression of the four lymphokine genes segregated independently among the clones and did not allow the division of T cells into subsets with distinct patterns of lymphokine synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dührsen U. In vitro growth patterns and autocrine production of hemopoietic colony stimulating factors: analysis of leukemic populations arising in irradiated mice from cells of an injected factor-dependent continuous cell line. Leukemia. 1988 Jun;2(6):334–342. [PubMed] [Google Scholar]
- Gearing D. P., Gough N. M., King J. A., Hilton D. J., Nicola N. A., Simpson R. J., Nice E. C., Kelso A., Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987 Dec 20;6(13):3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Piguet P. F., Vassalli P. Production of interleukin 2, interleukin 3, and interferon by mouse T lymphocyte clones of Lyt-2+ and -2- phenotype. J Immunol. 1984 Apr;132(4):1869–1871. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Carding S., Jones B., Murray J., Portoles P., Rasmussen R., Rojo J., Saizawa K., West J., Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988 Jan;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L. Secretion of interleukin 2, macrophage-activating factor, interferon, and colony-stimulating factor by alloreactive T lymphocyte clones. J Immunol. 1984 Jun;132(6):2924–2931. [PubMed] [Google Scholar]
- Kelso A., Metcalf D. Clonal heterogeneity in colony stimulating factor production by murine T lymphocytes. J Cell Physiol. 1985 Apr;123(1):101–110. doi: 10.1002/jcp.1041230115. [DOI] [PubMed] [Google Scholar]
- Kelso A., Owens T. Production of two hemopoietic growth factors is differentially regulated in single T lymphocytes activated with an anti-T cell receptor antibody. J Immunol. 1988 Feb 15;140(4):1159–1167. [PubMed] [Google Scholar]
- Killar L., MacDonald G., West J., Woods A., Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL 4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol. 1987 Mar 15;138(6):1674–1679. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Strath M., Warren D. J., O'Garra A., Kirkwood T. B. The production of lymphokines by primary alloreactive T-cell clones: a co-ordinate analysis of 233 clones in seven lymphokine assays. Immunology. 1985 Dec;56(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Funa K., Zalcberg-Quintana I., Xanthopoulos K. G., Kisielow P., Palacios R. Analysis by in situ hybridization of cells expressing mRNA for interleukin 4 in the developing thymus and in peripheral lymphocytes from mice. Proc Natl Acad Sci U S A. 1988 Jan;85(1):218–221. doi: 10.1073/pnas.85.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Löliger C., Kawai M., Suciu S., Gough N., Ostertag W. Identification of genes involved in growth autonomy of hematopoietic cells by analysis of factor-independent mutants. Cell. 1988 Jun 17;53(6):869–879. doi: 10.1016/s0092-8674(88)90329-7. [DOI] [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Dutton R. W., Tonkonogy S. L., English M. The role of IL4 and IL5: characterization of a distinct helper T cell subset that makes IL4 and IL5 (Th2) and requires priming before induction of lymphokine secretion. Immunol Rev. 1988 Feb;102:77–105. doi: 10.1111/j.1600-065x.1988.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Jabara H. H., DeKruyff R. H., Abbas A. K., Abrams J. S., Geha R. S. Functional heterogeneity among human inducer T cell clones. J Immunol. 1988 Jun 15;140(12):4211–4216. [PubMed] [Google Scholar]
- Yokota T., Arai N., de Vries J., Spits H., Banchereau J., Zlotnik A., Rennick D., Howard M., Takebe Y., Miyatake S. Molecular biology of interleukin 4 and interleukin 5 genes and biology of their products that stimulate B cells, T cells and hemopoietic cells. Immunol Rev. 1988 Feb;102:137–187. doi: 10.1111/j.1600-065x.1988.tb00744.x. [DOI] [PubMed] [Google Scholar]