Abstract
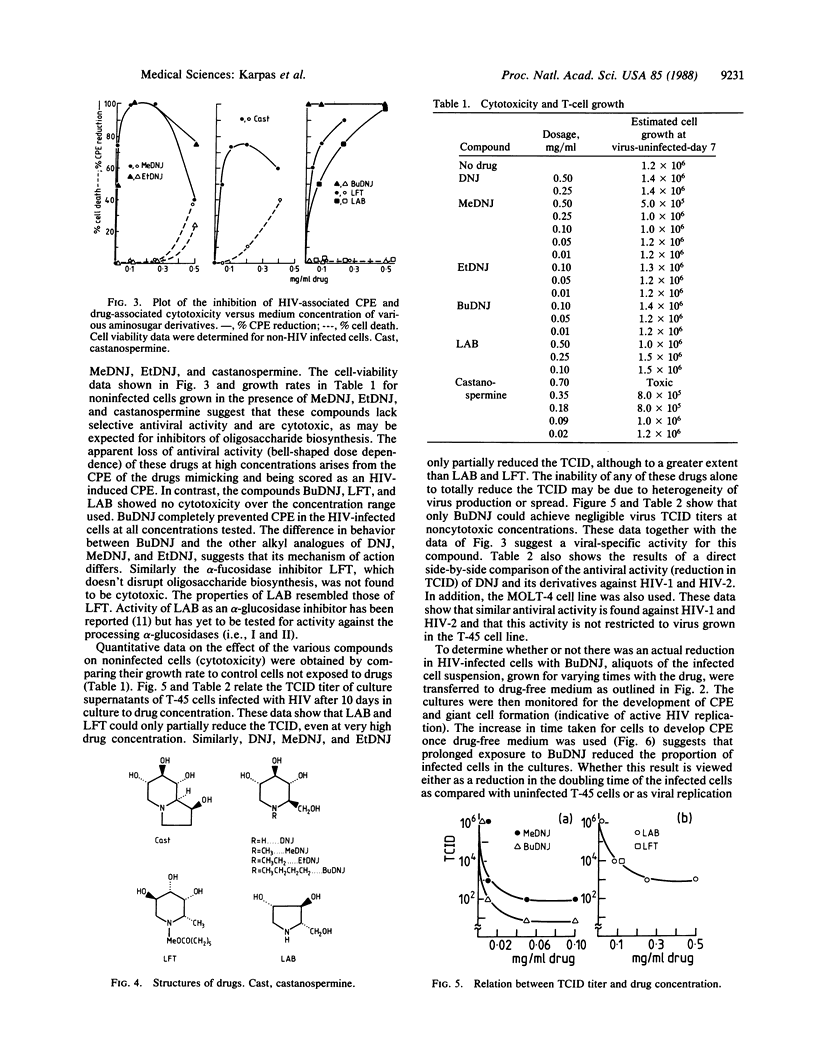
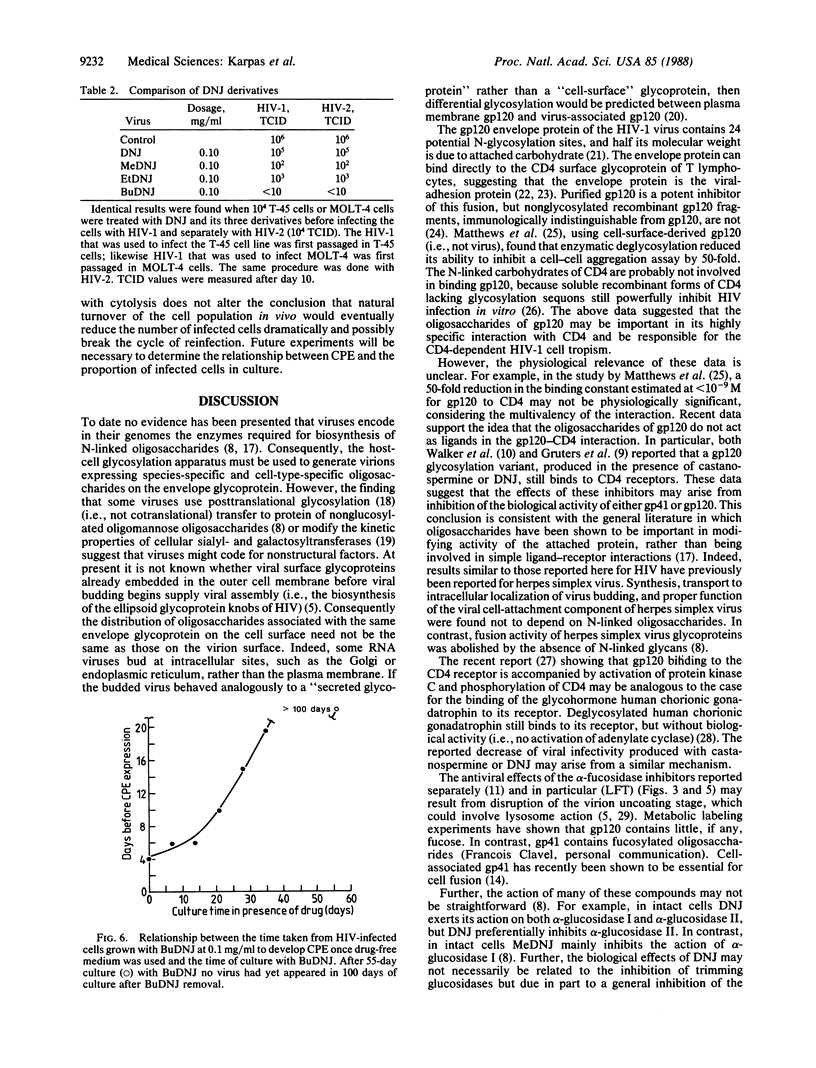
Recent data suggest that aminosugar derivatives which inhibit glycoprotein processing have potential anti-human immunodeficiency virus (HIV) activity. These inhibitory effects may be due to disruption of cell fusion and subsequent cell-cell transmission of the acquired immunodeficiency syndrome (AIDS) virus. Free virus particles able to bind CD4-positive cells are still produced in the presence of these compounds with only partial reduction of infectivity. We now report a method to score in parallel both the degree of antiviral activity and the effect on cell division of aminosugar derivatives. We find that (i) the compounds 1,4-dideoxy-1,4-imino-L-arabinitol and N-(5-carboxymethyl-1-pentyl)-1,5-imino-L-fucitol partially inhibit the cytopathic effect (giant cell formation, etc.) of HIV and yield of infectious virus; (ii) the compounds N-methyldeoxynojirimycin and N-ethyldeoxynojirimycin reduce the yield of infectious HIV by an order of four and three logarithms, respectively; and (iii) one compound, N-butyldeoxynojirimycin, of the 47 compounds previously screened reduces infectious viral particles by a logarithmic order greater than five at noncytotoxic concentrations. In addition, long-term growth of infected cells in the presence of N-butyldeoxynojirimycin gradually decreases the proportion of infected cells, leading to eventual elimination of HIV from culture. This result suggests that replication is associated with cytolysis. The ability to break the cycle of replication and reinfection has important implications in the chemotherapy of AIDS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Calvo F. O., Ryan R. J. Inhibition of adenylyl cyclase activity in rat corpora luteal tissue by glycopeptides of human chorionic gonadotropin and the alpha-subunit of human chorionic gonadotropin. Biochemistry. 1985 Apr 9;24(8):1953–1959. doi: 10.1021/bi00329a023. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Evidence for post-translational glycosylation of a nonglycosylated precursor protein of herpes simplex virus type 1. J Virol. 1984 Nov;52(2):630–637. doi: 10.1128/jvi.52.2.630-637.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Olofsson S., Romero P. A. Inhibitors of protein glycosylation and glycoprotein processing in viral systems. Pharmacol Ther. 1987;33(2-3):221–286. doi: 10.1016/0163-7258(87)90066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields A. P., Bednarik D. P., Hess A., May W. S. Human immunodeficiency virus induces phosphorylation of its cell surface receptor. Nature. 1988 May 19;333(6170):278–280. doi: 10.1038/333278a0. [DOI] [PubMed] [Google Scholar]
- Fleet G. W., Karpas A., Dwek R. A., Fellows L. E., Tyms A. S., Petursson S., Namgoong S. K., Ramsden N. G., Smith P. W., Son J. C. Inhibition of HIV replication by amino-sugar derivatives. FEBS Lett. 1988 Sep 12;237(1-2):128–132. doi: 10.1016/0014-5793(88)80185-6. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Guan J. L., Rose J. K. A membrane-anchored form but not the secretory form of human chorionic gonadotropin-alpha chain acquires polylactosaminoglycan. J Biol Chem. 1988 Apr 15;263(11):5314–5318. [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Karpas A., Gillson W., Bevan P. C., Oates J. K. Lytic infection by British AIDS virus and development of rapid cell test for antiviral antibodies. Lancet. 1985 Sep 28;2(8457):695–697. doi: 10.1016/s0140-6736(85)92934-4. [DOI] [PubMed] [Google Scholar]
- Karpas A. Unusual virus produced by cultured cells from a patient with AIDS. Mol Biol Med. 1983 Nov;1(4):457–459. [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Rivière Y., Dott K., Schmitt D., Girard M., Montagnier L., Lecocq J. Improved antigenicity of the HIV env protein by cleavage site removal. Protein Eng. 1988 Sep;2(3):219–225. doi: 10.1093/protein/2.3.219. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Leonard R., Zagury D., Desportes I., Bernard J., Zagury J. F., Gallo R. C. Cytopathic effect of human immunodeficiency virus in T4 cells is linked to the last stage of virus infection. Proc Natl Acad Sci U S A. 1988 May;85(10):3570–3574. doi: 10.1073/pnas.85.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Yoshima H., Kobata A. Carbohydrates of influenza virus hemagglutinin: structures of the whole neutral sugar chains. Biochemistry. 1983 Jan 4;22(1):188–196. doi: 10.1021/bi00270a028. [DOI] [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Merkle R. K., Elbein A. D., Heifetz A. The effect of swainsonine and castanospermine on the sulfation of the oligosaccharide chains of N-linked glycoproteins. J Biol Chem. 1985 Jan 25;260(2):1083–1089. [PubMed] [Google Scholar]
- Olofsson S., Khanna B., Lycke E. Altered kinetic properties of sialyl and galactosyl transferases associated with herpes simplex virus infection of GMK and BHK cells. J Gen Virol. 1980 Mar;47(1):1–9. doi: 10.1099/0022-1317-47-1-1. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Friedlander P., Herscovics A. Deoxynojirimycin inhibits the formation of Glc3Man9GlcNAc2-PP-dolichol in intestinal epithelial cells in culture. FEBS Lett. 1985 Apr 8;183(1):29–32. doi: 10.1016/0014-5793(85)80947-9. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Saunier B., Herscovics A. Comparison between 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin as inhibitors of oligosaccharide processing in intestinal epithelial cells. Biochem J. 1985 Mar 15;226(3):733–740. doi: 10.1042/bj2260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Schwarz P. M., Elbein A. D. The effect of glycoprotein-processing inhibitors on fucosylation of glycoproteins. J Biol Chem. 1985 Nov 25;260(27):14452–14458. [PubMed] [Google Scholar]
- Schweden J., Borgmann C., Legler G., Bause E. Characterization of calf liver glucosidase I and its inhibition by basic sugar analogs. Arch Biochem Biophys. 1986 Jul;248(1):335–340. doi: 10.1016/0003-9861(86)90429-7. [DOI] [PubMed] [Google Scholar]
- Scofield A. M., Fellows L. E., Nash R. J., Fleet G. W. Inhibition of mammalian digestive disaccharidases by polyhydroxy alkaloids. Life Sci. 1986 Aug 18;39(7):645–650. doi: 10.1016/0024-3205(86)90046-9. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Lüke W., Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988 Jan 7;331(6151):84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- Tyms A. S., Berrie E. M., Ryder T. A., Nash R. J., Hegarty M. P., Taylor D. L., Mobberley M. A., Davis J. M., Bell E. A., Jeffries D. J. Castanospermine and other plant alkaloid inhibitors of glucosidase activity block the growth of HIV. Lancet. 1987 Oct 31;2(8566):1025–1026. doi: 10.1016/s0140-6736(87)92588-8. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]


