Abstract
Mucosal surfaces of the body serve as the major portal of entry for human immunodeficiency virus (HIV). These tissues also house a majority of the body’s lymphocytes, including the CD4+ T-cells that are the major cellular target for HIV infection. Mucosal surfaces are defended by innate and adaptive immune mechanisms, including secreted antibodies and CD8+ cytotoxic T-cells (CTL). CTL in mucosal lymphoid tissues may serve to limit viral replication, decreasing the host’s viral burden as well as reducing the likelihood of sexual transmission to a naive host. This review summarizes recent literature on HIV-specific T-cell responses in mucosal tissues, with an emphasis on the gastrointestinal tract.
Keywords: CTL, gut, cytokine, Treg
Introduction
The genetic and immunologic correlates of protection from human immunodeficiency virus (HIV) infection and/or disease progression remain incompletely understood despite many years of intensive study. Most studies of HIV-specific immunity have focused primarily on peripheral blood, which is readily accessible for sampling. Nevertheless, the majority of the body’s T- and B-lymphocytes are housed in mucosal tissues lining the gastrointestinal tract [1]. These tissues also serve as a major site of HIV transmission, viral replication and CD4+ T-cell depletion. Because of the unique role of mucosal tissues in HIV transmission and pathogenesis, detailed studies of antiviral immune responses in these tissues can contribute important insights to our understanding of HIV pathogenesis. This review will provide an overview of recent literature on mucosal T-cell responses, with a major focus on CD8+ T-cells in HIV/SIV infection.
The gastrointestinal mucosa: inductive and effector sites
The gastrointestinal tract is thought of as the largest lymphoid organ in the body, with an estimated surface area 200 times that of skin [1]. The intestinal mucosa performs an important barrier function that permits peaceful coexistence with commensal flora while defending against potential pathogens [2]. Inductive sites for gut mucosal immunity include Peyer’s patches and lymphoid aggregates, where antigen is taken up and redirected towards priming of T and B-cell responses. Effector sites in the gut include the lamina propria and inter-epithelial lymphocytes (IEL) located at the basolateral surface of epithelial cells. Effector CD4+ T-cells are abundant in the lamina propria, while IEL are predominantly CD8+ T-cells [1, 3, 4].
Mucosal CD8+ T-cells differ from their counterparts in blood by increased expression of the IEL integrin CD103 (αEβ7), certain activation markers (CD69), chemokine receptors (CXCR4, CCR5), and a predominant effector memory phenotype (CD45RO+, CCR7−) [5–8]. Exposure to TGF-β in vitro can induce expression of CD103 and CD69 in PBMC [5], suggesting that locally-secreted cytokines may alter T-cell phenotype.
Recent studies in mice have led to a new appreciation for the role of mucosal dendritic cells in “imprinting” of newly primed lymphocytes to express trafficking molecules that will direct their eventual return to mucosal effector sites. Unlike most APCs in peripheral tissues, intestinal dendritic cells express enzymes critical for retinoic acid biosynthesis [9]. Retinoic acid induces the expression of integrin α4β7 on T and B-cells [10–13] (reviewed in [14]). Thus, lymphocytes primed in mucosal inductive sites receive an instructive signal directing them to express surface antigens that facilitate trafficking to mucosal effector sites. The ligand for α4β7 integrin, mucosal addressin cell adhesion molecule type 1 (MADCAM-1), is expressed on high endothelial venules in the gut mucosa. Engagement of MADCAM-1 by α4β7 facilitates T-cell extravasation from the bloodstream into mucosal tissues. Several chemokine receptors and their ligands also play a role in attracting lymphocytes to intestinal mucosa: CCR6/CCL20 [15], CCR9/TECK (CCL25) [16], and CCR10/MEC (CCL28) [17, 18]. Intriguingly, the activated α4β7 heterodimer can also bind HIV gp120 on infected CD4+ T-cells. This interaction then triggers activation of αLβ2 integrin (LFA-1) on the CD4+ T-cell surface, facilitating cell-cell interactions. Thus, activated α4β7 expressed by infected CD4 cells may increase cell-to-cell dissemination of HIV [19].
Acute HIV/SIV infection and the gut
As early as 1994, clinicians studying HIV-related diarrhea and wasting syndrome reported abnormalities in intestinal T-cell subsets in HIV-infected individuals [20, 21]. Studies of rhesus macaques infected with simian immunodeficiency virus (SIVmac) later revealed that acute infection results in rapid and profound CD4+ T-cell depletion, regardless of the route of transmission [22–24]. A recent resurgence of interest in this area has led to several detailed studies of acute HIV/SIV and the gastrointestinal tract, which have confirmed the rapid depletion of lamina propria CD4+ T-cells [24, 25]. This depletion may be mediated by direct infection [26], immune-mediated clearance of infected cells, bystander apoptosis [27], or a combination of mechanisms. During acute HIV/SIV infection, as lamina propria CD4+ T-cells are depleted, there is an expansion and/or influx of CD8+ T-cells [28] (Figure 1); however, these cells fail to clear infection or prevent widespread virus dissemination. This rapid depletion of gut CD4+ T-cells, and their slow reconstitution in patients on HAART, suggests that mucosal CD8+ T-cell responses may be inadequate, dysfunctional, and/or inhibited by inappropriate activity of regulatory T-cells (Treg).
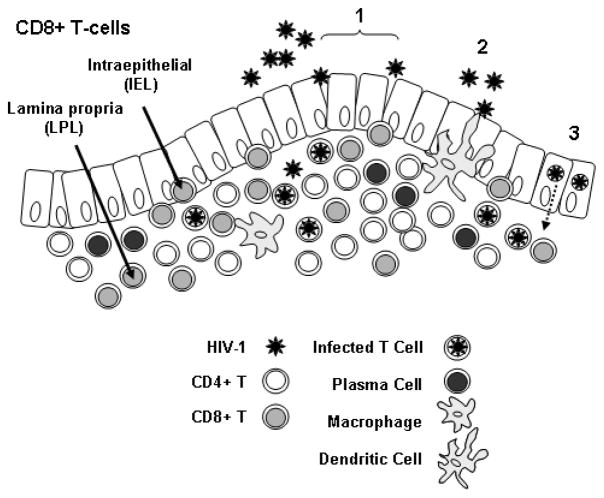
Figure 1. Mucosal CD8+ T-cells.
The figure shows an idealized rectal mucosa, with a single layer of columnar epithelium. HIV-1 may enter the mucosa through breaches in the epithelial layer (1); by binding to dendritic cell processes that extend through the epithelial layer to the lumen (2); via transcytosis across epithelial cells (3); or through a combination of mechanisms. CD8+ T-cells are present as intraepithelial cells (IEL) located between epithelial cells, and in the underlying layer as lamina propria lymphocytes (LPL). Also present in the lamina propria are CD4+ T-cells, macrophages, and antibody-producing plasma cells.
Are mucosal CD8+ T-cells “dysfunctional”?
Why are mucosal T-cells inefficient at clearing HIV/SIV infection? Despite expression of granzymes A and B, mucosal CD8+ T-cells express low levels of perforin as compared to CD8+ T-cells in PBMC [6, 29]; this is true of both healthy controls and individuals with chronic HIV infection and is therefore not directly linked to HIV. It is unclear whether this is due to the effects of the local ‘tolerogenic’ microenvironment; however, low perforin expression may restrict CTL cytotoxicity, limiting tissue damage and inflammation in the gut [6, 29]. Perforin expression is also typically low in CD8+ T-cells in other lymphoid sites such as lymph nodes and tonsil [30]. In contrast, perforin expression by gut T-cells is increased in inflammatory conditions such as Crohn’s disease [31], suggesting a link between high perforin expression and tissue damage.
Detailed kinetic studies of mucosal CD8+ T-cell function during acute HIV infection are lacking; however, in acute SIV infection, perforin is strongly expressed in gut by day 21 [29], consistent with the appearance of SIV-specific tetramer-binding CD8 T-cells in the colon at this time point [32]. Nevertheless, this perforin-positive CD8 T-cell response does not prevent the establishment and dissemination of SIV infection in the gut, and may be “too little and too late” [32]. In our studies of SIV infection, perforin expression (mRNA and protein) in the gut declined steadily after day 21 post-infection, and was indistinguishable from healthy control macaques by 180 days post-infection [29].
The PD-1/PDL-1 pathway may be important in regulating T-cell dysfunction during chronic viral infection [33–35]. In chronic HIV and SIV infection, mucosal T-cells appear to express higher levels of PD-1 than blood T-cells from the same individuals [36, 37]. This is consistent with the role of gut as a major site of HIV/SIV replication, but may also be related to the state of ‘partial activation’ typical of mucosal T-cells [5, 38].
Robust mucosal CD8+ T-cell responses in chronic HIV infection
Although the CD8+ T-cell response during acute infection is insufficient to eradicate infected cells, a robust and often polyfunctional CD8+ T-cell response continues to fight infection in the gut during the chronic phase. The persistence of virus in mucosal tissues throughout this phase of infection, including in patients on HAART [39], argues that the gut continues to be an important ‘battleground’ between the virus and the immune system.
Early studies of mucosal immunity to HIV and SIV relied upon 51Cr release assays to measure antigen-specific cytotoxic T cell activity [40–42]. These reports demonstrated that MHC class I restricted CTL were present in gastrointestinal mucosa of individuals with chronic HIV/SIV infection, but did not provide great detail about response specificity or breadth. In addition, these reports relied on mucosal cells that had been expanded and subjected to prolonged in vitro culture, so no conclusions could be drawn regarding the frequency of antigen-specific cells in vivo.
To address the frequency of HIV/SIV-specific CD8+ T-cells in mucosal tissues, several groups used MHC class I tetramer staining to quantify CD8+ T-cells specific for well-characterized immunodominant epitopes [7, 43]. These reports revealed that the frequency of Gag-specific CD8+ T-cells in both upper and lower GI tract during chronic infection was quite comparable to that observed in peripheral blood; at least, when immunodominant epitopes were studied. However, these studies looked only at tetramer binding and did not provide information on effector functions. Given that HIV/SIV-specific CD8+ T-cells in blood had been shown to be relatively ‘dysfunctional’ [44, 45], it was important to further explore the functions of mucosal CD8+ T-cells as well.
With the development of multiparameter cytokine flow cytometry (CFC), it became possible to assess the production of multiple cytokines, chemokines, and cytolytic granule constituents by antigen-specific T-cells in a single assay [46–48]. Importantly, CFC is performed on freshly isolated mucosal T-cells [49], without prior culture or in vitro expansion, so the results provide a reasonable estimate of the frequency and functionality of antigen-specific effector cells in a particular tissue. In a study of 28 patients with chronic HIV infection, we measured the production of IFN-γ, TNF-α, and release of the cytolytic granule constituent CD107 by CD8+ T-cells in blood and rectal mucosa in response to peptide stimulation [50]. In both blood and rectal mucosa, there was an immunodominant CD8+ IFN-γ response to Gag as compared to Pol and Env (P <0.01). In contrast, cytomegalovirus pp65 peptides elicited IFN-γ secretion strongly in blood but weakly in rectal CD8+ T cells (P = 0.015). Upon stimulation with HIV peptides, CD8+ T cells from both sites were capable of mounting complex responses, and in rectal mucosa CD107 release was frequently coupled with production of IFN-γ or TNF-α [50]. Thus, mucosal CD8+ T-cells can actively degranulate despite low levels of perforin expression. In a recent follow-up study, rectal HIV-specific CD8+ T-cells were also found to express MIP-1β and low levels of IL-2 [51, 52]. Expression of other chemokines or factors capable of inhibiting HIV replication by mucosal CD8+ T-cells has not been reported, nor has cell contact-dependent, non-cytolytic suppression been explored [53, 54]; these areas await further study. Taken together, these findings demonstrate that rectal CD8+ T cells are capable of robust and varied HIV-1-specific effector responses during chronic infection.
Mucosal T-cell responses are broad and largely shared with blood
An important question is the extent to which mucosal and blood T-cell populations overlap in terms of specificity and clonality. Musey and colleagues compared the TCR sequence and clonality of HIV-specific CTL that had been expanded from gastrointestinal mucosa, semen and cervix [55]. Their findings revealed that the majority of such clones were shared between mucosal sites and PBMC [55].
Due to the difficulty of obtaining large numbers of lymphocytes from mucosal biopsy tissue, comprehensive mapping of the fine specificity of mucosal responses has been challenging. However, relying on a polyclonal expansion approach, Ibarrondo and colleagues successfully mapped rectal HIV-specific CD8+ T cell responses to pools of overlapping peptides spanning the entire HIV genome [56]. These authors found a very similar pattern of responses in the two compartments, as well as a similar immunodominance hierarchy in terms of peptide pools; however, they did not map responses to specific epitopes. Subsequently, Lemongello et al. mapped responses to specific HIV peptides in Gag, Env and Nef [57]. This study reported a similar pattern of peptide recognition in both compartments, and determined that immunodominant peptide-specific responses were conserved between tissue sites [57]. Taken together, these studies reveal significant overlap in the specificity and clonality of CD8+ T cells isolated from mucosal tissues and blood of HIV-infected individuals.
Mucosal immunity in “long-term non-progressors” and “HIV controllers”
Crucial to our understanding of HIV pathogenesis is the study of individuals who remain healthy in the absence of antiretroviral therapy. Individuals who maintain normal CD4+ T-cell counts in the absence of therapy have been described as “long-term non-progressors”. Since many factors may contribute to limiting disease progression, investigators have increasingly defined HIV controllers based on plasma HIV RNA levels rather than on CD4 counts or survival time alone [58, 59]. Two distinct groups of HIV controllers have been described based on plasma viral load measurements: those who maintain HIV RNA below detectable limits (i.e., < 50 copies/mL), designated as “elite controllers”, and those with persistently detectable yet low plasma HIV RNA (i.e., 50–2,000 copies/mL), designated as “viremic controllers” [58]. Elite controllers are believed to represent fewer than 1% of the HIV infected population [58].
Although there have been isolated reports of strong mucosal CD8+ T-cell responses in LTNP and SIV/HIV controllers [50, 60], few studies have addressed these responses in detail. In one report, focused on gene expression analysis, differential expression of 34 genes associated with immune function was seen in jejunal biopsy tissue from 4 LTNP as compared to 11 patients with viral load >10,000 and progressive disease [61]. HIV RNA viral load in jejunal biopsies was low to undetectable in 3 of 3 LTNP, and mucosal Gag-specific CD8 T-cell responses were stronger in 3 LTNP than in 1 progressing individual [61].
Recently, we examined HIV Gag-specific CD8+ T-cell responses in blood and rectal mucosa of over 20 HIV controllers as compared to 14 non-controllers (VL >10,000 copies/vRNA/mL) and 10 antiretroviral treated individuals (VL <75 copies vRNA/mL) [62, 63]. Our hypothesis was that ‘polyfunctional’ T cells capable of producing multiple antiviral factors would be most abundant in mucosal tissues of HIV controllers. Using a 9-color flow cytometry panel measuring IFN-γ, TNF-α, IL-2, MIP-1β, and CD107, we found that mucosal responses were significantly stronger and more complex in controllers than in HAART-suppressed individuals (P=0.0004). Several controllers had unusually strong and complex mucosal CD8+ T-cell responses that were not mirrored in peripheral blood; these responses would have been overlooked if rectal mucosa had not been sampled (Figure 2). Furthermore, the frequency of 4-function HIV-specific CD8+ T-cells in rectal mucosa was significantly greater in controllers than non-controllers or patients on HAART (P<0.0001) [62, 63]. Many of the most robust mucosal responses were identified in subjects who had MHC class I alleles associated with non-progression (i.e., HLA-B57) [64–66]. The basis for HIV non-progression may be related to virologic, immunologic and/or genetic factors; nevertheless, these findings imply that mucosal T-cell responses play an important role in immune surveillance of gut mucosa.
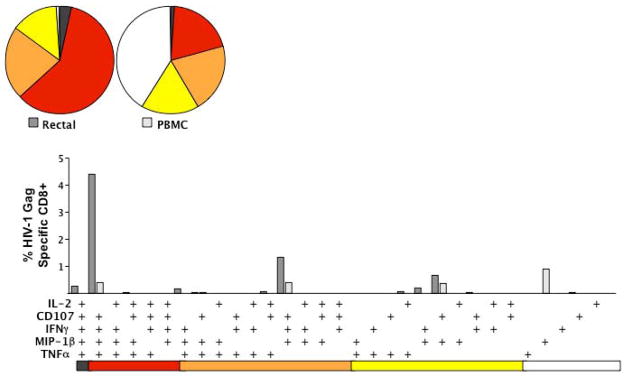
Figure 2. Nine-color, 5-function analysis of HIV Gag-specific T-cell responses in rectal mucosa.
The figure summarizes data for one patient, an HIV controller whose Gag-specific CD8+ T-cell response is substantially stronger (in terms of percent responding cells) and more polyfunctional in rectal mucosa than in blood. Bar graphs show the magnitude of the Gag-specific response in each of 31 functional categories. The legend shows the combination of functions evaluated in each category. These combinations are color-coded based on the number of functions and correspond to wedges in the pie charts (5-function responses are black, 4-function red, 3-function orange, 2-function yellow, and single-function white). The rectal Gag-specific CD8+ T-cell response (white bars) of this HIV controller was heavily biased towards polyfunctional cells secreting CD107, MIP-1β, TNF-α and IFN-γ. In contrast, the Gag-specific response in PBMC was largely monofunctional. Data for over 20 HIV controllers, compared to non-controllers and patients on HAART, are reported elsewhere [62, 63].
The question also arises as to the role of mucosal CD8+ T-cell responses in “highly exposed, persistently seronegative” (HEPS) cohorts. HIV-specific CD8+ T-cells have been detected in cervicovaginal mucosa of highly-exposed sex workers [67], and SIV-specific CD8+ T-cells have been reported in the vaginal mucosa of exposed, uninfected rhesus macaques [68]. However, to our knowledge there have been no published reports documenting HIV-specific CD8+ T-cells in gastrointestinal mucosa of HEPS individuals, or of macaques repeatedly exposed to SIV via the rectal route [69].
Do Treg modulate mucosal adaptive responses?
Given the role of immune activation in HIV/SIV pathogenesis, recent attention has focused on the balance between tolerogenic and pro-inflammatory T-cell responses in the intestinal mucosa. In the mouse, a tolerogenic cytokine environment dominated by retinoic acid and TGF-β induces differentiation of regulatory T-cells expressing the transcription factor Foxp3 [70, 71]. However, when both TGF-β and IL-6 are present, the cytokine balance favors induction of Th17-type responses [71, 72].
The role of Treg in HIV/SIV pathogenesis remains controversial; these cells may reduce immune activation, but may also limit HIV-specific adaptive responses [73]. Treg have been phenotypically identified in blood and mucosal tissues of HIV-infected humans [74, 75] and SIV-infected macaques [76, 77] by their expression of Foxp3, CTLA-4 and CD25. In acute SIV infection, Treg are reportedly depleted from ileal mucosa [78], but expanded in lymph nodes [76]. In chronic SIV infection, FoxP3 and CTLA-4 mRNA are increased in mucosal tissues of macaques with high viremia, and FoxP3 mRNA is positively correlated with SIV RNA levels in tissues [79]. However, to date very few studies have addressed the presence of Treg in mucosal tissues of HIV-infected humans [74, 75]. Assessing the functions of tissue Treg in humans is complicated by the difficulty of obtaining sufficient numbers of cells from biopsy samples. Thus, it remains to be formally demonstrated that mucosal Treg can actively limit HIV/SIV-specific CD8+ T-cell responses in tissues.
Conclusions
Given the recent failures of vaccine and microbicide trials, there is renewed interest in elucidating the role of mucosal immunity in HIV transmission and pathogenesis. The study of novel patient cohorts such as HIV controllers and exposed, seronegative individuals may shed light on this issue. Many HIV controllers have unusually robust, polyfunctional HIV-specific T-cell responses in mucosal tissues. Nevertheless, strong CD8+ T-cell responses, whether in mucosal tissues or blood, clearly do not account for all cases of HIV containment, suggesting that multiple mechanisms contribute to the controller phenotype. Additional studies will be required to address such issues as: (a) the role of Treg in modulating mucosal T-cell responses; (b) the role of innate mucosal effector cells in antiviral immunity, and (c) fully characterizing the immunologic, virologic and genetic correlates of protection in HIV controllers.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (NIH/NIAID R01 AI-057020), the California HIV/AIDS Research Program (CHRP, grant CH05-D-606), and the Pendleton Charitable Trust.
References
- 1.Mowat A, Viney J. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–66. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–64. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P, Pabst R. Let’s go mucosal: communication on slippery ground. Trends Immunol. 2004;25:570–7. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 5.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–83. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 6.Shacklett BL, Cox CA, Quigley MF, et al. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J Immunol. 2004;173:641–8. doi: 10.4049/jimmunol.173.1.641. [DOI] [PubMed] [Google Scholar]
- 7.Shacklett BL, Cox CA, Sandberg JK, Jacobson MA, Stollman NH, Nixon DF. Trafficking of HIV-1-specific CD8+ T-cells to gut-associated lymphoid tissue (GALT) during chronic infection. J Virol. 2003;77:5621–31. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 9.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 11.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–9. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora JR, Von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunology. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 15.Kondo T, Takata H, Takiguchi M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur J Immunol. 2007;37:54–65. doi: 10.1002/eji.200636251. [DOI] [PubMed] [Google Scholar]
- 16.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–8. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 18.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 19.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–9. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 20.Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37:524–9. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider T, Ullrich R, Bergs C, Schmidt W, Riecken EO, Zeitz M. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clin Exp Immunol. 1994;95:430–5. doi: 10.1111/j.1365-2249.1994.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattapallil JJ, Smit-McBride Z, McChesney M, Dandekar S. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1beta expression and display antiviral cytotoxic activity despite severe CD4(+) T-cell depletion in primary simian immunodeficiency virus infection. J Virol. 1998;72:6421–9. doi: 10.1128/jvi.72.8.6421-6429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–56. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veazey R, DeMaria M, Chalifoux L, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 25.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 26.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 28.Veazey RS, Gauduin MC, Mansfield KG, et al. Emergence and kinetics of simian immunodeficiency virus-specific CD8(+) T cells in the intestines of macaques during primary infection. J Virol. 2001;75:10515–9. doi: 10.1128/JVI.75.21.10515-10519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quigley MF, Abel K, Zuber B, Miller CJ, Sandberg JK, Shacklett BL. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. J Virol. 2006;80:3083–7. doi: 10.1128/JVI.80.6.3083-3087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson J, Behbahani H, Lieberman J, et al. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS. 1999;13:1295–303. doi: 10.1097/00002030-199907300-00005. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald TT, Di Sabatino A, Gordon JN. Immunopathogenesis of Crohn’s disease. JPEN J Parenter Enteral Nutr. 2005;29:S118–24. doi: 10.1177/01486071050290S4S118. discussion S24–5, S84–8. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds MR, Rakasz E, Skinner PJ, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–35. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 34.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 36.Hayes TL, Critchfield JW, Young DH, Shacklett BL. Effect of antiretroviral therapy on mucosal T-cells in HIV-infected patients. 13th International Congress of Mucosal Immunology; Tokyo, Japan: Society for Mucosal Immunology; 2007. [Google Scholar]
- 37.Velu V, Kannanganat S, Ibegbu C, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–28. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang EC, Borysiewicz LK. The role of CD8+, CD57+ cells in human cytomegalovirus and other viral infections. Scand J Infect Dis Suppl. 1995;99:69–77. [PubMed] [Google Scholar]
- 39.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J Infect Dis. 2008 doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 40.Shacklett BL, Beadle TJ, Pacheco PA, et al. Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology. 2000;270:317–27. doi: 10.1006/viro.2000.0299. [DOI] [PubMed] [Google Scholar]
- 41.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couedel-Courteille A, Le Grand R, Tulliez M, Guillet J, Venet A. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–7. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz JE, Veazey RS, Kuroda MJ, et al. Simian immunodeficiency virus (SIV)-specific cytotoxic T lymphocytes in gastrointestinal tissues of chronically SIV-infected rhesus monkeys. Blood. 2001;98:3757–61. doi: 10.1182/blood.v98.13.3757. [DOI] [PubMed] [Google Scholar]
- 44.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8+ T-cells produce antiviral cytokines but are impaired in cytolytic functions. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–101. [PubMed] [Google Scholar]
- 46.Maecker HT, Rinfret A, D’Souza P, et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 48.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 49.Shacklett BL, Yang OO, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection and vaccination. Journal of Immunological Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 50.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL. Multifunctional HIVgag Specific CD8+ T-cell Responses in Rectal Mucosa and PBMC During Chronic HIV-1 Infection. J Virol. 2007 doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Critchfield JW, Young DH, Hayes TL, Braun J, Garcia JC, Pollard RB, Shacklett BL. In: Barouch DH, Mascola JR, McElrath MJ, editors. Functionality of rectal HIV-1-specific CD8+ T-cells during chronic infection correlates with clinical status; Keystone Symposium, HIV Vaccines: Progress and Prospects (X7); Alberta: Banff; 2008. Abstract 352. [Google Scholar]
- 52.Critchfield JW, Young DH, Hayes TL, Braun JV, Garcia JC, Pollard RB, Shacklett BL. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS ONE. 2008 doi: 10.1371/journal.pone.0003577. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–81. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stranford SA, Skurnick J, Louria D, et al. Lack of infection in HIV-exposed individuals is associated with a strong CD8(+) cell noncytotoxic anti-HIV response. Proc Natl Acad Sci U S A. 1999;96:1030–5. doi: 10.1073/pnas.96.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musey L, Ding Y, Cao J, et al. Ontogeny and specificity of mucosal and blood human immunodeficiency virus-1 specific CD8+ cytotoxic T lymphocytes. J Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibarrondo FJ, Anton PA, Fuerst M, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–97. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemongello D, Morris MM, Walker DH, Garcia JC, Pollard RB, Shacklett BL. In: Ruprecht R, Franchini G, Barnett SW, editors. Mapping of HIV Gag immunodominant epitopes: a comparison of mucosal and peripheral blood responses, Abstract 306; Keystone Symposium on HIV Vaccines; Colorado: Keystone; 2006. [Google Scholar]
- 58.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–16. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Emu B, Sinclair E, Hatano H, et al. HLA Class I-Restricted T Cell Responses May Contribute to the Control of HIV Infection, but Such Responses are Not Always Necessary for Long-term Virus Control. J Virol. 2008 doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ling B, Veazey RS, Hart M, Lackner AA, Kuroda M, Pahar B, Marx PA. Early restoration of mucosal CD4 memory CCR5 T cells in the gut of SIV-infected rhesus predicts long term non-progression. AIDS. 2007;21:2377–85. doi: 10.1097/QAD.0b013e3282f08b32. [DOI] [PubMed] [Google Scholar]
- 61.Sankaran S, Guadalupe M, Reay E, George MD, Flamm J, Prindiville T, Dandekar S. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A. 2005;102:9860–5. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferre AL, Critchfield JW, Hunt PW, et al. In: Barouch DH, Mascola JR, McElrath MJ, editors. Polyfunctional T-cells in the rectal mucosa of HIV controllers; Keystone Symposium, HIV Vaccines: Progress and Prospects (X7); Alberta: Banff; 2008. Abstract 143. [Google Scholar]
- 63.Ferre AL, Hunt PW, Critchfield JW, et al. Mucosal Immune Responses to HIV-1 in Elite Controllers: A Potential Correlate of Immune Control. 2008 doi: 10.1182/blood-2008-10-182709. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Vazquez A, Mina-Blanco A, Martinez-Borra J, et al. Interaction between KIR3DL1 and HLA-B*57 supertype alleles influences the progression of HIV-1 infection in a Zambian population. Hum Immunol. 2005;66:285–9. doi: 10.1016/j.humimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–14. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–51. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 67.Kaul R, Plummer FA, Kimani J, et al. HIV-1-Specific Mucosal CD8+ Lymphocyte Responses in the Cervix of HIV-1- Resistant Prostitutes in Nairobi. J Immunol. 2000;164:1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 68.McChesney MB, Collins JR, Lu D, et al. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4(+)-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J Virol. 1998;72:10029–35. doi: 10.1128/jvi.72.12.10029-10035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Letvin NL, Rao SS, Dang V, et al. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J Virol. 2007;81:12368–74. doi: 10.1128/JVI.00822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 72.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Chougnet CA, Shearer GM. Regulatory T cells (Treg) and HIV/AIDS: summary of the September 7–8, 2006 workshop. AIDS Res Hum Retroviruses. 2007;23:945–52. doi: 10.1089/aid.2006.0259. [DOI] [PubMed] [Google Scholar]
- 74.Epple HJ, Loddenkemper C, Kunkel D, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072–8. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 75.Andersson J, Boasso A, Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–7. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 76.Estes JD, Li Q, Reynolds MR, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–12. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 77.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–17. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chase AJ, Sedaghat AR, German JR, Gama L, Zink MC, Clements JE, Siliciano RF. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2007;81:12748–57. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boasso A, Vaccari M, Hryniewicz A, et al. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J Virol. 2007;81:11593–603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]