Abstract
Purpose
Pro-inflammatory environments in the brain have been implicated in the onset and progression of neurological disorders. In the present study, we investigate the hypothesis that brain irradiation induces regionally specific alterations in cytokine gene and protein expression.
Materials and methods
Four month old F344 × BN rats received either whole brain irradiation with a single dose of 10 Gy γ-rays or sham-irradiation, and were maintained for 4, 8, and 24 h following irradiation. The mRNA and protein expression levels of pro-inflammatory mediators were analysed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA), and immunofluorescence staining. To elucidate the molecular mechanisms of irradiation-induced brain inflammation, effects of irradiation on the DNA-binding activity of pro-inflammatory transcription factors were also examined.
Results
A significant and marked up-regulation of mRNA and protein expression of pro-inflammatory mediators, including tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and monocyte chemoattractant protein-1 (MCP-1), was observed in hippocampal and cortical regions isolated from irradiated brain. Cytokine expression was regionally specific since TNF-α levels were significantly elevated in cortex compared to hippocampus (57% greater) and IL-1β levels were elevated in hippocampus compared to cortical samples (126% greater). Increases in cytokine levels also were observed after irradiation of mouse BV-2 microglial cells. A series of electrophoretic mobility shift assays (EMSA) demonstrated that irradiation significantly increased activation of activator protein-1 (AP-1), nuclear factor-κB (NF-κB), and cAMP response element-binding protein (CREB).
Conclusion
The present study demonstrated that whole brain irradiation induces regionally specific pro-inflammatory environments through activation of AP-1, NF-κB, and CREB and overexpression of TNF-α, IL-1β, and MCP-1 in rat brain and may contribute to unique pathways for the radiation-induced impairments in tissue function.
Keywords: radiation, brain inflammation, cytokines, AP-1, NF-κB, CREB
Introduction
It has been proposed that the acute inflammatory responses triggered by overexpression of pro-inflammatory mediators may be responsible for radiation-induced normal tissue injury (Denham and Hauer-Jensen 2002). For example, a marked elevation of cyclooxygenase (COX-1 and COX-2) activity and prostaglandin E2 (PGE2) synthesis in the mouse brain following ionising radiation augments brain inflammation through up-regulation of gene expression of a variety of pro-inflammatory molecules (Kyrkanides et al. 2002, Moore et al. 2005). Evidence from previous in vitro and in vivo studies has demonstrated that radiation-induced overexpression of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), exerts a crucial role in leukocyte recruitment and infiltration that lead to subsequent inflammatory injuries to a variety of tissues including intestine and lung, as well as various cell types such as vascular endothelial cells (Behrends et al. 1994, Hallahan et al. 1996, Gaugler et al. 1997, Molla et al. 2001, Baluna et al. 2006, Molla and Panes 2007). Enhanced expression of adhesion molecules was also observed in irradiated brain (Hong et al. 1995, Olschowka et al. 1997, Gaber et al. 2003, Baluna et al. 2006) and may contribute to brain damage and/or subsequent cognitive impairment.
Irradiation also has been reported to up-regulate expression of pro-inflammatory cytokines and chemokines. For example, a rapid induction of gene expression of the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in response to radiation has been implicated in radiotherapy-associated damage to both lung and brain (Hong et al. 1995, Hong et al. 1999, Gaber et al. 2003, Han et al. 2006). It was also found that both total-body irradiation (TBI) and localised irradiation of the right hind leg led to a significant increase in interleukin-6 (IL-6) levels in serum of rats (Haveman et al. 1998). Furthermore, Johnston et al. (2002) suggested potential mechanisms of radiation-induced pulmonary fibrosis based on their findings that the mRNA levels of chemokines, including monocyte chemoattractant protein-1 (MCP-1), and chemokine receptor families were elevated in fibrosis-sensitive C57BL/6 mice by thoracic irradiation.
These data provide robust but only partial evidence indicating that inflammation is one of the major consequences of irradiation and has a pivotal role in subsequent radiation-induced normal tissue injury. Unfortunately, in the majority of the aforementioned studies, the cytokine analyses are limited to gene expression (mRNA levels) without analysis of the corresponding protein levels, the time-course for the effects of irradiation are limited or differential changes in expression of cytokines in specific brain regions are not considered. Importantly, radiation has been shown to impair performance on spatial memory tasks that are hippocampally-dependent and analysis of the potential specific effects of irradiation on this tissue requires a clear understanding of the consequences of radiation on this important brain region.
In the present study, we examined the effects of whole brain irradiation on mRNA and protein expression of several pro-inflammatory mediators, (e.g., TNF-α, IL-6, IL-1β, and MCP-1) in both hippocampus and cortex. The potential contribution of microglia to radiation-induced TNF-α expression was also determined in vitro. Additionally, the DNA-binding activity of pro-inflammatory transcription factors, such as activator protein-1 (AP-1), nuclear factor-κB (NF-κB), and cAMP response element-binding protein (CREB), was assessed to define the molecular mechanisms of radiation-induced brain inflammation. Our results provide a clear time course for the acute effects of radiation on both cytokine and chemokine gene and protein expression in hippocampus and cortex. In addition, our results provide evidence for a differential induction of cytokines in specific brain regions that have importance for the neurological/neuropathological consequences of irradiation.
Materials and methods
Animals
Four month old Fisher 344-Brown Norway (F344 × BN) male rats were purchased from Harlan Laboratories, Inc. (Indianapolis, IN, USA). Animals were housed on a 12/12 light-dark cycle with food and water provided ad libitum. Animal care was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and this study was approved by the Institutional Animal Care and Use Committee.
For real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), the brains were rapidly removed and two different brain regions (hippocampus and cortex) were dissected, immediately frozen in liquid nitrogen, and stored at −80°C until analysis. For immunofluorescence staining, animals were given 350 μl of ketamine/xylazine (80/12 mg/ml, respectively) immediately prior to perfusion. Animals were then transcardially perfused with ice-cold phosphate buffered saline (PBS) and 6 unit/ml heparin (a total of 700 μl per animal), and the whole brains were rapidly removed, immediately frozen in liquid nitrogen, and stored at −80°C until analysis.
Cell cultures
The murine microglial cell line, BV-2 cells, was a generous gift from Dr Michael E. Robbins (Wake Forest University Medical Center, Winston-Salem, NC, USA). BV-2 cells are an immortalised cell line obtained by infecting mouse primary microglial cells with a v-raf/v-myc oncogene-carrying retrovirus (Blasi et al. 1990). BV-2 cells were cultured in Dulbecco's modified eagle medium (DMEM) with 5% fetal bovine serum (FBS), 100 unit/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in a humid atmosphere of 5% CO2 and 95% air.
Irradiation
Following an acclimatisation period of one week, the animals received either whole brain irradiation with a single dose of 10 Gy γ-rays or sham-irradiation. In the present study, a rat model of whole brain irradiation with a single dose of 10 Gy was chosen for three reasons: (1) It is known as the lowest dose to have a radiation effect (Voges et al. 1996, Kim et al. 2002), (2) it is well below the threshold for vascular changes, demyelination or radionecrosis (Hodges et al. 1997, Calvo et al. 1988, Monje et al. 2002), and (3) it is close to a clinically relevant dose in humans because rat brain is more resistant to radiation injury than human brain (Monje et al. 2002, Monje and Palmer 2003). Whole brain irradiation procedures were carried out as described previously (Shi et al. 2006, Schindler et al. 2008) with minor modifications. Rats were anesthetised using a 350 μl mixture of ketamine/xylazine (80/12 mg/kg body weight). Whole brain irradiation was performed in a 12,000 Ci self-shielded 137Cs irradiator (Gammacell 40 Exactor, Nordion International Inc; Kanata, Ontario, Canada) using lead and Cerrobend shielding devices to collimate the beam so that the whole rat brain, including the brain stem, was irradiated. Dosimetry was performed using thermoluminescent dosimeters placed in the skull of dead rats, and confirmed with ionisation chambers in tissue equivalent phantoms. The average dose rate to the midline of the brain for the two positions was ∼4 Gy/min with an 8% difference between the two positions. To ensure that each rat received the same midline brain dose, each lightly anesthetised (Ketamine/xylazine) animal had 5 Gy delivered to alternate sides of the head. A total dose of 10 Gy γ-rays at an average dose rate of 4.23 Gy/min. The eyes received about 15% of the brain dose, and the body received 1–3% of the brain dose. Control rats were anesthetised but not irradiated. The animals were maintained for 4, 8, and 24 h post-irradiation.
BV-2 cells were grown to 80–90% confluence and irradiated with a single dose of 10 Gy γ-rays using a 137Cs irradiator. All irradiations were performed at room temperature and control cells received sham-irradiation. After irradiation, the cells were returned to the CO2 incubator and maintained at 37°C in 5% CO2/95% air for 4 and 24 h post-irradiation.
Real-time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Quantitative real-time RT-PCR using fluorogenic 5′nuclease assay technology with TaqMan® probes and primers (Applied Biosystems, Foster City, CA, USA) were employed for gene expression analyses. Rat brains were homogenised with 1 ml of TRI Reagent (Sigma-Aldrich, St Louis, MO, USA) in a tissue homogeniser and total RNA was isolated from tissue homogenates as described previously (Toborek et al. 2002). In addition, total RNA was isolated from BV-2 using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the protocol of the manufacturer. 1 μg of total RNA was reverse transcribed at 25°C for 15 min, 42°C for 45 min, and 99°C for 5 min in 20 μl of 5 mM MgCl2, 10 mM Tris-HCl, pH 9.0, 50 mM KCl, 0.1% Triton X-100, 1 mM dNTP, 1 unit/μl of recombinant RNasin, 15 unit/μg of Avian Myeloblastosis Virus (AMV) reverse transcriptase, and 0.5 μg of random hexamers. Amplification of individual genes was performed on the Applied Biosystems 7300 Real-Time PCR System using TaqMan® Universal PCR Master Mix and a standard thermal cycler protocol (50°C for 2 min before the first cycle, 95°C for 15 sec and 60°C for 1 min, repeated 45 times). TaqMan® Gene Expression Assay Reagents for rat TNF-α, rat IL-1β, rat IL-6, rat MCP-1, rat glyceraldehydes-3-phosphate dehydrogenase (GAPDH), mouse TNF-α, and mouse GAPDH were used for specific probes and primers of PCR amplifications. The threshold cycle (CT), which indicates the fractional cycle number at which the amount of amplified target gene reaches a fixed threshold, was determined from each well using the Applied Biosystems Sequence Detection Software v1.2.3 and relative quantification was calculated by the comparative CT method as described previously (Livak and Schmittgen 2001, Deng et al. 2003, Lee et al. 2004). The data were analysed using the equation 2−ΔΔCT, where ΔΔCT = [CT of target gene − CT of housekeeping gene]treated group − [CT of target gene − CT of housekeeping gene]untreated control group. For the treated samples, evaluation of 2−ΔΔCT indicates the fold change in gene expression, normalised to a housekeeping gene (GAPDH), and relative to the untreated control.
Enzyme-Linked Immunosorbent Assay (ELISA)
Tissue homogenates from rat brain were prepared using the method recommended by R&D Systems (Minneapolis, MN, USA). Both hippocampus and cortex were homogenised in 1 ml of ice-cold PBS and stored overnight at −80°C. After three freeze-thaw cycles were performed, the homogenates were centrifuged for 5 min at 5,000 × g at 4°C. Supernatants were frozen immediately on dry ice and stored at −80°C until analysis. Protein concentrations of brain tissue homogenates were determined as described by Bradford (1976). The protein expression levels of pro-inflammatory mediators in brain tissue homogenates were determined by using Quantikine® Rat Immunoassay Kits for TNF-α, IL-1β, and IL-6 (R&D Systems) and Rat MCP-1 Immunoassay Kit (Biosource International, Camarillo, CA, USA) following to the manufacturer's protocols. TNF-α concentrations in cell culture supernatants were measured by using a Mouse TNF-α Quantikine® Immunoassay Kit (R&D Systems).
Immunofluorescence staining
Frozen tissues were cut into 20-μm sections using a Microm HM 550 cryostat (MICROM International GmbH, Walldorf, Germany) and mounted on Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA). Sections were fixed in 4% paraformaldehyde for 15 min at room temperature, rinsed with PBS three times for 5 min each, and incubated in 0.5% Triton X-100 for 15 min to permeabilise tissues for optimal staining. After washing with PBS three times, the sections were then incubated with 3% BSA in PBS for 1 h at room temperature to block non-specific binding of the antibodies, followed by incubation with the primary antibody, goat anti-TNF-α polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1/40 in 1.5% BSA, overnight at 4°C. Negative controls were prepared by incubation of tissue sections with non-immune goat serum (normal goat-IgG, Santa Cruz Biotechnology) instead of the primary antibody. Sections were washed three times with PBS and incubated with secondary antibody, bovine anti-goat IgG conjugated with Texas Red (Santa Cruz Biotechnology), diluted 1/100 in PBS in the dark for 1 h. Vectashield mounting medium (Vector Laboratories Inc, Burlingame, CA, USA) was added to prevent fading, and the slides were sealed with a cover slip. The slides were examined on a Zeiss AXIO Imager A1m fluorescence microscope (Carl Zeiss Micro-Imaging, Inc., Thornwood, NY, USA). Images were acquired with 10× objective by AxioCam MRc5 Digital Imaging System. Texas Red was assigned to the red channel of the generated RGB image.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts from hippocampus of each rat brain were prepared according to the method of Beg et al. (1993) with minor modification as described earlier (Toborek et al 2002). The tissues were homogenised in 1 ml of lysis buffer (10 mM Tris-HCl, pH 8.0, 60 mM KCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol, 100 μM phenylmethylsulfonyl fluoride, 0.1% NP-40), lysed for 5 min on ice, and centrifuged at 600 × g for 4 min at 4°C to collect nuclei. Then, the nuclear pellets were washed with 1 ml of lysis buffer without NP-40, lysed in 75 μl of nuclear extract buffer (20 mM Tris-HCl, pH 8.0, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol) for 10 min on ice, and centrifuged at 18,300 × g for 15 min at 4°C. Supernatants, which contain nuclear extracts, were frozen immediately on dry ice and transferred to −80°C until analysis. Protein concentrations of isolated nuclear extracts were determined as described by Bradford (1976).
Double-stranded oligonucleotides containing the consensus sequences of the binding sites for pro-inflammatory transcription factors AP-1, NF-κB, or CREB were purchased from Promega (Madison, WI, USA) and labeled with [γ-32P]-ATP using bacteriophage T4 polynucleotide kinase. The reaction mixture consisted of 70 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 5 mM dithiothreitol (DTT), 1.75 pmoles of double-stranded oligonucleotides, 30 μCi of [γ-32P]-ATP (GE Healthcare Bio-Sciences, Piscataway, NJ, USA), and 20 units of T4 polynucleotide kinase (Promega) in a total volume of 20 μl. The reaction was incubated for 1 h at 37°C. Following incubation, T4 polynucleotide kinase was inactivated by placing the tube for 10 min at 68°C. Unlabeled nucleotides were removed by gel filtration chromatography using mini Quick Spin Oligo Columns (Roche Applied Science, Indianapolis, IN, USA).
Binding reactions were performed in a 20 μl volume containing 6–10 μg of nuclear protein extracts, 10 mM Tris-Cl, pH 7.5, 50 mM NaCl, 1 mM EDTA, 0.1 mM dithiothreitol, 10% glycerol, and 2 μg of poly[dI-dC]. After adding the reagents, the mixture was incubated for 25 min at room temperature. Then, 40,000 cpm of 32P-labeled specific oligonucleotide probe was added, and the binding mixture was incubated for 25 min at room temperature. Competition studies were performed by the addition of a molar excess of unlabeled oligonucleotide to the binding reaction. Resultant protein-DNA complexes were electrophoresed on a non-denaturing 5% polyacrylamide gel using 0.25 × TBE buffer (50 mM Tris-Cl, 45 mM boric acid, 0.5 mM EDTA, pH 8.4) for 3 h at 150 V. The gel was transferred to Whatman® 3MM paper, dried on a gel dryer, and exposed to a X-ray film overnight at −80°C with an intensifying screen. All experiments were repeated using nuclear extracts from 4 rat hippocampi of each group and relative intensities of the bands corresponding to specific transcription factors were measured using UN-SCAN-IT gel™ image analysis software (Silk Scientific, Inc., Orem, UT, USA). The values of relative pixel intensity were subjected to statistical analyses.
Statistical analysis
The statistical analysis of data was completed using SigmaStat 3.5 (SPSS Inc., Chicago, IL, USA). Oneway analysis of variance (ANOVA) was used to compare mean responses among the treatments. For each endpoint, the treatment means were compared using the Bonferroni least significant difference procedure. Statistical probability of p < 0.05 was considered significant.
Results
Irradiation up-regulates TNF-α expression in rat brain
Quantitative real-time RT-PCR demonstrated a significant and marked increase in the mRNA expression levels of the pro-inflammatory cytokine, TNF-α, in hippocampus and cortex isolated from rat brains at 4, 8, and 24 h after a single dose of whole brain irradiation (Figure 1A). Up-regulation of TNF-α mRNA expression reached maximal levels within 4 h after irradiation (15-fold induction in hippocampus and 19-fold induction in cortex compared to the sham-irradiated control rats). Expression of GAPDH (a housekeeping gene), however, was not affected by irradiation (data not shown).
Figure 1.
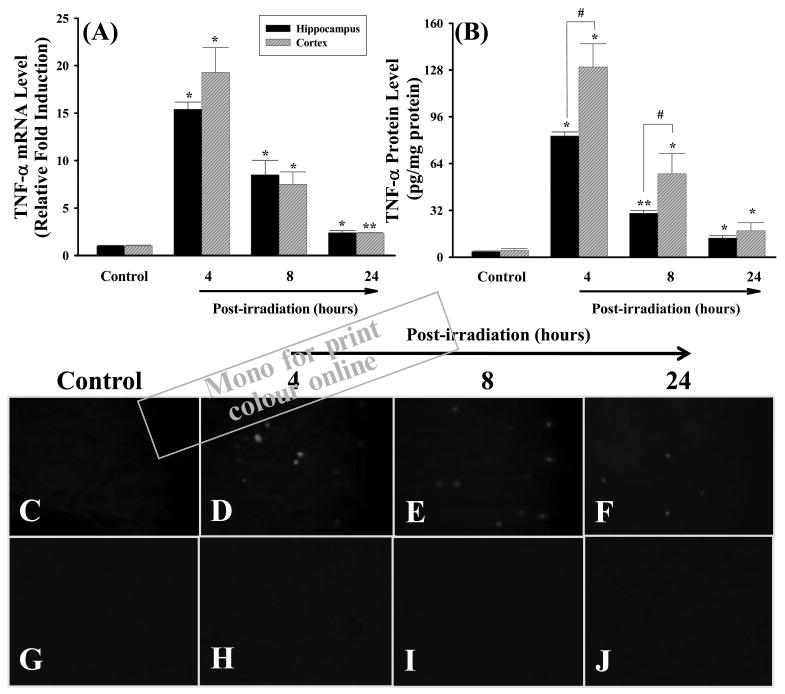
Irradiation up-regulates mRNA and protein expression of TNF-α in rat brain. F344 × BN rats (n = 4) received either whole brain irradiation with a single dose of 10 Gy or sham-irradiation. The animals were maintained for 4, 8, and 24 h post-irradiation, and the brains were rapidly removed and two different brain regions (hippocampus and cortex) were dissected. The mRNA expression levels of TNF-α in hippocampus and cortex were determined by quantitative real-time RT-PCR (panel A). Using the 2−ΔΔCT method as described in Materials and methods, the data are presented as fold change in gene expression normalised to a housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and relative to the sham-irradiated control. The protein expression levels of TNF-α in hippocampus and cortex were analysed by ELISA (panel B) and fluorescence microscopy (panel C–J). Panel C: sham-irradiation (Control); panel D: 4 h post-irradiation; panel E: 8 h post-irradiation; panel F: 24 h post-irradiation; panel G–J: negative controls. Magnification of the images (panel C–J) is 100×. Data shown are mean ± SEM for each group. *,**Statistically significant from control (*p < 0.05 and **p < 0.001). #Statistically significant from hippocampus (p < 0.05).
The quantitative sandwich enzyme immunoassay technique was employed to determine whether irradiation-mediated increases in TNF-α mRNA levels translate to elevated protein expression in hippocampal and cortical regions isolated from rat brains. As indicated in Figure 1B, very low expression levels of TNF-α protein were found in sham-irradiated control rats (3.6 pg/mg protein in hippocampus and 4.4 pg/mg protein in cortex). However, consistent with the gene expression data (Figure 1A), brain irradiation resulted in a significant and marked increase in TNF-α protein expression in hippocampus and cortex at 4 h (23- and 30-fold), 8 h (8.3- and 13-fold) and 24 h (3.6- and 4.1-fold) after irradiation (Figure 1B). TNF-α levels were significantly elevated in cortex compared to hippocampus at 4 and 8 h post-irradiation. In addition, the irradiation-mediated overexpression of TNF-α protein in rat brain was further confirmed by immunofluorescence straining. As shown in Figure 1C, no immunoreactivity of TNF-α protein was detected in sham-irradiated control rat brains. A marked increase in TNF-α immunoreactivity, however, was observed in rat brains at 4, 8, and 24 h after irradiation (Figure 1D–1F). In agreement with the results from ELISA (Figure 1B), the maximal immunoreactivity of TNF-α protein was formed 4 h after irradiation and maintained at a high level 8 h after irradiation. The TNF-α immunoreactivity was then decreased 24 h after irradiation. In contrast, negative control experiments did not show any positive staining for TNF-α protein at all studied time points (Figure 1G–1J).
Irradiation up-regulates TNF-α expression in microglia
To investigate the potential contribution of microglia to the induction of TNF-α expression in the brain after irradiation, BV-2, murine microglial cells, were exposed directly to a single dose of 10 Gy or sham-irradiation and maintained for 4 and 24 h post-irradiation. The mRNA and protein expression levels of TNF-α were analysed by real-time RT-PCR and ELISA. A significant up-regulation of mRNA and protein expression of TNF-α was observed in microglia at 4 and 24 h after irradiation compared with those determined in sham-irradiated control cells (Figure 2).
Figure 2.
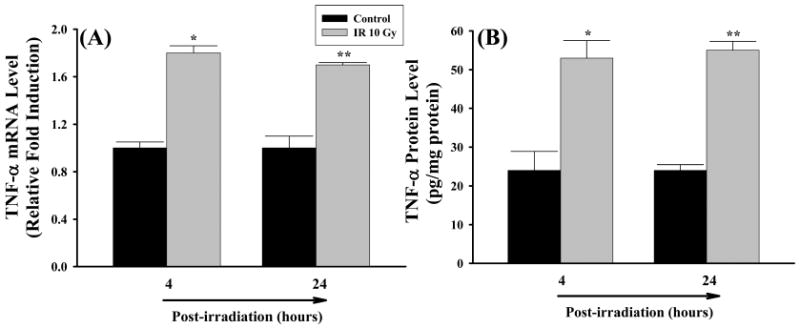
Irradiation up-regulates mRNA and protein expression of TNF-α in microglia. BV-2 cells received irradiation with a single dose of 10 Gy (IR 10 Gy) or sham-irradiation (Control). Cells were maintained for 4 and 24 h post-irradiation, and the mRNA and protein expression levels of TNF-α were analysed by quantitative real-time RT-PCR (panel A) and ELISA (panel B), respectively. Data shown are mean ± SEM for each group. *,**Statistically significant from control (*p < 0.05 and **p < 0.001).
Irradiation up-regulates expression of IL-1β and MCP-1 in rat brain
The effects of irradiation on mRNA and protein expression of IL-1β, IL-6, and MCP-1 were also investigated in rat brain. Figure 3A demonstrates that mRNA expression levels of the pro-inflammatory cytokine IL-1β were significantly higher in both hippocampal and cortical regions of irradiated rats compared to those of sham-irradiated controls at any time point. The greatest response was detected at 4 h after irradiation in hippocampus (a 20-fold induction) and 8 h after irradiation in cortex (a 19-fold induction). Significantly higher expression levels of IL-1β protein that remained for up to 24 h after irradiation were also observed in hippocampal and cortical regions isolated from irradiated rat brains (Figure 3B). IL-1β levels were markedly elevated in hippocampus compared to cortical samples. In addition, irradiation significantly up-regulated mRNA expression of another pro-inflammatory cytokine IL-6 in rat brain while an increased IL-6 mRNA expression was not translated to actual changes in protein levels (Figure 4). Furthermore, irradiation significantly and dramatically up-regulated mRNA expression of the pro-inflammatory chemokine MCP-1 in rat brain. The mRNA levels of MCP-1 in hippocampus and cortex were increased by 395- and 581-fold at 4 h, 252- and 347-fold at 8 h, and 11- and 22-fold at 24 h after irradiation (Figure 5A). Furthermore, expression levels of MCP-1 protein at 4 h and 8 h after irradiation were significantly greater in hippocampus and cortex than those of sham-irradiated control rat brains (Figure 5B).
Figure 3.
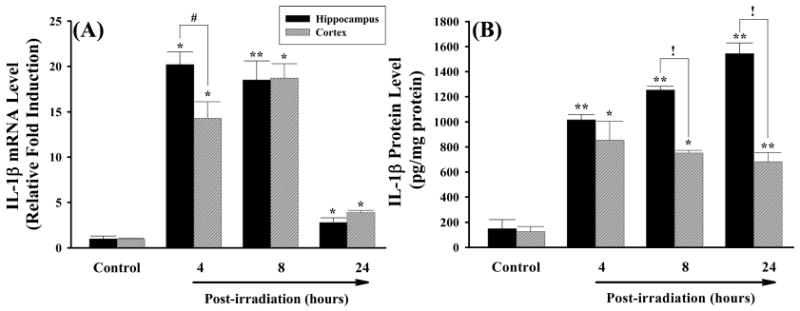
Irradiation up-regulates mRNA and protein expression of IL-1β in rat brain. Experiments were carried out as described in Figure 1. The mRNA expression levels of IL-1β in hippocampus and cortex were determined by quantitative real-time RT-PCR (panel A). The protein expression levels of IL-1β in hippocampus and cortex were determined by ELISA (panel B). Data shown are mean ± SEM for each group. *,**Statistically significant from control (*p < 0.05 and **p < 0.001). #,!Statistically significant from hippocampus (#p < 0.05 and !p < 0.001).
Figure 4.
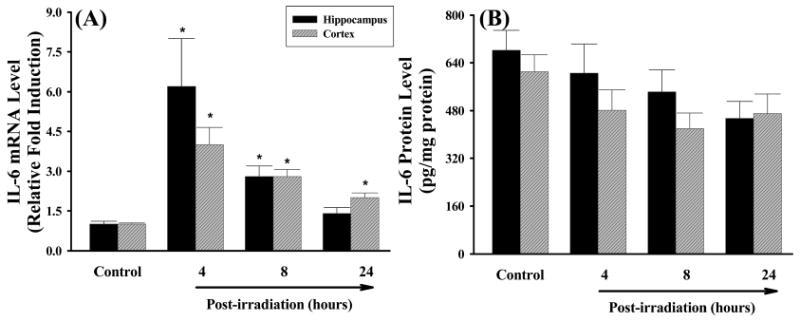
Effects of irradiation on mRNA and protein expression of IL-6 in rat brain. Experiments were carried out as described in Figure 1. The mRNA expression levels of IL-6 in hippocampus and cortex were determined by quantitative real-time RT-PCR (panel A). The protein expression levels of IL-6 in hippocampus and cortex were determined by ELISA (panel B). Data shown are mean ± SEM for each group. *Statistically significant from control (p < 0.05).
Figure 5.
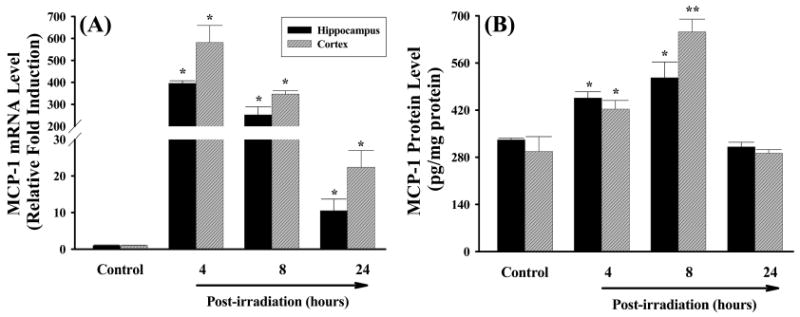
Irradiation up-regulates mRNA and protein expression of MCP-1 in rat brain. Experiments were carried out as described in Figure 1. The mRNA expression levels of MCP-1 in hippocampus and cortex were determined by quantitative real-time RT-PCR (panel A). The protein expression levels of MCP-1 in hippocampus and cortex were determined by ELISA (panel B). Data shown are mean ± SEM for each group. *,**Statistically significant from control (*p < 0.05 and **p < 0.001).
Irradiation activates pro-inflammatory transcription factors in rat brain
To elucidate the molecular mechanisms of irradiation-induced brain inflammation, the effects of irradiation on the DNA-binding activity of pro-inflammatory transcription factors in rat brain were examined by a series of EMSAs. These analyses were performed on nuclear protein extracts from hippocampal regions isolated from either irradiated rat brains or sham-irradiated controls. The effects of irradiation on the DNA-binding activity of AP-1 in rat brain are shown in Figure 6. A slight endogenous activity of AP-1 was observed in sham-irradiated control rat brains. In contrast, a significant increase in AP-1 DNA-binding activity was detected in irradiated rat brains (Figure 6A). AP-1 activation increased by 2.5-fold at 4 h and 2.8-fold at 8 h after irradiation compared to the sham-irradiated control rats and returned to the control levels at 24 h after irradiation (Figure 6B). The specificity of AP-1 DNA-binding was determined by competition experiments with molar excess of unlabeled oligonucleotide containing the consensus AP-1 binding sequence. As depicted in Figure 6A (lane 6), an excess amount of competitor oligonucleotide completely abolished the AP-1 DNA-binding activity.
Figure 6.
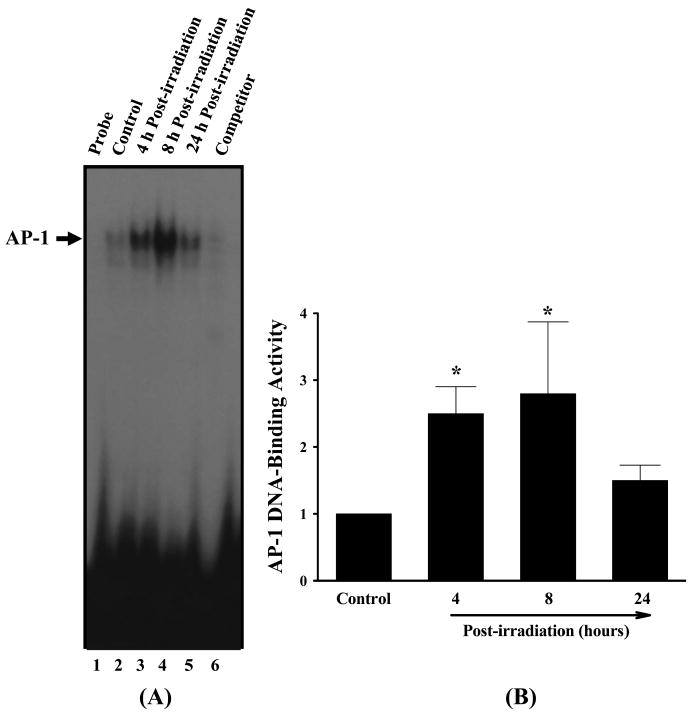
Representative autoradiogram of EMSA of the effects of irradiation on AP-1 DNA-binding activity in rat brain (panel A). F344 × BN rats (n = 4) received either whole brain irradiation with a single dose of 10 Gy or sham-irradiation. The animals were maintained for 4, 8, and 24 h post-irradiation and nuclear extracts were prepared from the brain hippocampus regions and analysed by EMSA. Competition studies were performed by adding excess unlabeled AP-1 probe. Densitometric quantification of the effects of irradiation on AP-1 DNA-binding activity in rat brain (panel B). Experiments were repeated four times, and the intensities of the AP-1-specific bands were measured and statistically analysed. The results are expressed as fold increase over control values. Data shown are the mean ± SEM for each group. *Statistically different from control (p < 0.05).
In addition to AP-1, the effects of irradiation on other pro-inflammatory transcription factors, including NF-κB and CREB, were studied. As illustrated in Figures 7 and 8, a prominent and significant stimulation of DNA-binding activities of NF-κB (3.3-fold increase) and CREB (2.0-fold increase) was observed 4 h after irradiation. This activation was maintained at a high level 8 h after irradiation, and no activation of NF-κB and CREB was detected 24 h after irradiation. The molar excess of each unlabeled oligonucleotide probe completely diminished the specific band that corresponded to either NF-κB (Figure 7A, lane 6) or CREB DNA-binding (Figure 8A, lane 6).
Figure 7.

Representative autoradiogram of EMSA of the effects of irradiation on NF-κB DNA-binding activity in rat brain (panel A). Experiments were carried out as described in Figure 6. Competition studies were performed by adding excess unlabeled NF-κB probe. Densitometric quantification of the effects of irradiation on NF-κB DNA-binding activity in rat brain (panel B). Experiments were repeated four times, and the intensities of the NF-κB-specific bands were measured and statistically analysed. The results are expressed as fold increase over control values. Data shown are the mean ± SEM for each group. *Statistically different from control (p < 0.05).
Figure 8.
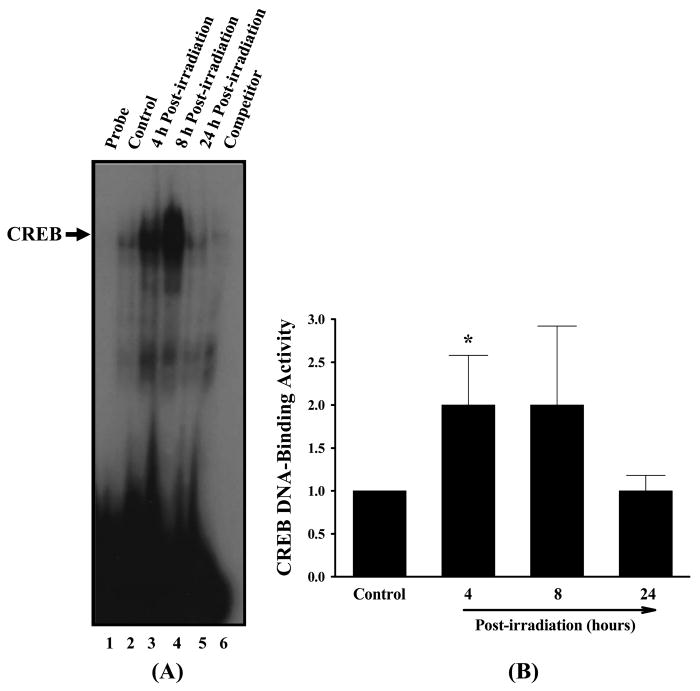
Representative autoradiogram of EMSA of the effects of irradiation on CREB DNA-binding activity in rat brain (panel A). Experiments were carried out as described in Figure 6. Competition studies were performed by adding excess unlabeled CREB probe. Densitometric quantification of the effects of irradiation on CREB DNA-binding activity in rat brain (panel B). Experiments were repeated four times, and the intensities of the CREB-specific bands were measured and statistically analysed. The results are expressed as fold increase over control values. Data shown are the mean ± SEM for each group. *Statistically different from control (p < 0.05).
Discussion
Recent evidence has identified inflammation as one of the important pathways leading to radiation-induced brain injury (Hong et al. 1995, Chiang et al. 1997, Olschowka et al. 1997, Gaber et al. 2003, Baluna et al. 2006). These early studies raise the possibility that a pro-inflammatory environment resulting from overexpression of inflammatory mediators may be responsible for many of the neurological/neuropathological complications occurring after irradiation (e.g., vascular rarefaction, necrosis, demyelination, vascular abnormality) (Sheline et al. 1980, Schultheiss and Stephens 1992, Denham and Hauser-Jensen 2002, Moulder and Cohen 2007). However, subsequent studies on the effects of irradiation on pro-inflammatory pathways in the brain are limited by the absence of a clear time course for cytokine induction, incomplete analyses of gene and protein expression and/or lack of regional specificity that may contribute to selective biochemical pathways to neurodegeneration. In the present study, we demonstrate that irradiation activates AP-1, NF-κB, and CREB and overexpression of TNF-α, IL-1β, and MCP-1 throughout the rat brain. Nevertheless, the time course for induction and maximal response were regionally specific with hippocampus exhibiting higher levels of IL-1β and cortex exhibiting elevated levels of TNF-α. Our results are consistent with the conclusion that elevated levels of specific cytokines and chemokines within each brain region are unique and may contribute to specific pathways for the decline in tissue function after whole brain irradiation.
In general, a cascade of inflammatory events is regulated through the production of pro-inflammatory mediators. Enhanced expression of pro-inflammatory cytokines, chemokines, and adhesion molecules, and their close interactions facilitate pro-inflammatory pathways by recruiting and transmigrating inflammatory cells from blood to tissues (Ross 1993, 1999, Lee et al. 2004). Several previous in vivo and in vitro studies have demonstrated that TNF-α and IL-1β are the most important pro-inflammatory cytokines that exert a central role in acute and chronic inflammation. It is well known that TNF-α and IL-1β strongly promote inflammatory responses in a wide spectrum of cell types, and overproduction of these cytokines has been implicated in a variety of human diseases including atherosclerosis, autoimmune disorders, and cancer (Dinarello 1996, Locksley et al. 2001). In addition, there are several reports demonstrating that overexpression of TNF-α and IL-1β genes may be associated with the molecular responses of the brain to irradiation. Whole brain irradiation significantly up-regulated TNF-α gene expression in the mouse brain (Gaber et al. 2003). Additionally, increased levels of TNF-α and IL-1β gene expression were observed as an initial response of the mouse and rat brains to brain irradiation (Hong et al 1995) and partial-body irradiation (Marquette et al. 2003). Furthermore, Chiang et al. (1997) reported that TNF-α mRNA was overexpressed 6 months after brain irradiation, suggesting that TNF-α may be involved in the late brain responses to irradiation. Up-regulation of TNF-α and IL-1β expression was also found in lung and intestine after irradiation (Hong et al. 1999, Linard et al. 2003, 2004). Despite the reports of increased gene expression, levels of TNF-α and IL-1β protein levels have not been reported. Importantly, the present study demonstrated for the first time a marked and significant increase in gene and protein expression of TNF-α and IL-1β in rat brain after whole brain irradiation and determined their specific time-courses. Since TNF-α and IL-1β are recognised as the crucial mediators of inflammatory events in brain, irradiation-mediated overexpression of these cytokines is likely to be an important contributing factor in the pathophysiological sequelae that occurs after whole brain irradiation.
The potential contribution of specific types of cells to the overexpression of pro-inflammatory mediators in the brain after irradiation, however, remains unclear. Microglia are the primary immune cells in the brain that release pro-inflammatory cytokines when they are activated by various environmental stimuli (Block et al. 2007). Therefore, we performed an in vitro study to further examine the role of microglia in irradiation-induced TNF-α expression in brain. The results of this analysis indicate a significant up-regulation of the mRNA and protein expression of TNF-α in irradiated microglia suggesting that irradiation-induced pro-inflammatory environments in the brain may be, at least in part, mediated through activation of microglia.
IL-6 is another multifunctional pro-inflammatory cytokine that plays a major role in the mediation of the inflammatory and immune responses initiated by infection or injury (Kishimoto 2005). Recent studies have suggested that elevated levels of IL-6 mRNA and protein expression may be responsible for the radiation-induced inflammation in the intestine and whole brain (Linard et al. 2003, 2004, Marquette et al. 2003). Additionally, both total-body and localised irradiation resulted in a small but significant increase in IL-6 levels in serum from rat blood (Haveman et al. 1998). Consistent with the previous studies, irradiation significantly up-regulated mRNA expression of IL-6 in rat brain, however, we did not find that increased IL-6 gene expression translated to actual changes in protein levels. Potentially, this translational block or delay may be due to different protein expression kinetics for pro-inflammatory cytokines in response to irradiation. Linard et al. (2003) demonstrated that no change in IL-6 protein expression was observed in rat ileal muscularis layer at 6 h after abdominal irradiation, while the IL-6 content increased significantly three days after irradiation. The potential biochemical mechanism(s) for the delayed protein expression of IL-6 in response to irradiation remain to be determined.
In addition to pro-inflammatory cytokines, chemokines are known to directly promote inflammatory responses. MCP-1 is a member of the CC chemokine family and has a critical role in monocyte chemotaxis and transmigration (Lee et al. 2003). Although recent evidence indicates that irradiation induces dermatitis and fibrosis in skin and lung by elevating the levels of MCP-1 (Johnston et al. 2002, Xiao et al. 2006), the molecular basis for the induction of this chemokine in irradiated brain has not yet been elucidated. Therefore, the results of the present study showing that irradiation significantly induced mRNA and protein expression of MCP-1 in hippocampus and cortex appear to be the first to document the stimulatory effects of irradiation on MCP-1 expression in the brain. Whether the irradiation-induced increase in MCP-1 results in fibrotic changes within the brain and contributes to neuropathology remains unknown.
Activation of AP-1 and NF-κB is considered to be a part of the general regulation of a number of pro-inflammatory gene expressions in response to various extracellular stimuli (Wung et al. 1997, Lakshminarayanan et al. 1998, Bouloumie et al. 1999, Guha et al. 2000, Lee et al. 2005). Evidence indicates that irradiation can cause an increase in AP-1 and/or NF-κB DNA-binding activity in several different types of cells including A549 lung epithelial cells, HeLa cells, KG-1 myeloid leukemia cells, astrocytes, and glioblastoma cells (Brach et al. 1991, Beetz et al. 2000, Mori et al. 2000, Park et al. 2001, Son et al. 2006). Additionally, both whole-body and abdominal irradiation increased inflammatory gene expression and activated NF-κB in rat intestine (Linard et al. 2003, 2004, Molla and Panes 2007). It was also reported that exposure of mice to total-body irradiation selectively activated NF-κB in the spleen, lymph nodes, and bone marrow, and subsequently increased mRNA expression of TNF-α, IL-1α, IL-1β, and IL-6 (Zhou et al. 2001). Furthermore, enhanced DNA-binding activities of AP-1 and NF-κB were found in the cerebral cortex collected from rat brains after whole brain irradiation (Raju et al. 2000). In agreement with these in vivo and in vitro studies, the present study further demonstrated a significant activation of AP-1 and NF-κB in rat hippocampus after irradiation. Recent evidence has also suggested that not only AP-1 and NF-κB but also CREB may belong to the family of transcription factors that play an important role in the expression of pro-inflammatory mediators (Grosch and Kaina 1999, Iwata et al. 1997). There are, however, no reports showing effects of irradiation on CREB activity in brain. Therefore, in the present study, DNA-binding activity of CREB was examined. To our knowledge, this is the first report to demonstrate that activation of CREB may be involved in irradiation-mediated cellular effects in the brain. Although these results provide evidence that activation of AP-1, NF-κB, and CREB may be responsible for irradiation-induced overexpression of pro-inflammatory mediators in brain, detailed signal transduction pathways leading to activation of these transcription factors in response to irradiation remain to be determined. In addition, it is necessary to find out whether radiation-mediated early inflammatory responses observed in the present study have a causal relationship with delayed injury to the brain.
Conclusions
The present study demonstrated that irradiation induces pro-inflammatory environments through activation of AP-1, NF-κB, and CREB and overexpression of inflammatory mediators throughout the brain but that the absolute levels of each cytokine and chemokine are unique to each brain region. Because brain inflammation is critically involved in the onset and progression of neurological disorders, these results may contribute to a deeper understanding of the pathophysiological mechanisms responsible for irradiation-induced brain injury at the cellular and molecular levels. Furthermore, the present study may provide a foundation for the development of novel strategies for prevention and treatment of irradiation-induced brain injury specifically targeted against pro-inflammatory pathways.
Acknowledgments
The editorial assistance of MaryAnn Sonntag in preparation of the manuscript is greatly appreciated. The project described was supported by Grant Number R01NS056218 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baluna RG, Eng TY, Thomas CR. Adhesion molecules in radiotherapy. Radiation Research. 2006;166:819–831. doi: 10.1667/RR0380.1. [DOI] [PubMed] [Google Scholar]
- Beetz A, Peter RU, Oppel T, Kaffenberger W, Rupec RA, Meyer M, van Beuningen D, Kind P, Messer G. NF-κB and AP-1 are responsible for inducibility of the IL-6 promoter by ionizing radiation in HeLa cells. International Journal of Radiation Biology. 2000;76:1443–1453. doi: 10.1080/09553000050176207. [DOI] [PubMed] [Google Scholar]
- Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: A mechanism for NF-kappa B activation. Molecular and Cellular Biology. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends U, Peter RU, Hintermeier-Knabe R, Eissner G, Holler E, Bornkamm GW, Caughman SW, Degitz K. Ionizing radiation induces human intercellular adhesion molecule-1 in vitro. Journal of Investigative Dermatology. 1994;103:726–730. doi: 10.1111/1523-1747.ep12398607. [DOI] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of Neuroimmunology. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nature Review Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. The FASEB Journal. 1999;13:1231–1238. [PubMed] [Google Scholar]
- Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. Journal of Clinical Investigation. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calvo W, Hopewell KW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. The British Journal of Radiology. 1988;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. International Journal of Radiation Biology. 1997;72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- Deng X, Li H, Tang YW. Cytokine expression in respiratory syncytial virus-infected mice as measured by quantitative reverse-transcriptase PCR. Journal of Virological Methods. 2003;107:141–146. doi: 10.1016/s0166-0934(02)00211-2. [DOI] [PubMed] [Google Scholar]
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury-a complex ‘wound’. Radiotherapy and Oncology. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Gaber MW, Sabek OM, Fukatsu K, Wilcox HG, Kiani MF, Merchant TE. Differences in ICAM-1 and TNF-alpha expression between large single fraction and fractionated irradiation in mouse brain. International Journal of Radiation Biology. 2003;79:359–366. doi: 10.1080/0955300031000114738. [DOI] [PubMed] [Google Scholar]
- Gaugler MH, Squiban C, van der Meeren A, Bertho JM, Vandamme M, Mouthon MA. Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. International Journal of Radiation Biology. 1997;72:201–209. doi: 10.1080/095530097143428. [DOI] [PubMed] [Google Scholar]
- Grosch S, Kaina B. Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress requires CREB. Biochemical and Biophysical Research Communications. 1999;261:859–863. doi: 10.1006/bbrc.1999.1125. [DOI] [PubMed] [Google Scholar]
- Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. Journal of Biological Chemistry. 2000;275:17728–17739. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Research. 1996;56:5150–5155. [PubMed] [Google Scholar]
- Han SK, Song JY, Yun YS, Yi SY. Effect of gamma radiation on cytokine expression and cytokine-receptor mediated STAT activation. International Journal of Radiation Biology. 2006;82:686–697. doi: 10.1080/09553000600930699. [DOI] [PubMed] [Google Scholar]
- Haveman J, Geerdink AG, Rodermond HM. TNF, IL-1 and IL-6 in circulating blood after total-body and localized irradiation in rats. Oncology Reports. 1998;5:679–683. doi: 10.3892/or.5.3.679. [DOI] [PubMed] [Google Scholar]
- Hodges H, Katzung N, Sowinski P, Hopewell JW, Wilkinson JH, Bywaters T, Rezvani M. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behavioural Brain Research. 1997;91:99–114. doi: 10.1016/s0166-4328(97)00108-3. [DOI] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. Induction of acute phase gene expression by brain irradiation. International Journal of Radiation Oncology, Biology and Physics. 1995;33:619–626. doi: 10.1016/0360-3016(95)00279-8. [DOI] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. International Journal of Radiation Biology. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- Iwata E, Asanuma M, Nishibayashi S, Kondo Y, Ogawa N. Different effects of oxidative stress on activation of transcription factors in primary cultured rat neuronal and glial cells. Brain Research Molecular-Brain Research. 1997;50:213–220. doi: 10.1016/s0169-328x(97)00190-3. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radiation-induced pulmonary fibrosis: Examination of chemokine and chemokine receptor families. Radiation Research. 2002;157:256–265. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lim DJ, Chung YG, Cho TH, Lim SJ, Kim WJ, Suh JK. Expression of TNF-α and TGF-β1 in the rat brain after a single high-dose irradiation. Journal of Korean Medical Science. 2002;17:242–248. doi: 10.3346/jkms.2002.17.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: From basic science to medicine – 40 years in immunology. Annual Review of Immunology. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, Kerry O'Banion M. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Research-Molecular Brain Research. 2002;104:159–169. doi: 10.1016/s0169-328x(02)00353-4. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan V, Drab-Weiss EA, Roebuck KA. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-κB to the interleukin-8 promoter in endothelial and epithelial cells. Journal of Biological Chemistry. 1998;273:32670–32678. doi: 10.1074/jbc.273.49.32670. [DOI] [PubMed] [Google Scholar]
- Lee YW, Eum SY, Nath A, Toborek M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovascular Research. 2004;63:139–148. doi: 10.1016/j.cardiores.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lee YW, Hennig B, Toborek M. Redox-regulated mechanisms of IL-4-induced MCP-1 expression in human vascular endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology. 2003;284:H185–192. doi: 10.1152/ajpheart.00524.2002. [DOI] [PubMed] [Google Scholar]
- Lee YW, Hirani AA, Kyprianou N, Toborek M. Human immunodeficiency virus-1 Tat protein up-regulates interleukin-6 and interleukin-8 expression in human breast cancer cells. Inflammation Research. 2005;54:380–389. doi: 10.1007/s00011-005-1371-8. [DOI] [PubMed] [Google Scholar]
- Linard C, Marquette C, Mathieu J, Pennequin A, Clarencon D, Mathe D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: Effect of an NF-kappaB inhibitor. International Journal of Radiation Oncology, Biology and Physics. 2004;58:427–434. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Linard C, Ropenga A, Vozenin-Brotons MC, Chapel A, Mathe D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappa B in rat ileal muscularis layer. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;285:G556–G565. doi: 10.1152/ajpgi.00094.2003. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Marquette C, Linard C, Galonnier M, Van Uye A, Mathieu J, Gourmelon P, Clarencon D. IL-1β, TNF-α and IL-6 induction in the rat brain after partial-body irradiation: Role of vagal afferents. International Journal of Radiation Biology. 2003;79:777–785. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- Molla M, Gironella M, Salas A, Miquel R, Perez-del-Pulgar S, Conill C, Engel P, Biete A, Pique JM, Panes J. Role of P-selectin in radiation-induced intestinal inflammatory damage. International Journal of Cancer. 2001;96:99–109. doi: 10.1002/ijc.1009. [DOI] [PubMed] [Google Scholar]
- Molla M, Panes J. Radiation-induced intestinal inflammation. World Journal of Gastroenterology. 2007;13:3043–3046. doi: 10.3748/wjg.v13.i22.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nature Medicine. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Palmer TD. Radiation injury and neurogenesis. Current Opinion in Neurology. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- Moore AH, Olschowka JA, Williams JP, Okunieff P, O'Banion MK. Regulation of prostaglandin E2 synthesis after brain irradiation. International Journal of Radiation Oncology, Biology and Physics. 2005;62:267–272. doi: 10.1016/j.ijrobp.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Mori K, Tani M, Kamata K, Kawamura H, Urata Y, Goto S, Kuwano M, Shibata S, Kondo T. Mitogen-activated protein kinase, ERK1/2, is essential for the induction of vascular endothelial growth factor by ionizing radiation mediated by activator protein-1 in human glioblastoma cells. Free Radical Research. 2000;33:157–166. doi: 10.1080/10715760000300711. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Seminar Radiation Oncology. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Olschowka JA, Kyrkanides S, Harvey BK, O'Banion MK, Williams JP, Rubin P, Hansen JT. ICAM-1 induction in the mouse CNS following irradiation. Brain, Behavior, and Immunity. 1997;11:273–285. doi: 10.1006/brbi.1997.0506. [DOI] [PubMed] [Google Scholar]
- Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CS, Grant S, Hagan MP, Ellis E, Fisher PB, Dent P. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001;20:3266–3280. doi: 10.1038/sj.onc.1204258. [DOI] [PubMed] [Google Scholar]
- Raju U, Gumin GJ, Tofilon PJ. Radiation-induced transcription factor activation in the rat cerebral cortex. International Journal of Radiation Biology. 2000;76:1045–1053. doi: 10.1080/09553000050111514. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis is an inflammatory disease. American Heart Journal. 1999;138:S419–420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. International Journal of Radiation Oncology, Biology and Physics. 2008;70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TE, Stephens LC. Invited review: Permanent radiation myelopathy. British Journal of Radiology. 1992;65:737–753. doi: 10.1259/0007-1285-65-777-737. [DOI] [PubMed] [Google Scholar]
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. International Journal of Radiation Oncology, Biology and Physics. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Brunso-Bechtold JK. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiation Research. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- Son EW, Rhee DK, Pyo S. Gamma-irradiation-induced intercellular adhesion molecule-1 (ICAM-1) expression is associated with catalase: Activation of Ap-1 and JNK. Journal of Toxicology and Environmental Health, Part A. 2006;69:2137–2155. doi: 10.1080/15287390600747759. [DOI] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods in Enzymology. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- Voges J, Treuer H, Sturm V, Büchner C, Lehrke R, Kocher M, Staar S, Kuchta J, Müller R. Risk analysis of linear accelerator radiosurgery. International Journal of Radiation Oncology, Biology and Physics. 1996;36:1055–1063. doi: 10.1016/s0360-3016(96)00422-1. [DOI] [PubMed] [Google Scholar]
- Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circulation Research. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Su Y, Yang S, Yin L, Wang W, Yi Y, Fenton BM, Zhang L, Okunieff P. Protective effect of esculentoside A on radiation-induced dermatitis and fibrosis. International Journal of Radiation Oncology, Biology and Physics. 2006;65:882–889. doi: 10.1016/j.ijrobp.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Zhou D, Yu T, Chen G, Brown SA, Yu Z, Mattson MP, Thompson JS. Effects of NF-κB1 (p50) targeted gene disruption on ionizing radiation-induced NF-κB activation and TNF-α, IL-1α, IL-1β and IL-6 mRNA expression in vivo. International Journal of Radiation Biology. 2001;77:763–772. doi: 10.1080/09553000110050047. [DOI] [PubMed] [Google Scholar]


