Abstract
Endothelial progenitor cells (EPC) may enhance blood vessel formation in a variety of clinical settings such as ischaemia and tumour angiogenesis as well as in tissue-engineered matrices. In the present study, we cultured a murine endothelial progenitor cell line, T17b, in vitro in cell culture as well as in an FDA-approved fibrin matrix and investigated cell proliferation, differentiation and secretion patterns of the angiogenic growth factor VEGF under hypoxia and differentiation. We show that T17b EPC remain viable for at least 8 days in the fibrin matrix where they proliferate and form clusters including lumen-like structures. Proliferation in fibrin clots overlayed with basal medium (BM) was confirmed morphologically and immunohistochemically by positive Ki67 staining, indicating mitotic activity. Significant cell proliferation and Ki-67 expression were absent when cells were incubated with dibutyryl-cAMP and retinoic acid (RA). Incubation with dibutyryl-cAMP and RA stimulated the expression of the EPC differentiation markers von Willebrand Factor (vWF) and VEGF receptor 2 (VEGFR-2), indicating successful differentiation in the fibrin clot. EPC differentiation induced by dibutyryl-cAMP and RA was confirmed in 2-D chamber slide cultures by positive vWF immunostaining, which was absent in BM controls. EPC chamber slides also displayed positive vWF staining when exposed to hypoxia under BM conditions, indicating EPC activation and differentiation could also be induced by hypoxia. Taken together, T17b EPC secrete increased levels of VEGF when submitted to either hypoxia or differentiation and can be differentiated into mature endothelial cells not only in cell and matrigel cultures but also in a fibrin matrix that is FDA approved for clinical application.
Keywords: angiogenesis, tissue engineering, embryonal endothelial progenitor cells
Introduction
The vascularization of large three-dimensional constructs is a major challenge in tissue engineering [1–3]. Thus far, experimental modulation of angiogenic processes in tissue-engineered three-dimensional matrices has mainly been attempted through delivery of angiogenic growth factors as recombinant proteins or by gene transfer [4, 5]. Endothelial progenitor cells (EPC) have been shown to enhance vascularization in various pathological conditions of tissue ischaemia such as myocardial infarction and peripheral vascular disease [6–8] and may also be used to induce blood vessel formation in tissue-engineered matrices, which could then be used to pre-fabricate organs or tissues for regenerative purposes [9].
Thus far, there is little knowledge on how EPC may support vascularization of these bioartificial constructs. There appear to be, however, several mechanisms by which EPC exert their therapeutic pro-angiogenic effects in animal models or humans. Upon vascular trauma, cytokines and growth factors such as granulocyte monocyte-colony stimulating factor (GM-CSF) and vascular endothelial-derived growth factor (VEGF) are released into the bloodstream and promote recruitment of EPC to the site of injury from the circulation and the bone marrow [10, 11]. Similarly, intravenous infusion of exogenous EPC after ischaemic injury results in their homing to ischaemic areas [8, 12] where they have been shown to directly contribute to blood vessel formation and increase overall density of blood vessels. The latter observation implies support of local angiogenesis by EPC in a paracrine manner, i.e. via secretion of proangiogenic factors including VEGF [12], which is known to be one of the most potent angioinductive growth factors. Even though delivery of VEGF both as a recombinant protein and via gene transfer has a certain angiogenic potential [13, 14], the number of local endothelial cells that can be stimulated by angioinductive molecules such as VEGF is limited [15]. Alternatively, formation of blood vessels can be stimulated by providing suitable cell populations such as EPC.
The T17b murine embryonal endothelial progenitor cell line has been initially characterized by Hatzopoulos et al. and shown to express early endothelial markers, differentiate to mature endothelial cells and form vascular structures in vitro and in vivo[16, 17]. Differentiation or activation of EPC was accompanied by induction of von Willebrand Factor (vWF) and VEGF receptor 2 (VEGFR-2) among other genes [16, 18]. T17b EPC are also a source of a multitude of secreted proteins that modulate angio-genesis and tissue regeneration [8]. In a rabbit model of chronic lower extremity ischaemia local retrograde intravenous infusion of T17b EPC significantly improved perfusion, capillary density and the number of collateral vessels. In the same study, systemic application improved organ function in a mouse model of myocardial infarction. Intravenously applied T17b EPC were integrated into the vasculature of pre-existing tumours with a predilection for hypoxic tumour areas where local expression of VEGF was particularly high. This co-localization of EPC and VEGF is particularly intriguing and may indicate that VEGF – the expression of which is up-regulated during hypoxia and tissue ischaemia [19]– recruits EPC via chemotaxis to ischaemic areas with high VEGF expression. In addition, EPC themselves may be the source of increased VEGF production in states and areas of hypoxia [12, 19]. VEGF stimulates proliferation of endothelial cells in an autocrine and paracrine fashion and has an antiapoptotic effect.
As previously demonstrated, T17b EPC do not express MHC I and cannot be detected by NK cells. Due to these immunological particularities, T17b will also be useful for xenogenic cell transplantation. Others and we (data not shown) have successfully transplanted these murine cells into rats without immune reaction and rejection. This makes them suitable for a variety of animal models where cell transplantation across species-barriers may be necessary and desirable.
In the present study, we aimed to characterize T17b EPC growth, morphology and VEGF secretion in vitro in cell culture and a 3-D fibrin matrix culture after hypoxia or differentiation to test their potential application in bioartificial constructs.
Materials and methods
Cell culture
T17b murine EPC were cultured in basal medium (BM) DMEM Glutamax (Invitrogen, Carlsbad, CA, USA) containing 20% foetal bovine serum, 100 U/ml Penicillin, 100 μg/ml Streptomycin, 0.1 mM (β-Mercaptoethanol, 1 mM non-essential amino acids and 2 mM HEPES buffer pH 7.5 (all purchased from Invitrogen). Cell differentiation was induced by incubation with differentiation medium (DM) where BM was supplemented with 0.5 mM dibutyryl cyclic AMP (cAMP) (Sigma Aldrich Chemie, Schnelldorf, Germany) and 1 μM retinoic acid (RA) (Sigma Aldrich Chemie) for 3 consecutive days. Cell culture supernatant was collected and stored at -80°C until further analyis. Cells were trypsinized and counted using the automatic CASY cell counting system (Schärfe System, Model DT, Reutlingen, Germany).
For characterization in 2-D cultures, T17b EPC were either seeded at 1 × 105 cells in cell culture flasks or on glass chamberslides at 2 × 104 cells per slide. The effect of hypoxia on VEGF expression was assessed by incubation under normoxic (20% O2) or hypoxic (1% O2) conditions for 48 hrs. To investigate the effect of differentiation on T17b EPC behaviour, cells were seeded at 5 × 105 cells and incubated with either DM or BM as a negative control for 4 subsequent days where medium was changed on a daily basis. In either case, T17b EPC culture supernatant was collected every 24 hrs and VEGF secretion was quantified using a VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Chamber slides seeded with T17b EPC were incubated with BM for 48 hrs to compare the effects of hypoxia to normoxic controls. To investigate the impact of differentiation, cells were incubated for 96 hrs. Chamber slides were subsequently processed for immunohistochemical staining.
T17b EPC fibrin matrices
For the 3-D fibrin matrix studies, 1 × 105 T17b EPC were re-suspended in 300 μl fibrin gel (10 mg/ml Fibrinogen, 2 IU/ml thrombin, Baxter Healthcare, Vienna, Austria, approved for clinical application). Cell-containing matrices were cultured in 24-well plates and overlayed with BM or DM. Supernatants were collected and medium was changed every other day. Fibrin matrices were harvested and underwent further processing for either homogenization and consecutive RNA extraction for subsequent PCR analysis or formalin fixation for (immuno-)histochemical analysis.
PCR analysis
Fibrin matrices were harvested after 5 days, weighed and frozen at -80° C until they were homogenized using a QIA Shredder (Qiagen, Hilden Germany) to allow for RNA extraction. RNA extraction was performed using the RNeasy mini kit (Qiagen). One microgram of total RNA from each sample underwent reverse transcriptase-polymerase chain reaction (RT-PCR) after DNAse treatment. Reverse transcription and cDNA synthesis was done using SuperScript™ III Reverse Transcriptase (Invitrogen) followed by RT-PCR analysis using 10X PCR RXM buffer, 50 mM MgCl2, Platinum TAQ DNA polymerase, dNTPs (25 mM each) according to the supplemented protocols. The following primer pairs were used for the amplification of murine reverse-transcribed products: vWF forward primer 5′-gtgaagattggctgcaacac-3′; backward primer, 5′-tgtgcttcaggaccacagag-3′; VEGFR-2 forward primer, 5′-ggaATTCAGGCATTGTACTGAGAG-3′; backward primer 5′-cggaTCCAAGTTGGTCTTTTCCTG-3′. The murine VEGFR-2 as well as GAPDH primer pairs have been published previously [18]. All agents were purchased from Invitrogen. Ten μl of each PCR mixture were electrophoresed in 1% agarose gel containing ethidium bromide for vWF. RT-PCR products were photographed under a UV transilluminator.
H&E histology and immunohistochemistry for vWF and Ki-67
Detection of von Willebrand Factor (vWF) was performed on chamber slides and Ki-67 immunostaining was done on sections derived from fibrin matrices. Matrices were fixed in 4% buffered formaldehyde, dehydrated and embedded in paraffin. Blocks were then cross-sectioned using a Leica microtome (Leica Microsystems, Bensheim, Germany) and stained with haematoxylin eosin. Images were generated using a Leica Microscope and digital camera under 100× or 400× magnifications.
Slides were rehydrated with TBS followed by antigen retrieval and two washes in TBS. Slides were then incubated overnight at 4°C with the primary antibody (Rabbit polyclonal to vWF, dilution 1:1000 Abcam Cambridge, UK # 6994; Rat Anti Mouse Ki67, dilution 1:50 Dako Cytomacion #M 7249). The secondary antibody (vWF; Goat anti-Rabbit IgG, biotinylated, Dako #E 0432, Ki-67: Polyclonal Rabbit Anti Rat IgG biotinylated, Dako Cytomacion #E 0468) and the Streptavidin/AP detection antibody (Dako Cytomation #D 0396, Glostrup, Denmark) were each diluted in normal goat serum and cycled three times for 30 min. Between incubation periods, sections were washed twice in TBS. All incubation steps took place in a humidified environment. Detection was performed by incubation with Liquid Permanent Red (Dako Cytomacion #K0640) according to the manufacturer's instructions and Hematoxilin Gill-III (Merck, Darmstadt, Germany) for counterstaining.
For semi-quantitative assessment of cell proliferation in the fibrin matrix, five regions of interest (ROI) per slide and study group were randomly selected and visualized under a 100-fold magnification. Cells as well as numbers of clusters (defined as more than five cells adjacent to each other) were counted by an independent blinded observer. Quantification of Ki-67 positive cells was carried out in an analogous fashion.
Statistical analysis
Results are given as mean ± standard deviation. Statistical analysis was performed using GraphPad prism software (GraphPad Software, San Diego, CA, USA). Two-tailed unpaired Student's t-test was applied for statistical analysis. The critical level of statistic significance chosen was P< 0.05.
Results
Hypoxia stimulates secretion of both VEGF and vWF from T17b EPC in 2-D cultures
Since hypoxia and ischaemia have been shown to induce VEGF expression, the capability of EPC to up-regulate VEGF secretion was investigated. Therefore, we submitted T17b EPC to a 48-hrs period of hypoxia at a concentration of 1% O2. VEGF secretion from hypoxic EPC was significantly stimulated compared to normoxic controls (Fig. 1A, left panel). Although the 48-hr production of VEGF reached 27.56 ± 0.75 pg/106 cells under normoxia, growth factor secretion from hypoxic T17b EPC was more than twice as high, reaching 59.57 ± 7.51 pg/106 cells. Cell morphology and cell proliferation were not changed by hypoxia (3.41 ± 0.52 × 105 cells versus 3.11 ± 0.41 × 105 cells, Fig. 1A, right panel). A 48-hr hypoxic incubation period induced vWF secretion from T17b EPC, as evidenced by a high percentage of positive staining (Fig. 1B), whereas staining was negative for cells incubated in normoxia (Fig. 1C).
1.
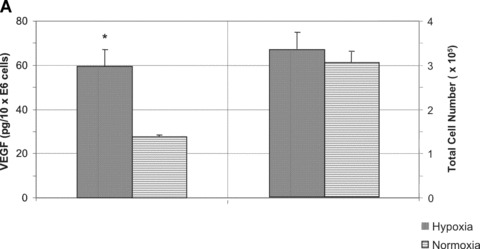
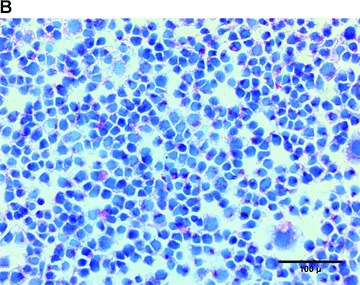
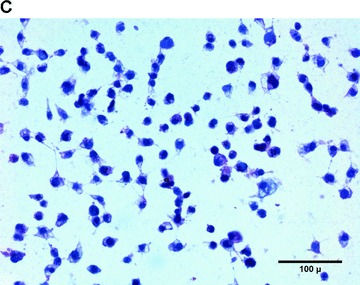
Hypoxia stimulates VEGF and vWF secretion from T17b EPC in 2-D cultures (A) 5 × 105T17b EPC were seeded in triplicates and cultured in BM and incubated either under normoxia or hypoxia (1% O2) for a total of 48 hrs without medium changes. VEGF secretion was quantified in cell culture supernatants. VEGF secretion was 59.57 pg/106 cells under hypoxia compared to 27.56 pg/106 cells compared to normoxic controls. *P< 0.05 versus normoxia. (B) T17b EPC were seeded in triplicates on glass chamber slides at 2 × 104 T17b cells per slide. A 48-hr hypoxic incubation period induced vWF secretion from T17b EPC, as evidenced by a high percentage of cells staining red positive for vWF. Also note the higher number of cells compared to normoxic controls in Panel C. (C) No vWF was seen in normoxic cells incubated in normoxia.
Differentiation with dibutyryl-cAMP and RA stimulates vWF and VEGF secretion in 2-D culture
Differentiation of T17b EPC in the presence of dibutyryl cAMP and RA resulted in morphologic changes as previously observed [16], yielding a phenotype characteristic of mature endothelial cells (data not shown). The levels of VEGF protein secretion were quantified in cell culture supernatants harvested at day 4 after initiation of differentiation by ELISA. VEGF secretion from differentiated T17b cells between day 3 and 4 was 11-fold higher compared to undifferentiated controls (95.31 ± 2.03 pg/106 cells versus 8.65 ± 0.49 pg/106 cells (Fig. 2A, left panel). Differentiation induced a proliferation arrest in 2-D cultures compared to ongoing proliferation in undifferentiated EPC (0.43 ± 0.06 × 106 cells versus 4.9 ± 0.6 × 106 cells, Fig. 2A, right panel).
2.
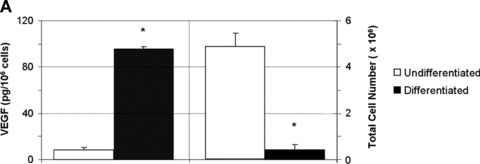
Differentiation with dibutyryl-cAMP and retinoic acid stimulates vWF expression and VEGF secretion in 2-D culture (A) 1 × 105 T17b EPC were seeded in triplicates and cultured either in basal culture medium (BM) or differentiation medium (DM), which was changed on a daily basis for a total of 4 days. VEGF concentrations were quantified in cell culture supernatants harvested at day 4 after initiation of differentiation. VEGF secretion between day 3 and day 4 after induction of differentiation was 95.31 pg/106 cells compared to 8.65/106 cells compared to undifferentiated controls, which constitutes an 11-fold increase in growth factor expression. *P< 0.05 versus undifferentiated cells. (B) T17b EPC were seeded on glass chamber slides at 2 × 104 T17b cells per slide. Successful differentiation of T17b EPC was induced by DM for 4 consecutive days, resulting in a high rate of vWF-secreting cells as demonstrated by abundant positive red staining. No specific staining indicating absence of vWF was noted in BM overlayed T17b (Panel 1.C).
T17b differentiation on chamber slide cultures with dibutyryl-cAMP and RA lead to an abundant presence of vWF (Fig. 2B).
Differentiation induces VEGF secretion from T17b EPC suspended in a fibrin matrix
To investigate their applicability in matrices used for tissue engineering, T17b EPC were suspended in a 3-D fibrin matrix approved for clinical application. Cells retained their vitality for an observation period of at least 8 days and displayed functionality, which was manifested by secretion of substantial amounts of VEGF into the supernatant medium of the fibrin matrix. In order to normalize secreted VEGF to the cell number in the matrix, total RNA was extracted and the relative amounts of VEGF secreted per μg of total RNA was determined. In consistence with the results observed in the 2-D cell culture, differentiated EPC secreted 37% higher VEGF levels per μg RNA compared to undifferentiated controls (1451 ± 102 pg/μg RNA versus 1062 ± 20.57 pg/μg RNA, P< 0.05, Fig. 3A). No VEGF expression was detected in cell-free matrices, which served as negative controls (Fig. 3A, right panel). RNA yield generated from BM matrices, on the other hand, was 4 times higher compared to DM matrices (0.444 ± 0.028 μg versus 0.112 μg ± 0.012, P< 0.05, Fig. 3B), indicating the number of cells present in DM matrices was significantly higher.
3.
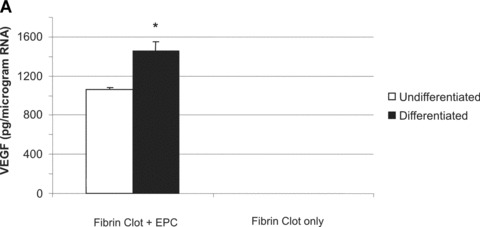
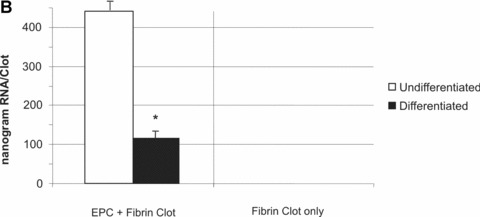
(A) 1 × 105 T17b EPC were suspended in a fibrin matrix and overlayed either with BM or DM. Medium was changed every 48 hrs. After 8 days, supernatant medium was collected for quantification of VEGF secretion by ELISA. RNA was extracted from fibrin clots and its concentration measured. Results are from triplicate samples and presented as picograms of VEGF expressed per microgram of T17b RNA present in the fibrin clots. VEGF expression was 37% higher in overlay medium from DM clots compared to BM clots (1.062 ± 20.57 ng versus 1.451 ± 102.85 ng). *P< 0.05 versus undifferentiated cells (B) RNA yield generated from BM clots was 4 times higher compared to DM clots (0.444 ± 0.028 μg versus 0.112 μg ± 0.012, P < 0.05, Fig. 3.B), indicating the number of cells present in DM clots was much higher. *P< 0.05 versus undifferentiated cells.
T17b morphology in 3-D fibrin matrix cultures
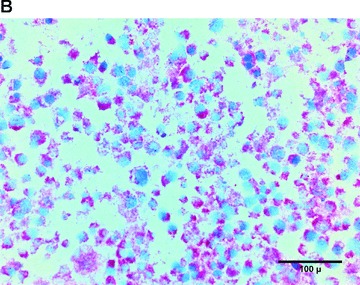
In the fibrin matrix, morphology and growth properties of T17 EPC were observed and their proliferation was quantified by immunohis-tochemical analysis of Ki-67. Upon differentiation with dibutyryl-cAMP and RA, EPC proliferation appeared to be inhibited with low cell numbers and little cluster formation. These findings were confirmed by the lack of Ki-67 positive T17b cells (Fig. 4A–C). In contrary, after incubation with BM, morphologic observation showed cell proliferation and growth in clusters, some of them forming lumen-like structures. Morphologic observations were endorsed by the presence of Ki-67-positive cells, indicating cell proliferation up to the end of the observation period on day 8 (Fig. 4D–F).
4.
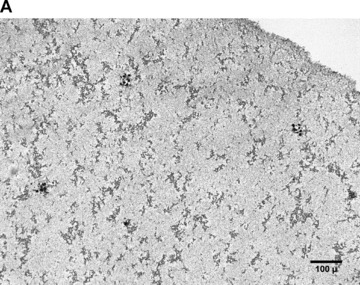
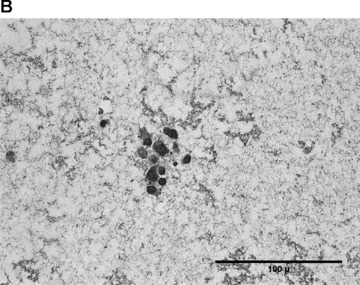
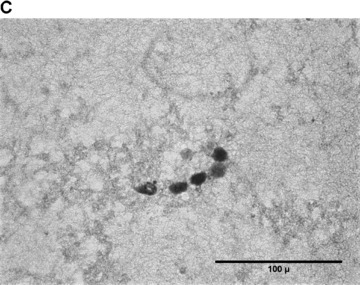
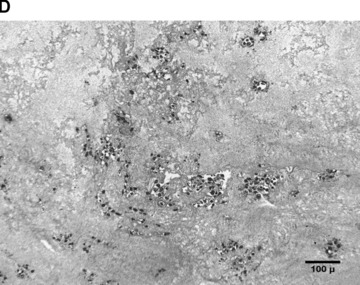
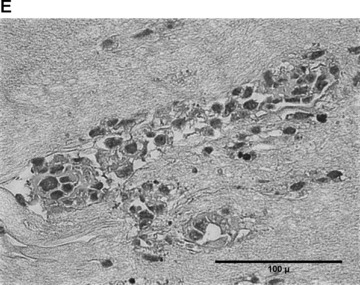
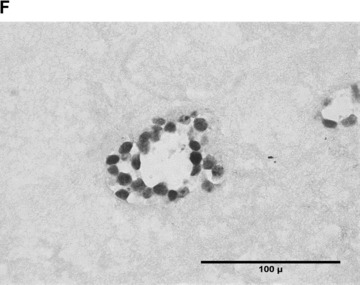
Morphology of T17b EPC suspended in fibrin matrices under BM or DM incubation. 1 × 105 cells were suspended in a fibrin matrix and over-layed with either BM or DM, which was changed every 48 hrs. Cell-containing matrices were harvested 8 days later and submitted to H&E standard histology for morphological analysis. Results are based on triplicate samples per group. (A-C) DM incubation in 100× (A) and 400× (B) magnification and Ki-67 expression (C). Upon differentiation with dibutyryl-cAMP and retinoic acid, EPC proliferation appeared to be inhibited with low cell numbers and little cluster formation. These findings were confirmed by the lack of Ki-67 positive T17b cells. (D-F) BM incubation in 100× (A) and 400× (B) magnification and Ki-67 expression (C). In contrary, after incubation with basal medium, morphologic observation showed cell proliferation and growth in clusters, some of them forming lumen-like structures. Morphologic observations were endorsed by the presence of Ki-67-positive cells, indicating cell proliferation up to the end of the observation period on day 8.
For semi-quantitative assessment of growth patterns, cells and cell clusters were counted in 5 randomly selected areas per slide per study group. DM leads to a reduction in cell number and cell clusters (Fig. 5A) from day 3 to day 8 (60 cells/ROI versus 46 cells/ROI, P< 0.05). On the other hand, BM incubation resulted in higher numbers of cells and cell clusters at both times and an increase of cell numbers and number of cell clusters (131 cell/ROI versus 166 cell/ROI, P< 0.05). The difference in cell numbers on day 8 between BM and DM groups was considered statistically significant (Fig. 5A). Similar differences were noticed when the number of cell clusters was counted, again reaching statistically significant differences on day 8 (Fig. 5B).
5.
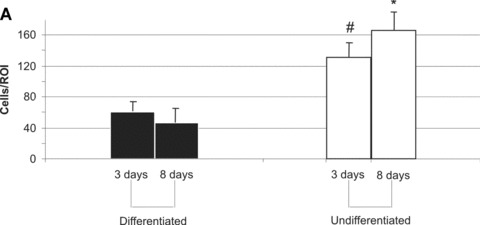
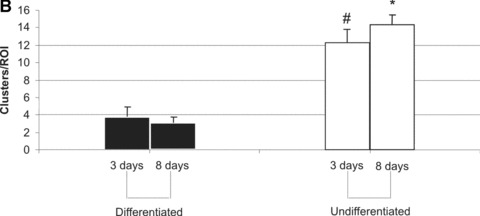
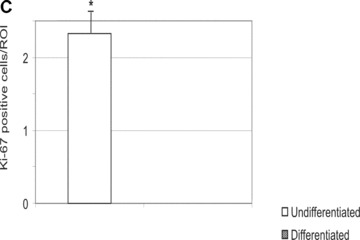
T17b cell proliferation (A) and cluster formation (B) in fibrin matrices. Five regions of interest (ROI) per slide and study group were randomly selected and visualized under a 100-fold magnification. Cells as well as numbers of clusters (defined as more than five cells adjacent to each other) were counted. Mean values and S.D. are shown. #P< 0.05 versus differentiated cells on day 3. *P< 0.05 versus differentiated cells on day 8.
T17b PCR in 3-D cultures
In order to investigate differentiation of T17b EPC in fibrin matrices, expression of the differentiation markers vWF and VEGFR-2 was analysed with PCR. In conformance with previously published results for 2-D cell cultures [16], we detected increased expression of vWF as well as VEGFR-2 after differentiation compared to undifferentiated controls (Fig. 6).
6.

vWF and VEGFR-2 detection in PCR from Fibrin-matrix suspended T17b EPC. Fibrin clots were harvested and RNA extracted followed by reverse transcription and PCR analysis for vWF and VEGFR-2 expression from EPC suspended in the fibrin matrix. GAPDH served as a control in both instances. vWF as well as VEGFR-2 are detectable in fibrin-suspended cells overlayed with BM, but both markers were strongly up-regulated after differentiation with dibutyryl-cAMP and RA. Results are representative of experiments executed in triplicates.
Discussion
EPC have been shown to play a key role in blood vessels formation in ischaemic conditions. By comparison, only few studies have investigated their behaviour in bioartificial matrices and their potential role in vascularization of these constructs, which remains one of the main challenges in tissue engineering.
In the present study, the murine embryonal endothelial progenitor cell line T17b was used because it offers significant experimental advantages compared to EPC of human origin. These cells are easy to grow, have been valuable in investigation of mediators, signalling pathways, and altered cellular properties that play a role in early vascular development and demonstrated angiogenic potential in a multitude of in vitro and in vivo studies [8, 12, 16–18]. A significant advantage over human EPC can be attributed to the fact that they can be transplanted xenogenically into immunocompetent recipients such as rats and rabbits [8], whereas the use of human EPC in vivo is restricted to immunodeficient animals such as nude mice. Due to size limitation of the animal, many surgical in vivo models of angiogenesis including the arteriovenous (AV) loop model in the rat for tissue engineering [27] are not applicable to a murine model, thus restricting attempts to generate larger units of vascularized bioartificial tissue in vivo.
In the present study, we investigated T17b murine embryonal EPC in terms of morphology, proliferative potential and VEGF secretion in response to hypoxia and differentiation in vitro in cell culture and a clinically approved fibrin matrix culture.
Hypoxia can stimulate VEGF secretion as well as vWF expression and cell proliferation in 2-D culture
VEGF is an important angiogenic factor in blood vessel formation and plays a key role in the angiogenic function of EPC [20]. Since previous studies showed co-localization of T17b EPC and VEGF expression preferentially in areas of tissue hypoxia [12], we examined the influence of hypoxia on VEGF secretion and cell proliferation. Indeed, hypoxia increased VEGF secretion from EPC by more than 100% compared to normoxic controls. This result is consistent with recent findings by Diez et al. who measured increased VEGF mRNA levels in hypoxic T17b EPC [21]. Although hypoxia did not induce changes in cell morphology consistent with differentiation, it was associated with an increased proliferation rate under certain conditions. Our results show that T17b cell proliferation was enhanced in chamberslide cultures but not in cell culture dishes, which may indicate that hypoxia could indeed stimulate EPC proliferation in association with certain in vitro surface properties or when cells are seeded at certain densities. A particularly interesting finding was that vWF expression was increased compared to normoxic controls. Indeed, vWF has been identified as one of the main differentiation markers in this embryonal endothelial progenitor cell line [16]. Hence, hypoxia has diverse effects on T17b EPC as it induces up-regulation of a gene indicating differentiation although the morphologic changes typical for differentiation are absent, and even appears to stimulate cell proliferation compared to normoxic EPC under certain conditions.
Differentiation by RA and dibutyryl-cAMP increases VEGF secretion as well as vWF expression and inhibits cell proliferation in 2-D culture
After induction of differentiation with cAMP and dibutyryl-cAMP, up-regulation of VEGF secretion was even more pronounced, with 11-fold higher growth factor levels in supernatant medium. Previous studies did not detect increased VEGF gene expression after differentiation [16, 18]. A possible explanation of our finding may be that even though differentiation does not significantly alter VEGF gene expression itself, it may stabilize VEGF mRNA and lead to increased VEGF expression and secretion on the protein level.
In contrary to hypoxia experiments where morphologic changes in T17b EPC were not observed, differentiation lead to morphological transformation consistent with a mature endothelial cell phenotype as previously published [16]. Addition of dibutyryl-cAMP and RA resulted in a profound increase of vWF expression, as evidenced by positive immunohistochemical staining for the majority of cells, without any increase in cellular proliferation.
VEGF protein levels and mRNA expression of vWF and VEGFR-2 are enhanced by RA and dibutyryl-cAMP
A detailed analysis on how differentiation of T17b EPC altered expression of genes crucial in angiogenesis and vasculogenesis has been carried out previously, showing up-regulation of vWF and VEGFR-2 [9]. Here, we demonstrated that VEGF protein levels are increased in supernatant after EPC activation with dibutyryl-cAMP and RA. To determine whether EPC suspended in a fibrin matrix retain the potential to react to the differentiation stimulus and express vWF and VEGFR-2, RNA was extracted from homogenized fibrin clots followed by PCR analysis of the cDNA products. We found a substantial increase in both vWF and VEGFR-2 gene expression. A possible explanation for the latter finding may be the increased VEGF levels we found in the matrix supernatants after differentiation, given that it has been shown that VEGF up-regulates its own receptor VEGFR-2 [22, 23].
Although previous work found undifferentiated T17b EPC to express only trace quantities of vWF mRNA and no detectable VEGFR-2 mRNA [16], a higher vWF mRNA signal along with detectable VEGFR-2 mRNA levels were present in EPC suspended in the fibrin matrix. This may indicate that the fibrin matrix by itself may have a certain differentiation-inducing effect.
EPC growth properties after suspension in a fibrin matrix
Analysis of T17b EPC behaviour in a fibrin matrix under selected conditions was performed for the first time to simulate their putative actions in the three-dimensional space in vivo. The fibrin matrix used in this study is FDA approved and has been used clinically, providing a particular clinical relevance to the present study. We have previously used this matrix in in vivo experiments alone and with growth factors [13, 24, 25]. Previous growth in matrigel culture showed a gradual phenotypic change towards the mature endothelial cell type over an observation period of 8 days with concomitant re-orientation of cells and formation of tubule-like structures [16]. However, use of Matrigel will never be approved in the clinical situation and therefore, use of a clinically approved fibrin matrix appears more relevant to potential therapeutic applications in the future. Here, we demonstrated survival of T17b cells for at least 8 days in a 3-dimensional fibrin matrix in vitro. After incubation with DM, cell proliferation was inhibited as seen by the absence of increase in cell numbers and number of clusters between days 3 and 8 in the follow-up period. In contrast, BM resulted in significant T17b cell proliferation, with an increase in cell numbers and cluster numbers from day 3 to day 8. The semi-quantitative cell count was confirmed by immunohistochemical for the proliferation marker Ki-67, staining positive with cells undergoing mitosis. These cells do not only form multicellular clusters, but also show circular arrangement of the cells with lumen-like structures in the centre.
Summary
In summary, we were able to show that T17b EPC can survive in a fibrin matrix and exhibit proliferation in this matrix over a period of 8 days. Differentiation can be induced by addition of DM, as demonstrated by reduced cell proliferation rate as well as by up-regulation of the differentiation markers vWF and VEGFR-2. Moreover, cell culture analysis demonstrated increased VEGF secretion upon differentiation as well as under hypoxic conditions. Taken together, these data indicate that EPC may be a promising tool that could be combined with other therapeutic strategies such as gene transfer [26] to enhance vascularization in several different (pre-)clinical settings where vascularization is desirable.
EPC-based therapy may be a valuable tool to induce formation of blood vessels in bioartificial matrices conducive towards tissue engineering. Their role in modulation of in vivo angiogenesis is currently under investigation in our laboratory. The use of EPC transplantation to enhance vascularization of bioartificial matrices embedded in an arteriovenous loop in a rat separation chamber model [27] may be a promising approach to enhance the angio-genic potential in this setting.
Acknowledgments
This study was supported by research grants from the Xue-Hong and Gans Georg Geis Foundation as well as the University of Erlangen (ELAN Program). The authors thank Katja Schubert and Ilse Arnold for their excellent technical assistance. This work contains parts of Matthias Hammon's doctoral thesis.
References
- 1.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:4–6. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiegel HC, Kaufmann PM, Bruns H, Kluth D, Horch RE, Vacanti JP, Kneser U. Review: hepatic tissue engineering. J Cell Mol Med. 2008;12:56–66. doi: 10.1111/j.1582-4934.2007.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polykandriotis E, Arkudas A, Horch RE, Stürzl M, Kneser U. Autonomously vascularised cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med. 2007;11:6–20. doi: 10.1111/j.1582-4934.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilising and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–46. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trentin D, Hall H, Wechsler S, Hubbell JA. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc Natl Acad Sci. 2006;103:2506–11. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–6. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 8.Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, Thalgott M, Buttner K, Browarzyk C, Mages J, Hoffmann R, Deten A, Lamparter M, Muller F, Beck H, Buning H, Boekstegers P, Hatzopoulos AK. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–88. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 9.Stahl A, Wu X, Wenger A, Klagsbrun M, Kurschat P. Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: a model for bone replacement grafts. FEBS Lett. 2005;579:5338–42. doi: 10.1016/j.febslet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Bengel FM, Schachinger V, Dimmeler S. Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging. 2005;32:S404–16. doi: 10.1007/s00259-005-1898-5. [DOI] [PubMed] [Google Scholar]
- 11.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 12.Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, Vlastos GA, Chan G, Fischer KD, Rattat D, Debatin KM, Hatzopoulos AK, Beltinger C. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5:477–88. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 13.Arkudas A, Tjiawi J, Bleiziffer O, Grabinger L, Polykandriotis E, Beier JP, Sturzl M, Horch RE, Kneser U. Fibrin Gel-immobilised VEGF and bFGFefficiently stimulate angiogenesis in the AV loop model. Mol Med. 2007;13:480–7. doi: 10.2119/2007-00057.Arkudas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalka C, Masuda H, Tomono T, Kalka-Moll W, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex-vivo expanded endothelial progenitor cells for therapeutic neovascularisation. Proc Natl Acad Sci. 1999;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Tepper OM, Galiano RD, Capla JM, Baharestani S, Kleinman ME, Pelo CR, Levine JP, Gurtner GC. Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg. 2004;113:284–93. doi: 10.1097/01.PRS.0000091169.51035.A5. [DOI] [PubMed] [Google Scholar]
- 16.Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–68. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 17.Bidzhekov K, Hautmann M, Semisch M, Weber C, Engelmann B, Hatzopoulos AK. Rafs constitute a nodal point in the regulation of embryonic endothelial progenitor cell growth and differentiation. J Cell Mol Med. 2007;11:1395–1407. doi: 10.1111/j.1582-4934.2007.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, Vestweber D, Hatzopoulos AK. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–65. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill M, Dias K, Hattori ML, Rivera D, Hicklin L, Witte L, Girardi R, Yurt H, Himel S, Rafii S. Vascular trauma induces rapid but transient mobilisation of VEGFR2+ AC133+ endothelial precursor cells. Circ Res. 2001;88:167–74. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 20.Smadja DM, Bieche I, Helley D, Laurendeau I, Simonin G, Muller L, Aiach M, Gaussem P. Increased VEGFR2 expression during human late endothelial progenitor cell expansion enhances in vitro angiogenesis with up-regulation of integrin a6. J Cell Mol Med. 2007;11:1149–61. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, Breier G, Gessler M. Hypoxia-mediated activation of DII4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Donner DB, Warren RS. Homeostatic modulation of cell surface KDR and Flt1 expression and expression of the vascular endothelial growth factor (VEGF) receptor mRNAs by VEGF. J Biol Chem. 2000;21:15905–11. doi: 10.1074/jbc.M001847200. [DOI] [PubMed] [Google Scholar]
- 23.Herve MA, Bluteau-Lozano H, Mourah S, Calvo F, Perrot Applanat M. VEGF 189 stimulates endothelial cell proliferation and migration in vitro and up-regulates the expression of Flk-1/KDR mRNA. Exp Cell Res. 2005;1:24–31. doi: 10.1016/j.yexcr.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Polykandriotis E, Tjiawi J, Euler S, Arkudas A, Hess A, Brune K, Greil P, Lametschwanother A, Horch RE, Kneser U. The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc Res. 2008;78:25–33. doi: 10.1016/j.mvr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Bach AD, Arkudas A, Tjiawi J, Polykandriotis E, Kneser U, Horch RE, Beier JP. A new approach to tissue engineering of vascularised skeletal muscle. J Cell Mol Med. 2006;10:716–26. doi: 10.1111/j.1582-4934.2006.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleiziffer O, Eriksson E, Yao F, Horch RE, Kneser U. Gene transfer strategies in tissue engineering. J Cell Mol Med. 2007;11:206–23. doi: 10.1111/j.1582-4934.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kneser U, Polykandriotis E, Euler S, Grabinger L, Heidner K, Amann M, Hess A, Stuerzl M, Horch RE. Induction of axial vascularization in processed bovine cancellous bone matrices using a microsurgically created arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]


