Abstract
This study tracks the fate of antigen-reactive B cells through follicular and extrafollicular responses and addresses the function of CD40 in these processes. The unique feature of this system is the use of transgenic B cells in which the heavy chain locus has been altered by site-directed insertion of a rearranged VH DJH exon such that they are able to clonally expand, isotype-switch and follow a normal course of differentiation upon immunization. These Ig transgenic B cells when adoptively transferred into non-transgenic (Tg) mice in measured amounts expanded and differentiated distinctively in response to T cell-independent (TI) or T cell-dependent (TD) antigens. The capacity of these Tg B cells to faithfully recapitulate the humoral immune response to TI and TD antigens provides the means to track clonal B cell behavior in vivo. Challenge with TI antigen in the presence of agonistic anti-CD40 mAb resulted in well-defined alterations of the TI response. In vivo triggering of Tg B cells with TI antigen and CD40 caused an increase in the levels IgG produced and a broadening of the Ig isotype profile, characteristics which partially mimic TD responses. Although some TD characteristics were induced by TI antigen and CD40 triggering, the Tg B cells failed to acquire a germinal center phenotype and failed to generate a memory response. Therefore, TD-like immunity can be only partially reconstituted with CD40 agonists and TI antigens, suggesting that there are additional signals required for germinal center formation and development of memory.
Keywords: B lymphocyte, Isotype switching, Memory, Adoptive transfer, Transgenic mouse
1 Introduction
The use of receptor transgenic (Tg) T and B cells has revolutionized our capacity to visualize cell-mediated and humoral-mediated immune responses in vivo. The transfer of small numbers of easily identifiable Tg lymphocytes into non-Tg recipients has allowed the tracking of their fate and function in an environment where the Tg lymphocytes are not overly abundant. Over the past few years, a number of reports have described the creation of Ig Tg mice in which the heavy chain locus has been altered by site-directed insertion of a rearranged VH DJH exon such that the B cells are able to isotype-switch the transgene. Unlike mice with randomly inserted Ig genes, these novel mice offer the potential to track the fate of Ag-specific B cells that can clonally expand, differentiate, affinity mature and isotype-switch in vivo. QM (quasi-monoclonal) mice were generated by the site-directed insertion of a rearranged VDJ into the heavy chain locus with specificity to the hapten, nitrophenol (NP) [1, 2]. These mice are heterozygous at the heavy chain locus with one allele containing a rearranged VDJ exon encoding an H-chain V region specific for NP, and bears the 17.2.25 idiotype. The other heavy chain allele is inactivated by the deletion of all JH gene segments. In addition, these Tg mice have been backbred onto Jk knockout mice, thereby allowing only the production of NP-specific λ -bearing Ab. This report is the first to document that QM Tg B cells faithfully recapitulate responses to T cell-independent (TI) and T cell-dependent (TD) Ag. Detailed analysis of their expansion, differentiation, Ig production, and memory B cell formation upon immunization is presented.
This system is exploited to begin the study of the signals that combine to induce the differentiation of follicular mantle B cells to germinal center (GC) B cells and memory B cells. We have used an initiating signal of a TI Ag followed by a CD40 agonist (anti-CD40) to isolate the effects of CD40 triggering on the fate of actively expanding B cells in vivo. The combined administration of the TI Ag, NP-Ficoll, and anti-CD40 resulted in increased levels of NP-specific Ab and a broadening of the Ig-isotype profile, yet did not induce a GC phenotype nor elicit B cell memory to NP. These results show that signaling through CD40 can reconstitute certain components of a TD humoral immune response; however, the acquisition of additional features (like GC phenotype and B cell memory) require other signals yet to be identified.
2 Results
2.1 QM Tg B cells markedly expand in response to both TD and TI antigens
Functional studies utilizing Ig transgenic B cells specific for hen egg lysozyme [3, 4] or H-2k MHC class I molecules [5] have elucidated the requirement of both Ig engagement and cognate T cell help for efficient B cell activation. However, these studies have provided little information with regard to identifying potential signals leading to B cell differentiation, due to the fact that the Ig transgenic B cells used in these model systems were incapable of isotype switching and limited in their capacity to undergo somatic mutation. Therefore, we have developed a similar strategy utilizing QM Tg B cells which retain the capacity to isotype switch and undergo somatic mutation to study the factors involved in regulating B cell differentiation [1, 2]. The Ab produced by these cells are of the Igha allotype and recognize the NP hapten, thus they are easily identified by virtue of their binding to NP-PE or a specific anti-VH17.2.25 idiotype mAb. To evaluate the regulation of clonal expansion and differentiation, small yet detectable numbers of Tg B cells were transferred into H-2- and sex-matched recipient mice as described in Sect. 4.2. The first series of experiments tested whether these Tg B cells could expand in vivo in response to the TI antigen, NP-Ficoll and the TD antigen, NP-KLH. Fig. 1A shows a plot from a representative experiment and clearly identifies the Tg B cell population (NP+ B220+) in spleen and lymph nodes taken from naive mice. Likewise, Tg B cells were readily distinguished in both TD and TI immunized mice and exhibited a frequency that was enhanced compared to naive controls. To elucidate the kinetics of Tg B cell expansion and contraction, parallel experiments were set up to determine the total number of splenic and lymph node Tg B cells on days 3, 5, and 7 post-immunization. Results shown in Fig. 1 demonstrate that Tg B cell expansion from TI (panel B) and TD (panel C) immune mice peaks at day 5 post-immunization and rapidly declines thereafter. Levels of Tg B cell expansion showed approximately a three- to sixfold increase over basal levels observed in naïve mice on day 5. In contrast, the expansion of endogenous NP-specific B cells from recipients that did not receive Tg B cells (no cells) was minimal. To determine that the Tg B cell expansion from mice challenged with NP-KLH was dependent on carrier-specific T cells, adoptive transfer experiments were also performed using recipient mice that were primed with an irrelevant carrier protein (data not shown). Results from these studies revealed that Tg B cells failed to expand, demonstrating that the response to NP-KLH is T cell dependent and also required the hapten to be physically linked to the carrier. These findings are consistent with a previous report that utilized HEL-specific B cells in a similar system [3].
Fig. 1.
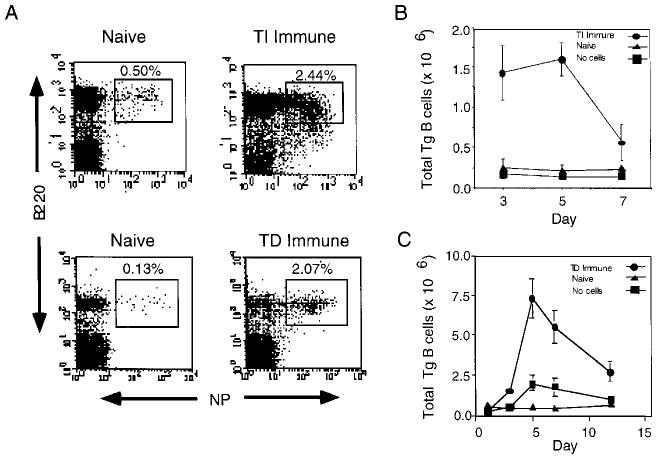
Expansion of Tg B cells in response to NP-Ficoll and NP-KLH. Recipients were challenged with either PBS (Naive), NP-Ficoll (TI Immune), or NP-KLH (TD Immune). (A) Spleen cells from naive and TI Immune recipients or lymph node cells from naive and TD Immune recipients were stained with NP-PE and B220 CyC on day 5 post-immunization. Kinetics of Tg B cell expansion from TI Immune (B) and TD Immune (C) recipients are shown on the indicated days. Mice that did not receive Tg B cells (no cells) were challenged with NP-Ficoll (B) or NP-KLH (C) and spleen or lymph node cells were used to control for the endogenous B cell response. Cell numbers are representative of three mice per group, and are normalized to the total number of spleen cells collected. Plots are representative of ten experiments.
2.2 QM Tg B cells faithfully recapitulate TD and TI responses in vivo
A hallmark of B cell differentiation is the capacity of antigen-activated B cells to undergo Ig class switching, and therefore express downstream isotypes bearing identical antigen-specificity [6]. Previous reports have demonstrated that the humoral immune responses to the TI and TD forms of the hapten NP are dramatically different. Like most TI antigens, NP-Ficoll induces primarily IgM Ab and to a lesser extent IgG; in contrast, IgG Ab tend to dominate the primary response to NP when coupled to a protein carrier [7, 8]. To test whether QM Tg B cells have the ability to isotype switch in response to NP-Ficoll, adoptive transfer experiments were performed and surface phenotype of Tg cells was assessed. On day 5 post-immunization, splenic B cells were stained with IgM-, IgD-, and IgG1-specific mAb to determine levels of Ig expression. Tg B cells (B220+NP+) from naive recipients expressed high levels of both IgM and IgD but no measurable IgG above background as shown in Fig. 2A. In contrast, Tg cells isolated from immune recipients showed decreased levels of IgM and IgD. While the relative intensity of IgM appears to decrease, levels remain significantly higher than those seen with IgD expression. Interestingly, the analysis of IgG1 expression levels on Tg cells from immune mice demonstrated that a small population of cells stained positive for IgG1, and suggest that a portion of Tg B cells have the capacity to undergo isotype-switching in response to NP-Ficoll. It is not surprising that the majority of Tg cells remain IgG-negative and represent either the IgM-positive population or B cells which have terminally differentiated into Ab-secreting cells. Consistent with the above findings, is the observation that Tg B cells also down-regulate IgM and IgD in response to NP-KLH (Fig. 2B). IgG1-bearing QM cells were likewise observed in recipients challenged with NP-KLH but not in naive controls.
Fig. 2.
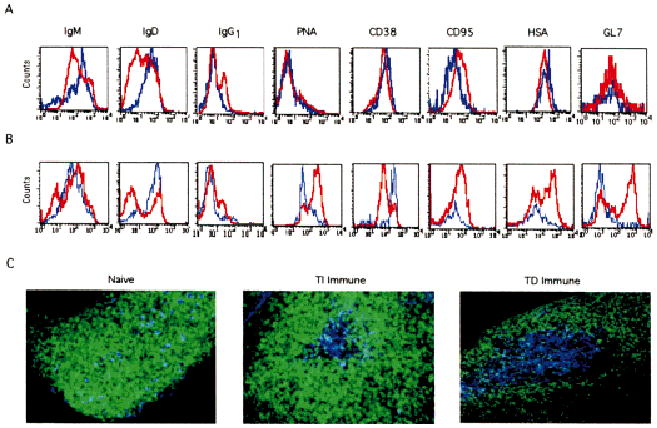
Phenotypic analysis of TI and TD immune Tg B cells. On day 5 post-immunization with either NP-Ficoll (A) or NP-KLH (B), spleen and lymph node cells were stained with IgM, IgD, IgG1, PNA, CD38, GL7, HSA, and CD95 in combination with B220 CyC and NP-PE. Histograms shown represent the relative intensity of the indicated marker on B220+NP+ gated cells from naive (blue line) and immune (red line) mice. (C) Intact spleens from naive and TI immune recipients, or inguinal lymph nodes from TD immune recipients were sectioned as described and stained with IgD FITC (green) and anti-NP idiotype 17.2.25 Cy5 (blue), followed by confocal image analysis. Objective: ×20, zoom 1.5. Data are representative of six experiments.
One characteristic of a TI immune response is the lack of GC formation [9, 10]. Thus, it was predicted that QM Tg B cells would not acquire a GC phenotype in response to NP-Ficoll. Tg B cells from immune recipients lacked peanut agglutinin (PNA) staining and failed to down-regulate CD38 expression (Fig. 2A), a phenotype otherwise consistent with murine GC B cells [11, 12]. Like their naive counterparts, Tg B cells from recipients challenged with NP-Ficoll failed to express the GC marker, GL7 [13]. Tg B cells, however, were capable of exhibiting a GC phenotype when recipients were challenged with NP-KLH (Fig. 2B). The majority of Tg cells bind PNA, down-regulate CD38, and express GL7. Together, these data are consistent with previous reports on TI immune responses [9, 10], and indicate that Ag-activated Tg B cells undergo isotype-switching in the absence of GC formation. The up-regulation of CD95 on Tg B cells from immune mice, regardless of the source of Ag, further confirmed that these cells were Ag-activated in comparison to mature resting Tg B cells from naïve mice. Of interest, is the observation that levels of HSA expression increased on Tg B cells isolated from recipients that were challenged with NP-KLH but not NP-Ficoll. Up-regulation of HSA has been previously reported to be a marker of memory B cells [14]. The elevation of HSA observed in response to NP-KLH may reflect those Tg B cells that have committed to the memory B cell compartment. The observation that NP-KLH but not NP-Ficoll can generate a Tg B cell memory response (see Fig. 6) may correlate with the differential expression of HSA.
Fig. 6.
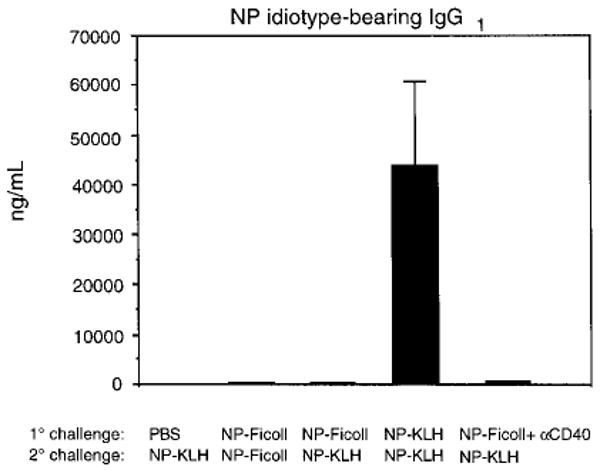
Ig secretion analysis of Tg B cells upon secondary challenge with low dose Ag. Secondary adoptive transfer experiments were carried out as described. Recipient mice were challenged i.p. with either NP-Ficoll (25 μg) or a low dose of NP-KLH (1.0 μg). Sera was collected 5 days later and assayed for NP-idiotype IgG1 by ELISA. Data are representative of three independent experiments.
Studies were undertaken to examine the histology of viable intact spleen or lymph node sections from recipient mice. On day 5 post-immunization, tissue sections taken from intact spleens of naive and TI immune mice, and inguinal lymph nodes from TD immune mice were prepared. Fig. 2C shows two micrographs representing a follicle from either a naive or TI immune spleen section. A dense population of mature IgD+ B cells (green) were seen surrounded by red pulp (not shown) from spleens of naive recipients. Scattered throughout the primary follicles from naive mice are detectable numbers of transferred Tg B cells that concomitantly stain for IgD (light blue). In contrast, follicles examined from TI immune mice consistently showed a central pocket of Tg B cells (bright blue) that lacked IgD expression. Follicles from lymph nodes taken from TD immune mice revealed clusters of anti-NP idiotype bearing Tg B cells that also exhibited decreased levels of IgD; however, B cell clusters that formed in response to NP-KLH but not NP-Ficoll exhibited a GC phenotype by losing CD38 expression (see Fig. 4C).
Fig. 4.
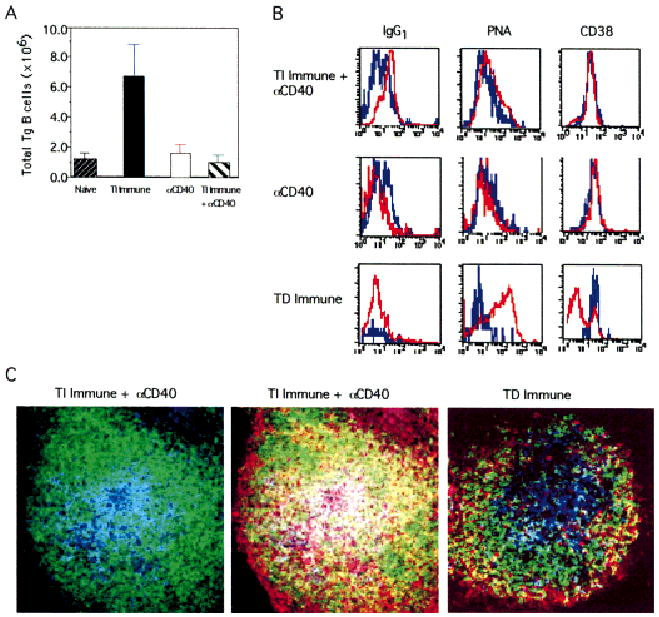
Expansion and phenotype of Tg B cells in response to NP-Ficoll plus anti-CD40 treatment. On day 5 post-immunization, splenocytes from recipient mice injected with PBS (Naive), NP-Ficoll (TI Immune), FGK115 (αCD40), or NP-Ficoll plus FGK115 (TI Immune + αD40) were stained with B220 and NP. (A) Expansion of Tg B cells. (B) Histograms represent relative intensities of the indicated marker on NP+B220+ gated B cells from naïve mice (blue line) and TI immune mice treated with anti-CD40, TD immune mice, or mice treated with anti-CD40 alone (red lines). (C) Spleen sections from TI immune + anti-CD40 mice were stained with IgD FITC (green), anti-NP Id 17.2.25 Cy5 (blue), and CD38 PE (red). Both micrographs depict the same follicle and show a two-color analysis with IgD and NP-Id (left panel), and a three-color analysis with all three markers (middle panel). As a control, a lymph node section from recipients challenged with NP-KLH is shown in right panel. Objective: ×20, zoom 1.5. Data are representative of three independent experiments.
2.3 QM Tg B cells isotype switch to produce NP-specific IgG in vivo
A distinguishing factor of using QM Tg cells, as opposed to conventional Tg B cells, is their ability to isotype switch. We therefore sought to examine the capacity of QM Tg B cells to produce NP-specific IgG in vivo upon challenge with NP-Ficoll. Serum from mice was tested for Ag-specific total IgG by ELISA. As shown in Fig. 3, NP-specific IgG was detected in immune but not naive mice. Recipients that did not receive Tg cells and were challenged with Ag failed to generate NP-specific IgG, and indicated that the endogenous host response is below the level of detection at this time point. Previous studies have reported that the earliest NP-specific IgG measured in a primary response of C57BL/6 mice is 9 days after challenge [8]. Collectively, these findings demonstrate that Ag-specific humoral responses can be measured by adoptively transferring QM Tg B cells into syngeneic recipients and show a characteristic TI phenotype consistent with previous reports.
Fig. 3.
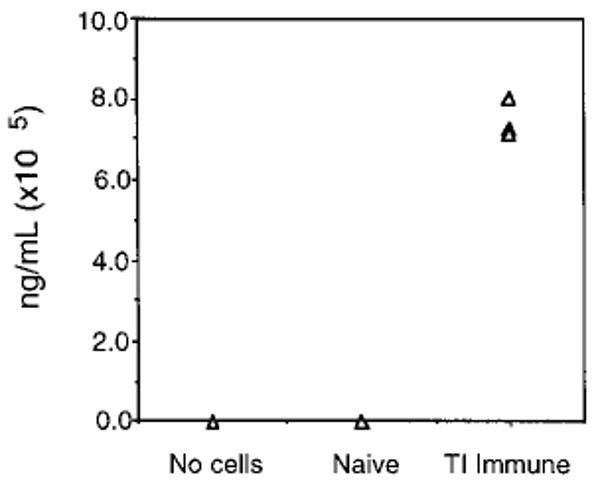
Detection of total NP-specific IgG. On day 5 post-immunization, sera was collected and assayed for NP-specific IgG by ELISA. Each symbol represents one mouse per group. One of five experiments.
2.4 Triggering via CD40 synergizes with TI signals to enhance IgG production
Since CD154 is an integral signal in the development of TD responses, we sought to examine whether triggering by a TI Ag and CD40 could reconstitute TD responses. To address this issue, adoptive transfer experiments were performed in the presence of an agonistic anti-CD40 mAb to mimic T cell help. To test whether CD40 ligation could alter the profile of NP-specific IgG Ab, sera was collected on day 5 post-immunization and assayed for NP-specific IgG1, IgG2a, and IgG3. Table 1 depicts levels of NP-specific IgG isotypes from a representative experiment. TI immune mice treated with anti-CD40 produced 24-fold greater amounts of Ag-specific IgG1, and 80-fold greater amounts of IgG2a than mice challenged with NP-Ficoll alone. High levels of IgG1 and IgG2a are consistent with humoral immune responses to TD Ag. This is further supported by elevated levels of NP-specific IgG1 and IgG2a in recipients that were challenged with NP-KLH (TD immune). Mice treated with anti-CD40 alone produced levels of IgG1 and IgG2a equivalent to naïve recipients, further supporting the fact that administration of this dose of anti-CD40 mAb does not elicit polyclonal B cell activation. Similar levels of IgG3 were observed between naïve and immune mice; however, a complete absence of IgG3 was seen in anti-CD40-treated mice. Strikingly, TI immune mice treated with anti-CD40 generated approximately a 8-fold increase in IgG3 over naive and immune groups. Studies using a variety of experimental models for TI Ag have previously reported that the IgG3 subclass is distinctly generated [15, 16]. These findings demonstrate that CD40 ligation can augment the production of Ag-specific Ab and alter the isotype.
Table 1.
IgG isotypes induced by CD40 ligation in response to NP-Ficolla)
| Recipients | NP-specific IgG isotypes (ng/ml) | ||
|---|---|---|---|
| IgG1 | IgG2a | IgG3 | |
| Naive | n.d.b) | 61 ± 17 | 6,729 ± 887 |
| TI Immune | 6,120 ± 5,316 | 2,550 ± 1803 | 7,257 ± 1700 |
| Anti-CD40 | 203 ± 55 | 22 ± 16 | n.d. |
| TI Immune + anti-CD40 | 148,006 ± 72,510 | 203,081 ± 76,175 | 54,727 ± 17,381 |
| TD Immune | 710,016 ± 131,302 | 985,402 ± 45,787 | 270,258 ± 27,546 |
NP-specific IgG1, IgG2a, and IgG3 were measured by ELISA as previously described. One of four independent experiments.
(n.d., not detected).
2.5 CD40 ligation fails to enhance QM Tg B cell expansion in response to TI Ag
Since we observed differences in Ig secretion driven by CD40 ligation, we sought to address whether CD40 engagement could influence the expansion and differentiation of QM Tg B cells in response to NP-Ficoll. On day 5 post-immunization, splenocytes were stained with B220 and NP and analyzed by flow cytometry. The bar graph depicted in Fig. 4A shows an 8-fold expansion of Tg B cells from immune mice compared to naive mice. These results are consistent with those observed in Fig. 1. Interestingly, levels of Tg B cell expansion in TI immune + anti-CD40 recipients are markedly decreased in numbers compared to levels from mice immunized with Ag alone. Thus, the elevated levels of NP-specific IgG were not due to an overt expansion of differentiated B cells. On the contrary, it appears that anti-CD40 may have enforced the terminal differentiation of Tg B cells and their departure from the spleen. Present efforts have demonstrated that low numbers of Tg B cells are found in the bone marrow of mice treated with anti-CD40, and are observed in peripheral blood of these animals (manuscript in preparation). A dose response showed one administration of FGK115 at 10 μg per mouse to be effective in mediating B cell activation and inducing only modest splenomegaly, whereas higher doses (100 μg) caused marked splenomegaly (data not shown).
To test whether the NP-specific IgG induced by CD40 ligation was a result of GC formation, the surface phenotype of Tg B cells was examined using a panel of markers. Histograms shown in Fig. 4B depict representative expression levels of the indicated markers on Tg B cells from mice challenged with NP-Ficoll plus anti-CD40. Tg B cells lacked PNA staining and failed to down-regulate constitutive levels of CD38 expression. For a positive control, Tg cells from mice challenged with NP-KLH exhibited a marked increase in PNA binding and loss of CD38 expression. Kinetic analyses further demonstrated that Tg B cells lacked a GC phenotype on days 3 and 7 post-immunization (data not shown). Of equal interest is the finding that Tg B cells from immune mice treated with anti-CD40 showed a marked increase in levels of IgG1 expression compared to naive cells. Surprisingly, anti-CD40 treatment alone failed to induce IgG1 expression on Tg B cells. These results indicate that Ag-activated Tg B cells can undergo significant levels of isotype switching upon CD40 engagement in the absence of GC formation. To confirm the results of the flow cytometric analysis, immunohistochemical analysis was performed on spleen sections obtained from these mice. Fig. 4C shows two micrographs representing the same follicle and displays IgD FITC (green) and NP-Id Cy5 (blue) either in the absence or presence of CD38 PE (red). Findings demonstrated that as for immune mice (Fig. 2C), Tg B cells from TI immune + anti-CD40 mice also localize to the center of follicles. In addition, Tg B cells stain strongly positive for CD38 that is consistent with the phenotypic analysis shown in Fig. 4B. These results indicate that Ag-activated Tg B cells fail to generate a GC phenotype upon CD40 ligation. For comparison, a lymph node tissue section taken from mice challenged with NP-KLH likewise shows a central cluster of Tg B cells in the follicle. In contrast to Tg cells within the follicle of spleen sections, lymph node Tg B cells lack CD38 expression. Taken together, the results from flow cytometric and immunohistochemistry analyses demonstrate that GC are not formed in TI immune recipients, regardless of anti-CD40 treatment.
2.6 Exclusive targeting of CD40 agonist to QM Tg B cells induces them to produce expanded levels of IgG antibodies
To determine whether the induction of anti-NP Ab was due to a direct effect of CD40 triggering of the Tg B cells, QM Tg B cells were adoptively transferred into wild-type C57BL/6 and CD40-deficient C57BL/6. Fig. 5 shows that CD40 treatment enhances the production of NP idiotype-specific IgG, regardless of whether the recipients were wild-type or CD40-deficient mice. Similar to findings shown in Fig. 3, wild-type naive, no cells, and anti-CD40-treated recipients failed to produce detectable levels of NP idiotype-specific Ig. These observations strongly suggest that anti-CD40 treatment is exerting its effect directly on Tg B cells and not via an indirect effect on other CD40-bearing accessory cells.
Fig. 5.
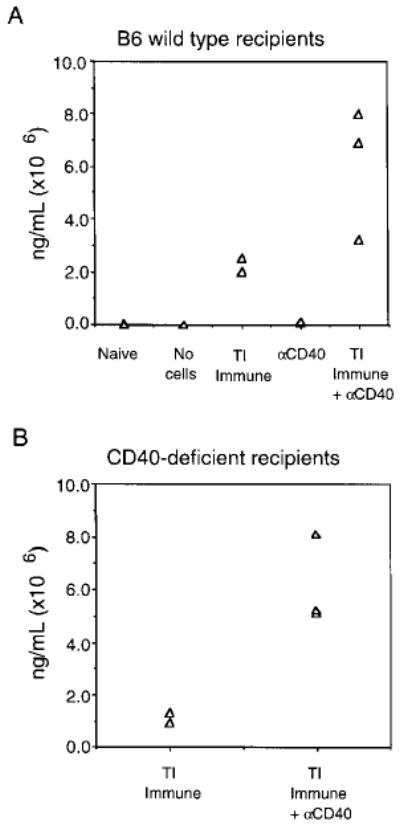
Levels of NP-specific IgG from Tg B cells adoptively transferred into CD40-deficient mice. Wild-type C57BL/6 (panel A) or CD40−/− B6 (panel B) recipient mice were challenged with PBS (Naive), NP-Ficoll (TI Immune), FGK115 alone (αCD40), or NP-Ficoll plus FGK115 (TI Immune + αCD40). As a control, mice that did not receive any Tg B cells were challenged with NP-Ficoll to assess the endogenous response (no cells). On day 5 post-immunization, sera was collected and assayed for NP-specific IgG by ELISA. Data are representative of two experiments.
2.7 Combined triggering via mIg and CD40 fails to generate a QM Tg B cell memory response
Perhaps the most distinctive difference between TD and TI responses is the capacity of the former to elicit B cell memory. Since it has been demonstrated that CD40-CD154 interactions are essential for the development of memory B cells [17, 18], we tested whether the combined administration of anti-CD40 and NP-Ficoll could induce the generation of NP-specific memory B cells. Adoptive transfer experiments were carried out as previously described. Levels of NP-specific IgG1 from the various groups of mice are shown in Fig. 6. Animals that received either PBS or NP-Ficoll during the primary immunization and NP-KLH during the secondary challenge failed to generate significant levels of NP idiotype-bearing IgG1. As anticipated, mice that received NP-Ficoll during primary and secondary challenge failed to generate significant levels of NP idiotype-specific IgG1. This finding is consistent with previous literature reporting that TI Ag such as NP-Ficoll do not induce a secondary memory response. As a positive control, mice that received NP-KLH during both primary and secondary immunizations produced marked levels of NP idiotype-specific IgG1. Animals that received NP-Ficoll plus anti-CD40 treatment during the primary immunization and were given NP-KLH during the secondary challenge failed to generate levels of IgG1 substantially above control groups of mice. This finding is not attributed to a decrease in the frequency of Tg B cells since comparable numbers of splenic Tg B cells were readily detected by flow cytometric analysis from all groups of mice (data not shown). These studies indicate that in vivo treatment with anti-CD40 fails to induce a memory response to NP-Ficoll.
3 Discussion
This study presents a novel Ig Tg system that faithfully recapitulates Ag-specific B cell responses to TI and TD Ag. Such a system provides a unique approach to track the fate of Ag-reactive B cells through follicular and extrafollicular pathways. Furthermore, this system offers the means to incisively evaluate the impact of extrinsic factors on the course of B cell immunopoiesis. Since CD40-CD154 interactions are centrally involved in TD B cell activation, we sought to investigate how the engagement of CD40 alters the course of QM Tg B cell growth and differentiation in vivo. The data shows that QM Tg B cells expand and produce NP-specific Ab in response to NP-Ficoll. The co-administration of an agonistic anti-CD40 mAb induces an increase in Ab titer and a broadening in IgG isotype profiles of the NP-specific Ab response. However, co-engagement of CD40 and mIg in vivo does not induce a phenotypic differentiation of Tg B cells towards GC B cells nor the functional differentiation of the Tg B cells into memory B cells, as was observed upon immunization with a TD Ag. Thus, CD40 triggering and mIg engagement can elicit some, but not all characteristics of TD humoral immune responses.
The data present insights into the phenotypic description of B cells responding to TI Ag. Tg B cells that were stimulated with NP-Ficoll expanded extensively by day 3, peaked on day 5 and declined thereafter. Immunization with NP-Ficoll caused a reduction in expression of IgM and IgD, and an increase in IgG1 and CD95. A hallmark of TD immune responses is the formation of GC within follicles of secondary lymphoid tissues. Within these specialized microenvironments, proliferation of Ag-specific B cells occurs [19, 20] and phenotypic changes of cell surface molecules also take place. B cells recruited to GC bind PNA [11, 21–23], up-regulate GL7 expression [13], and down-regulate CD38 expression [12]. Recipients that were challenged with NP-Ficoll displayed no change in the GC markers, PNA, CD38 or GL7 on the responding Tg B cell population. Following immunization, Tg B cells were observed histologically as a population of B cell aggregates expanding within the follicle. Tg B cells from immune mice localized to the center of follicles and down-regulated IgD expression. These observations are consistent with previous reports that demonstrate that most TI Ag induce only minimal [24] or no GC formation [25]. Of interest, however, was the finding that anti-CD40 treatment of immune mice similarly failed to induce a GC phenotype on Tg B cells. These observations were further confirmed by histological examination of spleen sections taken from identical recipients. Previous reports have provided strong evidence lending support to the requirement of CD40-CD154 interactions in GC formation. Mice genetically deficient in either CD40 or CD154 or mice treated with anti-CD154 fail to develop GC [9, 18, 26]. Our present data indicate that enhancement of Ab production and isotype switching can occur upon CD40 ligation even in the absence of a GC reaction and Ag-primed T cells. These in vivo results confirm in vitro studies showing that CD40 signaling and mIg engagement are not sufficient to induce a GC phenotype [27]. There have been a number of instances in which TI Ab have been shown to induce GC. Wang et al. [24] reported that immunization of mice with alpha(1–6)dextran induced the appearance of a distinct population of DEX+PNA+ splenic B cells as well as DEX+PNA− cells in the red pulp of immune mice. Recently, MacLennan et al. [28] demonstrated that immunization of QM mice with NP-Ficoll induces splenic GC. This report shows that low doses of NP-Ficoll or a high precursor frequency of Ag-reactive B cells skews the in vivo TI response of a QM mouse to a GC-type response. Our findings are in contrast to this study; however, these differences likely reflect an Ag threshold where GC formation in QM mice is a result of extensive BCR cross-linking when given NP-Ficoll. An important finding from the MacLennan study was that neither T cells nor CD154 was critical for GC formation. This finding dismisses the role of CD154 in GC formation.
The data presented here show that the co-engagement of mIg and CD40 is sufficient to induce the NP-specific Ab titer and, moreover, broaden the Ig isotypes in the absence of GC formation and phenotype. The induction of Ag-specific Ab in our studies was dependent on concurrent engagement of mIg and CD40. Furthermore, titration of anti-CD40 dosage was critical to illuminate this synergy, since higher doses of anti-CD40 mAb induced massive splenomegaly and heightened levels of polyspecific immunoglobulin (data not shown). It is likely that high doses of anti-CD40 activates a large spectrum of CD40-bearing cells and produce a myriad of secondary effects. However, we have shown that adoptive transfer experiments utilizing CD40-deficient recipient mice suggest that treatment with a low dose of anti-CD40 mAb (10 μg) acts directly on the Tg B cells to enhance Ab titer. These studies extend the data reported by Heath et al. [29] that show anti-CD40 co-administered with pneumococcal polysaccharide can induce a protective, isotyped-switched response.
The observation that co-engagement of CD40 and mIg induces an increase in isotype-switched B cell responses suggests that these events occur separately from the generation of B cell memory. Our studies that assessed secondary immune responses demonstrated that CD40 ligation of Tg B cells in vivo is insufficient in eliciting memory B cell differentiation yet adequate to augment Ig isotype-switching. These data are intriguing and imply that signals in addition to CD40 engagement are required for the development of B cell memory. Our lab has previously reported that in vivo blockade of CD40-CD154 interactions prevents the generation of memory responses [18], and probably is attributed to the disruption of GC formation. One attractive hypothesis is that during the GC reaction, Ag-specific helper T cells provide cognate factors in addition to CD154 that promote memory B cell differentiation. If this were the case, the failure of anti-CD40 treatment to generate a QM Tg B cell memory response could be interpreted as the lack of helper T cell recruitment during the primary response. Recent studies have identified human splenic memory B cells by the expression of the CD27 cell surface Ag, a member of the TNF receptor family [30, 31]. These studies demonstrated that B cells staining positively for CD27 also possessed characteristics unique to memory B cells, such as the expression of somatically mutated Ig variable region genes and localization to the marginal zone of splenic follicles. It is tempting to speculate that perhaps engagement of CD27 through its CD70 counter-receptor expressed on activated T cells is an additional signal required for memory B cell differentiation. Studies designed to address this question are currently in progress.
Immune responses of infants and young children to TI Ag such as encapsulated bacterial pathogens are extremely poor [32–34]. One reason for this weak response is thought to be associated with an underdeveloped marginal zone within the white pulp of the spleen. Vaccination to such Ag is likely to be ineffective based on the assumption that marginal zone B cells are involved in the generation of a memory response. In mice, neonatal B cells have been shown to be defective in Ab secretion in response to TI-2-like Ag [35]; however, co-engagement of the BCR and CD40 restored the ability of these cells to secrete IgM. Our studies demonstrate that in vivo anti-CD40 treatment can partially reconstitute TD humoral immune responses in mice challenged with a TI Ag; however, CD40 ligation alone fails to induce GC formation and memory. The identification of additional signals that regulate B cell differentiation will be likely candidates in serving as immunological agents in designing vaccines to TI Ag.
4 Material and methods
4.1 Mice
CD40−/− C57BL/6, C57BL/6, (C57BL/6×129.J) F1 mice, 6–8 weeks old, were bred and maintained in the specific pathogen-free animal facility at Dartmouth Medical School, Lebanon, NH. The generation of QM mice has been described previously [2]. QM mice were subsequently backbred to the C57BL/6 JH−/− Jk−/− strain for two to four generations to produce QM C57BL/6×129.J and QM F4 C57BL/6×129.J, respectively.
4.2 B cell preparation and adoptive transfer
Spleen cells isolated from QM mice were treated with ammonium chloride-Tris buffer (ACT) and T cell-depleted using anti-Thy-1.2 plus anti-CD4 mAb and rabbit complement (Accurate Chemical, Westbury, NY). An aliquot of cells was stained in 5% BCS in BSS with NP and B220 for 20 min at 4°C, washed and analyzed by flow cytometry (Becton Dickinson FACScan) to determine percentage of Tg B cells. In all experiments, normal rat serum was added to minimize nonspecific staining. Tg B cells (3×106) were adoptively transferred i.v. into sex-matched (C57BL/6×129.J) F1 recipient mice. As indicated, Tg B cells from QM F4 mice were similarly isolated and transferred into wild-type C57BL/6 and CD40−/− C57BL/6 mice.
4.3 Immunization and anti-CD40 treatment
NP-Ficoll and NP-KLH were purchased from Solid Phase Sciences (San Rafael, CA). To examine TI responses, recipients were challenged with PBS (naive) or 25 μg NP-Ficoll (immune) i.p. 24 h after transfer. FGK115, an agonistic rat IgG2a anti-mouse CD40 (a gift from Dr. A.G. Rolink, Basel Institute for Immunology, Switzerland), was HPLC purified and administered at 10 μg i.p. at the time of immunization. On day 5, spleens were removed and sera collected. To examine TD responses, recipients were primed with 100 μg KLH (Calbiochem, La Jolla, CA) in complete Freund's adjuvant (CFA) by i.p. injection 1–2 weeks before use to prime the endogenous T cell compartment. Tg B cells were adoptively transferred as previously described. After 24 h, mice were immunized with 125 μg soluble NP-KLH s.c. into each flank. At various times thereafter, mice were bled and the draining lymph nodes were removed for analysis. For detecting memory responses, Tg B cells were adoptively transferred into recipients and, after 24 h, mice were immunized i.p. with PBS, 250 μg NP-KLH, or 25 μg NP-Ficoll in the absence or presence of 10 μg FGK115. On day 14 post-immunization, splenocytes from three mice per group were pooled, ACT-treated, and T cell-depleted. Splenic B cells were transferred (30×106) i.v. into secondary F1 recipients that had been KLH-primed (100 μg i.p.) in CFA. After 24 h, mice were challenged i.p. with either NP-Ficoll (25 μg) or a low dose of NP-KLH (1.0 μg) previously determined not to elicit an endogenous response. On day 5 after secondary challenge, sera was collected and assayed for NP-idiotype IgG by ELISA.
4.4 Flow cytometric reagents
The following mAb were utilized for multicolor flow cytometric analysis. 6B2 CyC, a rat IgG2a anti-mouse B220 and Jo2, a hamster IgG anti-mouse CD95 were purchased from PharMingen (San Diego, CA); FITC-conjugated Ab90, a rat IgG2a anti-mouse CD38, and M1/69, a rat IgG anti-mouse CD24 (heat stable Ag) were generously provided by Dr. Tom Waldschmidt, University of Iowa (Iowa City, IA). Hδa/1, a rat IgG2a anti-mouse IgDa; biotin-conjugated rat anti-mouse IgG1 was purchased from Zymed (San Francisco, CA). Rat IgM anti-mouse GL7 was provided by Dr. Tom Waldschmidt. FITC-conjugated PNA was purchased from Sigma Chemical Co. PE-conjugated NP was made by the protocol established by Genosys Biotechnologies (The Woodlands, TX). Streptavidin-PE and FITC were obtained from Southern Biotechnologies (Birmingham, AL). The anti-VH17.2.25 cell line (R2348.8) was generously provided by Dr. Theresa Imanishi-Kari (UCSF, San Francisco, CA). For removal of cytophilic Ig, cells were washed in 5% FCS in BSS and resuspended in low pH (pH 4.0) acetate buffer containing 0.05 M sodium acetate, 0.085 M NaCl, and 0.005 M KCl and 2% FCS in dH2O. Samples were incubated on ice for 1.0 min, followed by the addition of an equal volume of 0.1 M Tris buffer pH 8.0 containing 2% FCS, washed, and stained. A minimum of 150,000 events per sample were collected on a Becton Dickinson FACScan. Fluorescence signals were collected using four-decade logarithmic amplification. Data were analyzed using CellQuest software (Becton Dickinson), and final graphic output was performed with Canvas software.
4.5 ELISA reagents
NP30-BSA was used to capture NP-specific Ab in a sandwich-based ELISA. NP-BSA was prepared according to Genosys Biotechnologies. Goat anti-mouse IgG, IgG1, IgG2a, and IgG3 conjugated to AP were used for detection (Southern Biotechnology Associates, Birmingham, AL) and subsequent development with phosphatase substrate (Sigma). Absorbance was read using an MR700 plate reader (Dynatech Laboratories, Chantilly, VA). As indicated, some ELISA utilized biotin-conjugated anti-idiotype V17.2.25 for detection, followed by alkaline phosphatase strepavidin (Zymed). Serum from a hyperimmunized transgenic QM mouse was used as a standard in all assays for quantification (in ng/ml).
4.6 Confocal image analysis
On day 5 post-immunization, intact spleens or lymph nodes were harvested and embedded in 4.0% agarose in BSS. Sections, 500 μm thick, were cut using a vibrating microtome (Microcut H12; Energy Beam Sciences, Agawam, MA) and placed in 1.0% BSA in PBS staining buffer. Sections were stained using a three-color protocol with IgD FITC, CD38 PE, and Cy5-conjugated anti-NP idiotype 17.2.25. To block nonspecific binding, an excess of mIgG (100 μg/ml) was included in the staining process. Samples were analyzed on a Bio-Rad 1024 confocal microscope (Bio-Rad Labs, Hercules, CA).
Acknowledgments
The authors would like to thank Dr. A. G. Rolink for generously providing the FGK115 (anti-mouse CD40) mAb and Dr. T. Imanishi-Kari for the kind gift of the R2348.8 cell line (anti-VH17.2.25). The C57BL/6 JH−/− and Jk−/− mice were kindly supplied by Dr. D. Nemazee. This work was supported by grants from the National Institutes of Health to R. J. Noelle (AI-26296), L. D. Erickson (T32AI07363), and M. Wabl (AI-41570). L. A. Vogel was supported by a postdoctoral fellowship award from the Arthritis Foundation.
Abbreviations
- QM
Quasi-monoclonal
- Cy5
Cyanine
- CyC
Cychrome
- PNA
Peanut agglutinin
- TI
T cell-independent
- TD
T cell-dependent
- Tg
Transgenic
- FDC
Follicular dendritic cells
- GC
Germinal center
References
- 1.Cascalho M, Wong J, Wabl M. VH gene replacement in hyperselected B cells of the quasimonoclonal mouse. J Immunol. 1997;159:5795–5801. [PubMed] [Google Scholar]
- 2.Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 3.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 4.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, Howard M, Goodnow CC. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 6.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 7.Maizels N, Bothwell A. The T cell-independent immune response to the hapten NP uses a large repertoire of heavy chain genes. Cell. 1985;43:715–720. doi: 10.1016/0092-8674(85)90244-2. [DOI] [PubMed] [Google Scholar]
- 8.Lalor PA, Nossal GJ, Sanderson RD, McHeyzer-Williams MG. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992;22:3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- 9.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 10.Castigli E, Alt FW, Davidson L, Bottaro A, Mizoguchi E, Bhan AK, Geha RS. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci USA. 1994;91:12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coico RF, Bhogal BS, Thorbecke GJ. Relationship of germinal centers in lymphoid tissue to immunologic memory. VI. Transfer of B cell memory with lymph node cells fractionated according to their receptors for peanut agglutinin. J Immunol. 1983;131:2254–2257. [PubMed] [Google Scholar]
- 12.Oliver AM, Martin F, Kearney JF. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J Immunol. 1997;158:1108–1115. [PubMed] [Google Scholar]
- 13.Laszlo G, Hathcock KS, Dickler HB, Hodes RJ. Characterization of a novel cell-surface molecule expressed on subpopulations of activated T and B cells. J Immunol. 1993;150:5252–5262. [PubMed] [Google Scholar]
- 14.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation II. Heat-stable antigen hi splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 15.Slack J, Der-Balian GP, Nahm M, Davie JM. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980;151:853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlmutter RM, Hansburg D, Briles DE, Nicolotti RA, Davie JM. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- 17.Foy TM, Aruffo A, Ledbetter JA, Noelle RJ. In vivo CD40-gp39 interactions are essential for thymus-dependent immunity. II. Prolonged in vivo suppression of primary and secondary humoral immune responses by an antibody targeted to the CD40 ligand, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–164. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroese FG, Wubbena AS, Seijen HG, Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987;17:1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- 20.Kosco MH. Germinal centers and the immune response. Res Immunol. 1991;142:219. doi: 10.1016/0923-2494(91)90061-m. [DOI] [PubMed] [Google Scholar]
- 21.Butcher EC, Rouse RV, Coffman RL, Nottenberg CN, Hardy RR, Weissman I. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982;129:2698. [PubMed] [Google Scholar]
- 22.Platt FM, Cebra-Thomas JA, Baum CM, Davie JM, McKearn JP. Monoclonal antibodies specific for novel murine cell surface markers define subpopulations of germinal center cells. Cell Immunol. 1992;143:449–466. doi: 10.1016/0008-8749(92)90039-r. [DOI] [PubMed] [Google Scholar]
- 23.Rose ML, Birbeck MSC, Wallis VJ, Forrrester JA, Davies AJS. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980;284:364. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Wells SM, Stall AM, Kabat EA. Reaction of germinal centers in the T cell-independent response to the bacterial polysaccharide alpha(1–6)dextran. Proc Natl Acad Sci USA. 1994;91:2502–2506. doi: 10.1073/pnas.91.7.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissman IL, Gutman GA, Friedberg SH, Jerabek L. Lymphoid tissue architecture. III. Germinal centers, T cells, and thymus-dependent vs thymus-independent antigens. Adv Exp Med Biol. 1976;66:229–237. doi: 10.1007/978-1-4613-4355-4_35. [DOI] [PubMed] [Google Scholar]
- 26.Renshaw BR, Fanslow WR, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahvis GP, Cerny J. Induction of germinal center B cell markers in vitro by activated CD4+ T lymphocytes: the role of CD40 ligand, soluble factors, and B cell antigen receptor cross-linking. J Immunol. 1997;159:1783–1793. [PubMed] [Google Scholar]
- 28.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dullforce P, Debbie C, Sutton DC, Heath AW. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nature. 1998;4:88–92. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- 30.Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas RM, Paton JC, Duncan SJ, Hansman DJ. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 33.Kayhty H, Karanko V, Peltola H, Makela PH. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984;74:857–865. [PubMed] [Google Scholar]
- 34.Lee HJ, Kang JH, Henrichsen J, Konradsen HB, Jang SH, Shin HY, Ahn HS, Choi Y, Hessel L, Nam SW. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in healthy children and in children at increased risk of pneumococcal infection. Vaccine. 1995;13:1533–1538. doi: 10.1016/0264-410x(95)00093-g. [DOI] [PubMed] [Google Scholar]
- 35.Snapper CM, Rosas FR, Moorman MA, Mond JJ. Restoration of T cell-independent type 2 induction of Ig secretion by neonatal B cells in vitro. J Immunol. 1997;158:2731–2735. [PubMed] [Google Scholar]


