Abstract
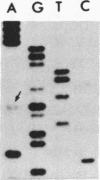
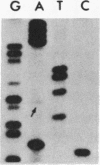
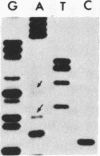
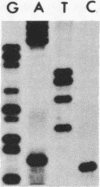
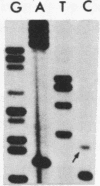
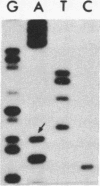
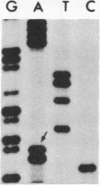
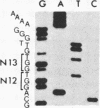
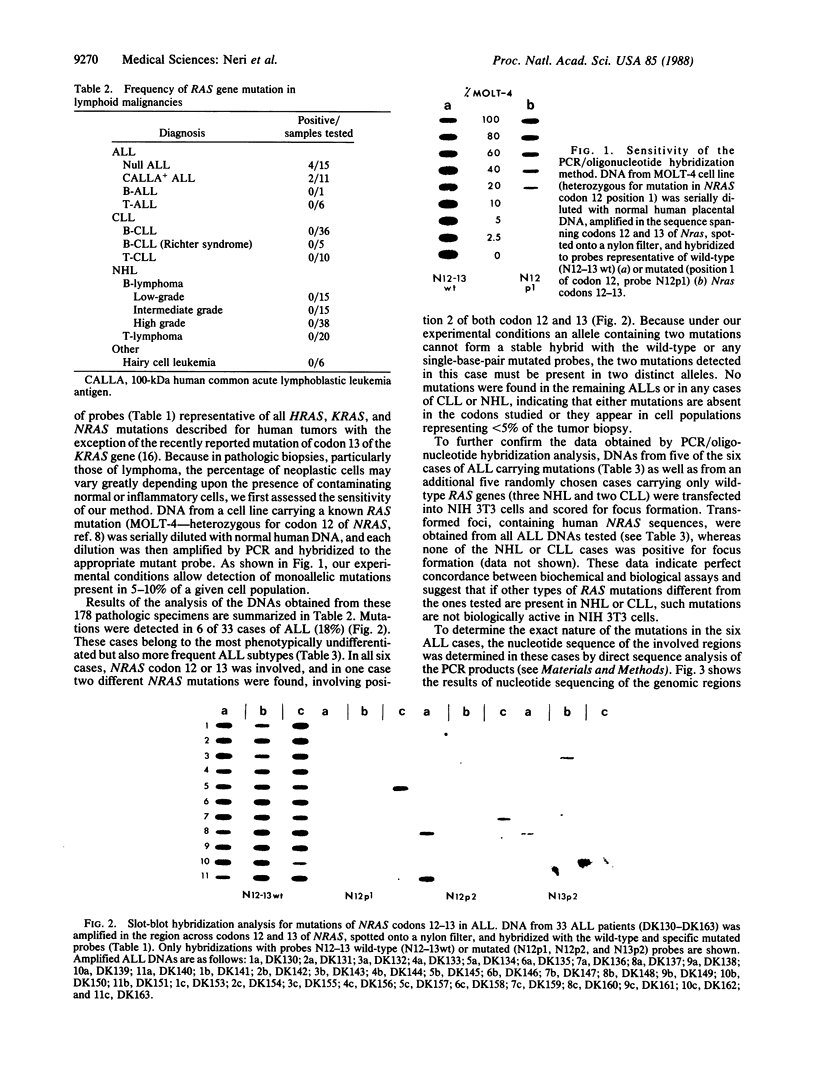
We investigated the frequency of mutations activating RAS oncogenes in human lymphoid malignancies, including B- and T-cell-derived acute lymphoblastic leukemia, chronic lymphocytic leukemia, and non-Hodgkin lymphoma. By the polymerase chain reaction/oligonucleotide hybridization method, DNA from 178 cases was analyzed for activating mutations involving codons 12 and 61 of the HRAS, KRAS and NRAS genes and codon 13 of the NRAS gene. Mutations involving codons 12 or 13 of the NRAS gene were detected in 6 of 33 cases of acute lymphoblastic leukemia (6/33, 18%), whereas no mutations were found in non-Hodgkin lymphoma or chronic lymphocytic leukemia. Direct nucleotide sequence analysis of polymerase chain reaction products showed that the mutations involved a G----A transition in five of the six cases of acute lymphocytic leukemia. In four cases the mutations seemed to occur in only a fraction of the neoplastic cells, and one case displayed two distinct NRAS mutations, most likely present in two distinct cell populations. These results indicate the following: (i) RAS oncogenes are not found in all types of human malignancies, (ii) significant differences in the frequency of RAS mutations can be found among subtypes of neoplasms derived from the same tissue, (iii) in lymphoid neoplasms the NRAS mutation correlates with the most undifferentiated acute lymphocytic leukemia phenotype, and (iv) NRAS mutations present in only a fraction of malignant cells may result from either the selective loss or the acquisition of mutated alleles during tumor development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., van der Eb A. J., Janssen J. W., Delwel R., Löwenberg B., Colly L. P. Mutations in N-ras predominate in acute myeloid leukemia. Blood. 1987 Apr;69(4):1237–1241. [PubMed] [Google Scholar]
- Diamond L. E., Guerrero I., Pellicer A. Concomitant K- and N-ras gene point mutations in clonal murine lymphoma. Mol Cell Biol. 1988 May;8(5):2233–2236. doi: 10.1128/mcb.8.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Tronick S. R., Gol R. A., Pierce J. H., Aaronson S. A. Transforming genes of human hematopoietic tumors: frequent detection of ras-related oncogenes whose activation appears to be independent of tumor phenotype. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4926–4930. doi: 10.1073/pnas.80.16.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Differentiation-linked leukemogenesis in lymphocytes. Science. 1986 Nov 7;234(4777):697–704. doi: 10.1126/science.3535067. [DOI] [PubMed] [Google Scholar]
- Hirai H., Kobayashi Y., Mano H., Hagiwara K., Maru Y., Omine M., Mizoguchi H., Nishida J., Takaku F. A point mutation at codon 13 of the N-ras oncogene in myelodysplastic syndrome. Nature. 1987 Jun 4;327(6121):430–432. doi: 10.1038/327430a0. [DOI] [PubMed] [Google Scholar]
- Hirakawa T., Ruley H. E. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J. W., Lyons J., Steenvoorden A. C., Seliger H., Bartram C. R. Concurrent mutations in two different ras genes in acute myelocytic leukemias. Nucleic Acids Res. 1987 Jul 24;15(14):5669–5680. doi: 10.1093/nar/15.14.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Pelicci P. G., Dalla-Favera R. T-cell receptor beta chain gene rearrangements: genetic markers of T-cell lineage and clonality. Hum Pathol. 1986 Jun;17(6):546–551. doi: 10.1016/s0046-8177(86)80125-3. [DOI] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Hjelle B., Morgan R., Hecht F., Bishop J. M. Mutations of the Kirsten-ras proto-oncogene in human preleukaemia. Nature. 1987 Nov 12;330(6144):186–188. doi: 10.1038/330186a0. [DOI] [PubMed] [Google Scholar]
- McMahon G., Davis E., Wogan G. N. Characterization of c-Ki-ras oncogene alleles by direct sequencing of enzymatically amplified DNA from carcinogen-induced tumors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4974–4978. doi: 10.1073/pnas.84.14.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. J., Cunningham J. M., Parada L. F., Dautry F., Lebowitz P., Weinberg R. A. The HL-60 transforming sequence: a ras oncogene coexisting with altered myc genes in hematopoietic tumors. Cell. 1983 Jul;33(3):749–757. doi: 10.1016/0092-8674(83)90017-x. [DOI] [PubMed] [Google Scholar]
- Padua R. A., Barrass N., Currie G. A. A novel transforming gene in a human malignant melanoma cell line. Nature. 1984 Oct 18;311(5987):671–673. doi: 10.1038/311671a0. [DOI] [PubMed] [Google Scholar]
- Rodenhuis S., Bos J. L., Slater R. M., Behrendt H., van 't Veer M., Smets L. A. Absence of oncogene amplifications and occasional activation of N-ras in lymphoblastic leukemia of childhood. Blood. 1986 Jun;67(6):1698–1704. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shen W. P., Aldrich T. H., Venta-Perez G., Franza B. R., Jr, Furth M. E. Expression of normal and mutant ras proteins in human acute leukemia. Oncogene. 1987 May;1(2):157–165. [PubMed] [Google Scholar]
- Trahey M., Milley R. J., Cole G. E., Innis M., Paterson H., Marshall C. J., Hall A., McCormick F. Biochemical and biological properties of the human N-ras p21 protein. Mol Cell Biol. 1987 Jan;7(1):541–544. doi: 10.1128/mcb.7.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Senn H. P., Jiricny J., Signer E., Moroni C. Glycine-cysteine substitution at codon 13 of the N-ras proto-oncogene in a human T cell non-Hodgkin's lymphoma. Oncogene. 1987;1(4):457–461. [PubMed] [Google Scholar]