Abstract
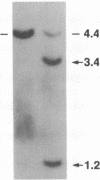
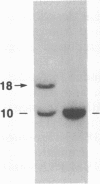
We describe a t(14;14)(q11;q32) translocation in a patient with T-cell chronic lymphocytic leukemia and ataxia-telangiectasia (AT). By using a battery of joining (J)-segment probes from the T-cell receptor (TCR) alpha-chain locus TCRA, three distinct J alpha rearrangements were observed. One rearrangement reflected a normal TCRA variable (V) region V alpha-to-J alpha recombination. The second rearrangement was caused by the translocation even itself, which joined a DNA segment from 14q32 centromeric to the immunoglobulin heavy chain locus (IGH) and a J alpha gene located approximately 75 kilobases (kb) 5' of the TCRA constant region gene (C alpha). A third rearrangement involved a 17-kb internal deletion 3' to the translocation, a rearrangement within the J alpha locus that has been observed once before in a patient with AT. Analysis of these three rearrangements underscores the increase in aberrant locus-specific recombination in lymphocytes from patients with AT. Furthermore, these studies support the view that a growth-effecting gene is present in the 14q32 region that participates in the leukemogenic process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurias A., Couturier J., Dutrillaux A. M., Dutrillaux B., Herpin F., Lamoliatte E., Lombard M., Muleris M., Paravatou M., Prieur M. Inversion (14)(q12qter) or (q11.2q32.3): the most frequently acquired rearrangement in lymphocytes. Hum Genet. 1985;71(1):19–21. doi: 10.1007/BF00295660. [DOI] [PubMed] [Google Scholar]
- Aurias A., Croquette M. F., Nuyts J. P., Griscelli C., Dutrillaux B. New data on clonal anomalies of chromosome 14 in ataxia telangiectasia: tct(14;14) and inv(14). Hum Genet. 1986 Jan;72(1):22–24. doi: 10.1007/BF00278811. [DOI] [PubMed] [Google Scholar]
- Aurias A., Dutrillaux B., Buriot D., Lejeune J. High frequencies of inversions and translocations of chromosomes 7 and 14 in ataxia telangiectasia. Mutat Res. 1980 Feb;69(2):369–374. doi: 10.1016/0027-5107(80)90101-3. [DOI] [PubMed] [Google Scholar]
- Aurias A., Dutrillaux B., Griscelli C. Tandem translocation t(14;14) in isolated and clonal cells in ataxia telangiectasia are different. Hum Genet. 1983;63(4):320–322. doi: 10.1007/BF00274754. [DOI] [PubMed] [Google Scholar]
- Aurias A., Dutrillaux B. Probable involvement of immunoglobulin superfamily genes in most recurrent chromosomal rearrangements from ataxia telangiectasia. Hum Genet. 1986 Mar;72(3):210–214. doi: 10.1007/BF00291879. [DOI] [PubMed] [Google Scholar]
- Baer R., Boehm T., Yssel H., Spits H., Rabbitts T. H. Complex rearrangements within the human J delta-C delta/J alpha-C alpha locus and aberrant recombination between J alpha segments. EMBO J. 1988 Jun;7(6):1661–1668. doi: 10.1002/j.1460-2075.1988.tb02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Baer R., Heppell A., Taylor A. M., Rabbitts P. H., Boullier B., Rabbitts T. H. The breakpoint of an inversion of chromosome 14 in a T-cell leukemia: sequences downstream of the immunoglobulin heavy chain locus are implicated in tumorigenesis. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9069–9073. doi: 10.1073/pnas.84.24.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Brito-Babapulle V., Pomfret M., Matutes E., Catovsky D. Cytogenetic studies on prolymphocytic leukemia. II. T cell prolymphocytic leukemia. Blood. 1987 Oct;70(4):926–931. [PubMed] [Google Scholar]
- Croce C. M., Isobe M., Palumbo A., Puck J., Ming J., Tweardy D., Erikson J., Davis M., Rovera G. Gene for alpha-chain of human T-cell receptor: location on chromosome 14 region involved in T-cell neoplasms. Science. 1985 Mar 1;227(4690):1044–1047. doi: 10.1126/science.3919442. [DOI] [PubMed] [Google Scholar]
- Denny C. T., Yoshikai Y., Mak T. W., Smith S. D., Hollis G. F., Kirsch I. R. A chromosome 14 inversion in a T-cell lymphoma is caused by site-specific recombination between immunoglobulin and T-cell receptor loci. Nature. 1986 Apr 10;320(6062):549–551. doi: 10.1038/320549a0. [DOI] [PubMed] [Google Scholar]
- Filipovich A. H., Heinitz K. J., Robison L. L., Frizzera G. The Immunodeficiency Cancer Registry. A research resource. Am J Pediatr Hematol Oncol. 1987 Summer;9(2):183–184. doi: 10.1097/00043426-198722000-00017. [DOI] [PubMed] [Google Scholar]
- Graham M., Adams J. M. Chromosome 8 breakpoint far 3' of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986 Nov;5(11):2845–2851. doi: 10.1002/j.1460-2075.1986.tb04578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska F. G., Finver S., Tsujimoto Y., Croce C. M. The t(8; 14) chromosomal translocation occurring in B-cell malignancies results from mistakes in V-D-J joining. Nature. 1986 Nov 13;324(6093):158–161. doi: 10.1038/324158a0. [DOI] [PubMed] [Google Scholar]
- Hara J., Benedict S. H., Champagne E., Mak T. W., Minden M., Gelfand E. W. Comparison of T cell receptor alpha, beta, and gamma gene rearrangement and expression in T cell acute lymphoblastic leukemia. J Clin Invest. 1988 Apr;81(4):989–996. doi: 10.1172/JCI113453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis R. J., Kennaugh A. A., Butterworth S. V., Taylor A. M. Growth of large chromosomally abnormal T cell clones in ataxia telangiectasia patients is associated with translocation at 14q11. A model for other T cell neoplasia. Hum Genet. 1987 Aug;76(4):389–395. doi: 10.1007/BF00272451. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Gatti R. A., Sears T. S., White R. L. Inverted duplication of JH associated with chromosome 14 translocation and T-cell leukemia in ataxia-telangiectasia. Am J Hum Genet. 1986 Dec;39(6):787–796. [PMC free article] [PubMed] [Google Scholar]
- Kennaugh A. A., Butterworth S. V., Hollis R., Baer R., Rabbitts T. H., Taylor A. M. The chromosome breakpoint at 14q32 in an ataxia telangiectasia t(14;14) T cell clone is different from the 14q32 breakpoint in Burkitts and an inv(14) T cell lymphoma. Hum Genet. 1986 Jul;73(3):254–259. doi: 10.1007/BF00401239. [DOI] [PubMed] [Google Scholar]
- Kirsch I. R., Morton C. C., Nakahara K., Leder P. Human immunoglobulin heavy chain genes map to a region of translocations in malignant B lymphocytes. Science. 1982 Apr 16;216(4543):301–303. doi: 10.1126/science.6801764. [DOI] [PubMed] [Google Scholar]
- Levitt R., Pierre R. V., White W. L., Siekert R. G. Atypical lymphoid leukemia in ataxia telangiectasia. Blood. 1978 Nov;52(5):1003–1011. [PubMed] [Google Scholar]
- Luria S., Gross G., Horowitz M., Givol D. Promoter and enhancer elements in the rearranged alpha chain gene of the human T cell receptor. EMBO J. 1987 Nov;6(11):3307–3312. doi: 10.1002/j.1460-2075.1987.tb02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Hieter P. A., Hollis G. F., Swan D., Otey M. C., Leder P. Chromosomal location of human kappa and lambda immunoglobulin light chain constant region genes. J Exp Med. 1982 May 1;155(5):1480–1490. doi: 10.1084/jem.155.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Swan D. C., Santos E., Barbacid M., Tronick S. R., Aaronson S. A. Localization of the normal allele of T24 human bladder carcinoma oncogene to chromosome 11. Nature. 1982 Dec 23;300(5894):773–774. doi: 10.1038/300773a0. [DOI] [PubMed] [Google Scholar]
- McCaw B. K., Hecht F., Harnden D. G., Teplitz R. L. Somatic rearrangement of chromosome 14 in human lymphocytes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2071–2075. doi: 10.1073/pnas.72.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon P. J. Ataxia-telangiectasia: an inherited disorder of ionizing-radiation sensitivity in man. Progress in the elucidation of the underlying biochemical defect. Hum Genet. 1987 Mar;75(3):197–208. doi: 10.1007/BF00281059. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L., Albertson D. G., Sherrington P. D., Rabbitts T. H. Analysis of a T-cell tumor-specific breakpoint cluster at human chromosome 14q32. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9171–9175. doi: 10.1073/pnas.85.23.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengle-Gaw L., Willard H. F., Smith C. I., Hammarström L., Fischer P., Sherrington P., Lucas G., Thompson P. W., Baer R., Rabbitts T. H. Human T-cell tumours containing chromosome 14 inversion or translocation with breakpoints proximal to immunoglobulin joining regions at 14q32. EMBO J. 1987 Aug;6(8):2273–2280. doi: 10.1002/j.1460-2075.1987.tb02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Lefranc M. P., Stinson M. A., Sims J. E., Schroder J., Steinmetz M., Spurr N. L., Solomon E., Goodfellow P. N. The chromosomal location of T-cell receptor genes and a T cell rearranging gene: possible correlation with specific translocations in human T cell leukaemia. EMBO J. 1985 Jun;4(6):1461–1465. doi: 10.1002/j.1460-2075.1985.tb03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G., Isobe M., Pegoraro L., Finan J., Nowell P. C., Croce C. M. Molecular analysis of a t(7;14)(q35;q32) chromosome translocation in a T cell leukemia of a patient with ataxia telangiectasia. Cell. 1988 Apr 8;53(1):137–144. doi: 10.1016/0092-8674(88)90495-3. [DOI] [PubMed] [Google Scholar]
- Sadamori N., Kusano M., Nishino K., Tagawa M., Yao E., Yamada Y., Amagasaki T., Kinoshita K., Ichimaru M. Abnormalities of chromosome 14 at band 14q11 in Japanese patients with adult T-cell leukemia. Cancer Genet Cytogenet. 1985 Jul;17(3):279–282. doi: 10.1016/0165-4608(85)90019-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster R. N., Minowada J., Suciu-Foca N., Minden M., Mak T. W. Rearrangement and expression of the alpha, beta, and gamma chain T cell receptor genes in human thymic leukemia cells and functional T cells. J Exp Med. 1986 Jun 1;163(6):1491–1508. doi: 10.1084/jem.163.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano S. R., Lange B. J. Ataxia-telangiectasia and acute lymphoblastic leukemia. Cancer. 1980 Apr 1;45(7):1675–1678. doi: 10.1002/1097-0142(19800401)45:7<1675::aid-cncr2820450725>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The arrangement of immunoglobulin and T cell receptor genes in human lymphoproliferative disorders. Adv Immunol. 1987;40:247–321. doi: 10.1016/s0065-2776(08)60241-2. [DOI] [PubMed] [Google Scholar]
- Welch J. P., Lee C. L., Beatty-DeSana J. W., Hoggard M. J., Cooledge J. W., Hecht F., McCaw B. K., Peakman D., Robinson A. Non-random occurrence of 7-14 translocations in human lymphocyte cultures. Nature. 1975 May 15;255(5505):241–245. doi: 10.1038/255241a0. [DOI] [PubMed] [Google Scholar]