Abstract
Many age-associated degenerative diseases commonly involve degradation of the extracellular matrix and aberrant matrix metalloproteinase-1 (MMP-1) expression. In diverse cell lines MMP-1 or interstitial collagenase (CL) expression is tightly regulated through a network of signals involving reactive oxygen species (ROS). However, whether the in vivo age-associated increase in CL expression is also sensitive to ROS-mediated signaling has not been established. To evaluate the contribution of ROS to the age-dependent increase in CL we monitored the levels of murine CL in two well-established models of oxidant stress. Analysis of murine CL levels in mice deficient in either of the intracellular superoxide dismutases (Sod2+/− or Sod1−/−) revealed its age- and redox-dependent expression relative to WT controls. Both age- and redox-dependent increases in murine CL expression were associated with elevations in phosphorylation of the MAP Kinases, Erk, JNK and p38. CL expression was highest in renal and skeletal muscle tissue from the aged Sod1−/− mice and was associated with a decrease in collagen staining. These findings suggest that MAPK signaling and CL production are both age- and redox-responsive. The redox sensitivity of age-associated CL expression suggests that degenerative disease associated with aberrant matrix remodeling and oxidant stress may be amenable to antioxidant-based therapies.
Keywords: Ageing, Collagenase, MMP-13, Superoxide dismutase, Oxidants
1. Introduction
Matrix metalloproteinase-13 (MMP-13) is a murine interstitial collagenase (CL) and the functional homologue of human matrix metalloproteinase-1 (MMP-1). MMPs are a broad family of 23 Zn2+-binding, Ca2+-dependent endopeptidases that are responsible for degrading various components of the extracellular matrix (ECM). The MMP family is divided into seven classes depending on their substrate specificity. The various MMP family members are involved in a wide number of physiological processes including cell migration, tissue remodeling, embryogenesis and organ morphogenesis (Nagase et al., 2006). MMPs are expressed at low levels under normal physiological conditions and can be induced or activated when required by diverse stimuli such as mechanical stimulation, growth factors, cytokines, ultra-violet radiation and infection (Lee et al., 1996; Vincent et al., 2002; Ito et al., 1990). Apart from normal physiological processes (Parks et al., 2004) augmented activity of this family of enzymes is associated with many age-related disease pathologies including: chronological skin ageing and photoageing (Hornebeck, 2003); periodontitis (Nomura et al., 1998); lung emphysema (Imai et al., 2001); atherosclerosis (Nikkari et al., 1995); tumor metastasis (Brinckerhoff et al., 2000), both osteo- and rheumatoid arthritis (Burrage et al., 2006) and renal disease (Catania et al., 2007). In addition, fibroblasts obtained from patients suffering from various premature ageing syndromes secrete elevated levels of MMP-1 (Kumar et al., 1992). These observations indicate there is a strong association between age and aberrant MMP-1 expression.
Tight regulatory control of MMP is maintained by controlling its transcription, translation, activation and by tissue inhibitors of metalloproteinases (Vincenti and Brinckerhoff, 2002). MMP-1 transcription involves signaling via members of the phosphoinositoyl-3-kinase (PI-3-Kinase) and MAP Kinase (MAPK) pathways as well as various protein kinase C isoforms (Lechuga et al., 2004; Shum et al., 2002). Both MMP expression and activity can also be regulated by reactive oxygen species (ROS) (Brenneisen et al., 1997; Nelson et al., 2006).
ROS have been proposed to be key players in the process of ageing and are at the foundation of the free radical theory of ageing proposed by Denham Harman (Harman, 1956). Age-dependent increases in ROS production, resulting from metabolic defects or diminished antioxidant scavenging, are responsible for oxidation of cellular biomolecules (Cand and Verdetti, 1989). MMPs have also shown to be directly activated by ROS (Shah et al., 1987). Lipid and organic peroxides are involved in UV-induced MMP-1 expression (Polte and Tyrrell, 2004). Treatment of brain microvascular endothelial cells with peroxynitrite can induce MMP-1 and -9 and increase the activity of MMP-1, -2 and -9 (Gursoy-Ozdemir et al., 2004). MMP induction contributes to degradation of the blood–brain barrier during neurodegenerative and neuroinflammatory disorders (Haorah et al., 2007). High endogenous levels of ROS in tumor cells correlates with the increased activity of MMP-2 and -9 (Burlaka et al., 2006). ROS associated induction of MMP-1 and -2 mRNA can be blocked by catalase (Zaw et al., 2006). Cytokine-dependent induction of MMP-1 is also ROS mediated (Lo and Cruz, 1995). We have identified MMP-1 as a redox-sensitive protease that is sensitive to increases in the steady state production of H2O2 which can be reversed by coexpression of catalase (Nelson et al., 2006). All of these reports indicate that there is a strong link between ROS production and MMP induction. Thus, ROS are linked to both the ageing process and induction of MMPs. However, whether a correlation exists between oxidant stress, ageing and MMP induction is not known. The current study aims to define if CL is regulated in an age- and redox-dependent fashion by using, Mn (Sod2+/−) and CuZn (Sod1−/−) superoxide dismutase-deficient mice, two well-established models of oxidant stress. Both these mouse models have been widely used to study the effect of oxidant stress on ageing and are well characterized in terms of their antioxidant profiles and associated lipid, protein and DNA damage (Huang et al., 1997; Van Remmen et al., 1999; Elchuri et al., 2005; Muller et al., 2006). The current study demonstrates that murine MMP-13, the functional homolog of human MMP-1, is increased in an age-dependent manner in all tissues tested. Furthermore, the ROS-dependent regulation of MMP-13 appears to be tissue specific and is associated with age and redox-dependent increases in the activity of signaling networks that drive CL expression. Our findings suggest that antioxidant-based therapies may be particularly useful in ameliorating degenerative diseases associated with the aberrant expression of interstitial collagenase.
2. Materials and methods
2.1. Animal tissues
Frozen tissues were obtained from the Sod1−/− or Sod2+/− mice and their aged-matched WT counterparts from the laboratory of Dr. Van Remmen at University of Texas Health Science center at San Antonio, TX. These models were originally generated in the lab of Dr. CJ Epstein's and have been previously described in detail (Huang et al., 1997; Elchuri et al., 2005; Muller et al., 2006). Tissues used for analysis were based on availability. Heart and kidney tissues were obtained from young (6 months) and old (26–28 months) Sod2+/− and WT mice while heart, kidney, liver and skeletal muscles were used for the Sod1−/− and WT mice. The Sod1−/− mice have a decreased lifespan compared to their WT controls and were provided from 16 to 18 month old mice, which are technically middle-aged and for this manuscript have been described as old. All young mice were 6 months old. Aged Sod1−/− mice display a severe loss of muscle mass, thus, MMP-13 analysis was restricted to animals of 12–14 months. Tissues were shipped frozen and were maintained at −80 °C until analyzed.
2.2. Preparation of tissue lysates
Tissue lysates were prepared in 500 μl lysis buffer (1× PBS, pH 7.4, 1% NP-40, 0.5% Sodium deoxycholate, 0.1% Sodium Dodecyl Sulphate with protease inhibitor cocktail (Roche) and 2 mM Sodium orthovanadate and pulse homogenized in a bead beater. The homogenates were cleared by centrifugation at 14,000 rpm for 10 min. The supernatant was collected, aliquoted and frozen for future analysis.
2.3. Murine MMP-13 pulldown and immunoblot
Protein concentrations were determined using BCA protein assay kit (Pierce). Tissue lysates equivalent to 100 μg protein were resuspended in 1 ml of lysis buffer and incubated overnight on a rotator at 4 °C with 40 μl of heparin–Sepharose beads (Amersham Biosciences) to bind CL. Following incubation, beads were centrifuged at 1000 rpm for 5 m and resuspended in 40 μl of 1× HBSS and boiled with denaturing loading dye to release the bound MMP-13 Samples were centrifuged at 14,000 rpm for 5 m to pellet Sepharose beads. Boiled supernatants were cleared of beads using microcon ultrafiltration tubes and loaded on 4–12% gradient gels (Invitrogen) and electrophoresed. Proteins were immunoblotted onto nitrocellulose using Invitrogen transfer system at 30 V for 1 h at room temperature or 6 min transfer using the iBLOT®. Membranes were blocked in 5% milk, Tris buffered saline containing 0.1% Tween (TTBS) (pH 7.6) for one hour at room temperature with gentle rocking. Membranes were incubated with monoclonal MMP-1 antibody (R&D Systems) at 1:400 dilution in 5% milk in TTBS overnight at 4 °C. Following incubation, membranes were washed three times with 1×-TTBS and incubated with horseradish peroxidase-conjugated anti-mouse secondary antibody (Amersham Biosciences) (1:4000 dilution in 5% milk-TTBS) for one hour at room temperature. The membrane was washed three times for 15 min with 1×-TTBS and developed using Pierce Femto Supersignal chemiluminescent substrate for 3 min and exposed to Kodak MS radiographic film (Kodak, Rochester, NY). Data was normalized to GAPDH from lysates of tissues that were used for the MMP-13 pulldown.
2.4. Western blot
Protein concentrations were determined as above and 25 μg total protein was normally used for immunoblotting. The following antibodies were used: p-p38 (9211), total p38 (9212), total Erk (9102), total JNK (9258), p-Akt S473 (9271) and total Akt (9272) from Cell Signaling Technologies; MMP-1 (MAB901) from R&D Systems, GAPDH (4300) from Ambion; p-Erk (sc-7383), p-JNK (44-682G) from Biosource. Secondary antibodies anti-mouse (NA931) and anti-rabbit (NA934) were from Amersham.
2.5. Trichrome staining and quantification
Paraffin embedded tissue sections of 4–6 μM were mounted on slides. Tissue sections were stained using Masson's Trichrome stain kit according to manufacturers instruction (American MasterTech). The different stains represent: red for cytoplasm, keratin and muscle; blue for collagen and mucus; and black for nuclei. For each slide ≥4 pictures were imaged. Red and blue staining was digitally extracted from all images with Adobe Photoshop and quantified using ImageJ. Blue staining intensity was normalized to overall red stain. Glomerular trichrome staining was similarly quantified and normalized to area analyzed. Quantification is representative of 30 glomeruli for each cohort of mice analyzed. Data was normalized to WT control values.
2.6. Statistical analysis
ANOVA with α = 0.05 was used for processing the data. Two-sample t test was used as posttest.
3. Results
3.1. MMP-13 increases with age in renal and cardiac tissue of both WT and Sod2+/− mice
MMP-13 levels were determined in kidney and heart lysates of both young (6 months) and old (26–28 months) WT and Sod2+/− mice. MMP-13 levels increased with age in both the WT and Sod2+/− heart (Fig. 1a) and kidney (Fig. 1b) when compared to their age-matched controls. While not achieving statistical significance, there was a more robust increase in MMP-13 expression in the aged Sod2+/− relative to the WT animals.
Fig. 1.
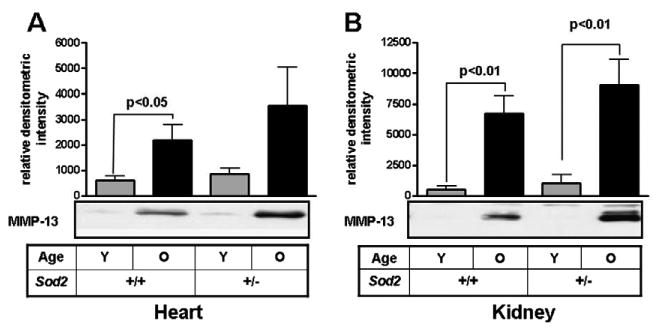
MMP-13 production increases with age in tissues from WT and Sod2+/− mice. MMP-13 levels were determined in heart (A) and kidney (B) tissues from WT and Sod2+/− mice either young (6 months) or old (26–28 months) by heparin–Sepharose pulldown and Western blotting. Data normalized to GAPDH as loading control determined from tissue lysates. Values are reported as relative densitometric intensities and represented as ± SE of mean. n = 4 for each group of heart tissues. n = 6 for young WT, n = 7 for old WT and young Sod2+/−, n = 10 for old Sod2+/−.
3.2. MMP-13 expression increases with age in kidney, liver and skeletal muscles of WT and Sod1−/− animals
MMP-13 immunoreactive protein was monitored in kidney and liver of young (5–6 months) and old (16–18 months) Sod1−/− and aged-matched WT control animals. For skeletal muscles 12–14 month old mice were analyzed due to severe muscle atrophy in the older animals. MMP-13 increased with age in both WT and Sod1−/− animals in all tissues analyzed. MMP-13 levels were increased in cardiac tissue with age which was exacerbated in the Sod1−/− mice, however statistical significance could not be assessed due to the limited sample number (data not shown). Age-dependent increases in MMP-13 were observed in both renal (Fig. 2a) and hepatic (Fig. 2b) tissue lysates from both the WT and Sod1−/− mice. Loss of Sod1 significantly enhanced age-associated renal MMP-13 expression. Skeletal muscle MMP-13 levels were also increased in both the old WT and Sod1−/− over their respective young controls (Fig. 2c). More striking was the increase in MMP-13 in the old Sod1−/− relative to age-matched controls. These findings suggest that the kidney and the skeletal muscles of the mice may be more susceptible to redox-dependent increases in MMP-13 expression resulting from a CuZnSod-deficiency.
Fig. 2.
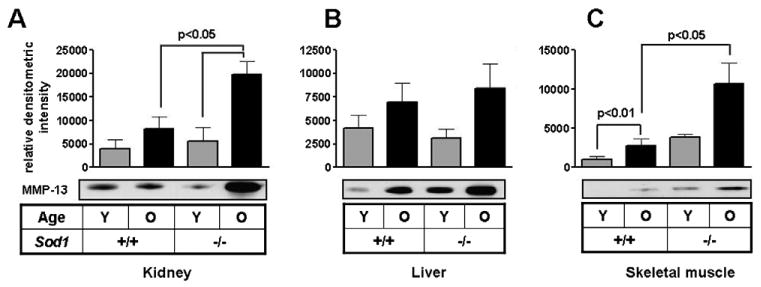
MMP-13 expression increases in an age-dependent manner in WT and Sod1−/− mice tissues. MMP-13 levels were determined in kidney (A), liver (B), and skeletal muscle (C) tissues from WT and Sod1−/− mice either young (6 months) or middle-aged (16–18 months, 12–14 months for skeletal muscles) by heparin–Sepharose pulldown and Western blotting. MMP-13 levels were normalized to GAPDH levels in the lysates. Values are reported as relative densitometric intensities and represented as ± SE of mean. n = 4 for each group of kidney, liver and skeletal muscle tissues.
3.3. Age and redox-dependent changes in MAP Kinase activation
MMP expression is controlled by the various MAP Kinase family members. To determine if increases in MAPK phosphorylation were associated with elevated CL levels we monitored the phosphorylation of Erk, JNK and p38 in tissue lysates from young and old SOD deficient mice and their aged matched controls. The prominent MAP Kinase family members Erk (Fig. 3a), JNK (Fig. 3b) and p38 (Fig. 3c) displayed increases in their phosphorylation with age in heart tissues in both WT and Sod2+/− mice compared to young mice. Renal tissues from these mice also showed an increased in phosphorylation of Erk and JNK (data not shown).
Fig. 3.
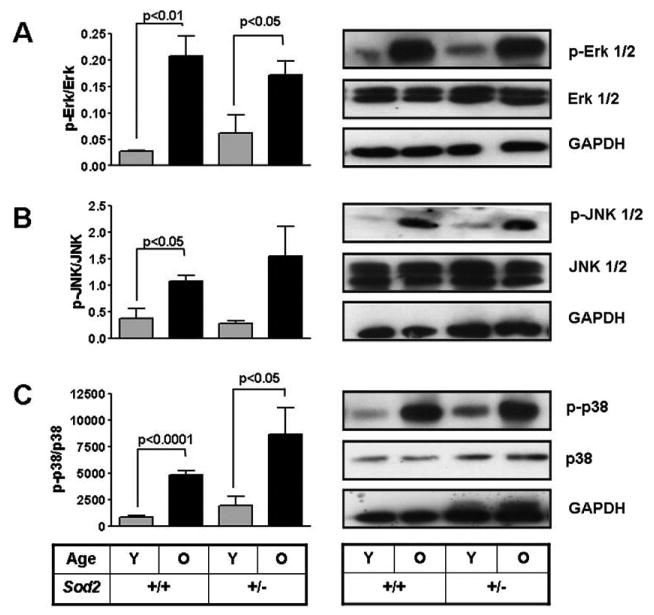
Phosphorylation of MAP Kinases increases with age in heart tissues from WT and Sod2+/− mice. Phosphorylation of different members of the MAP Kinase pathway Erk (A), JNK (B) and p38 (C) was determined by Western blotting. Values reported as relative densitometric intensity and represented as mean ± SE. n = 4 for each group in p-Erk. n = 4 for young and old WT and n = 5 for young and old Sod2+/− in p-JNK, n = 2 for all groups in p-p38.
Erk phosphorylation did not vary significantly between the different groups in the kidney tissues of WT and Sod1−/− mice (Fig. 4a). Interestingly, phospho-JNK levels in kidney increased in both young and old Sod1−/− mice relative to controls. JNK phosphorylation was low in both young and old WT kidney tissues (Fig. 4b). Age-dependent increases in p38 phosphorylation were only observed in renal tissues (Fig. 4c). In addition, the phosphorylation state of both JNK and p38 was increased in the aged Sod1−/− relative to their controls.
Fig. 4.
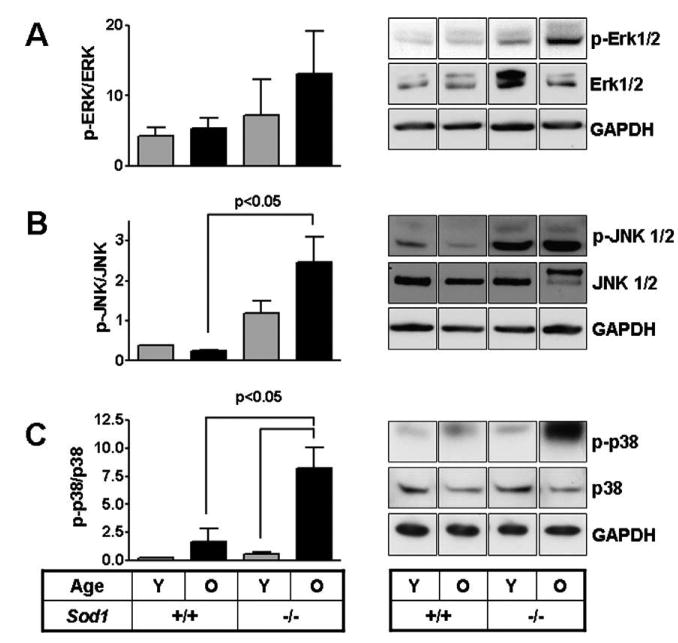
MAP Kinase phosphorylation in kidney tissues of WT and Sod1−/− mice. Phosphorylation status of different members of the MAP Kinase pathway Erk (A), JNK (B) and p38 (C) ws = as determined by Western blotting and normalized to total protein. Values reported as relative densitometric intensity and represented as mean ± SE. n = 4 for each group. Immunoblot panels for each row are from the same autoradiographic exposure and are representative of three independent samples, with the exception of a young+/+ samples for JNK and p38.
3.4. Akt phosphorylation changes with age in but is not redox-responsive
The PI3Kinase/Akt axis also participates in regulating MMP expression. Analysis of Akt from the distinct tissue samples indicate that its phosphorylation was increased with age in both WT and Sod2+/− heart tissues over their respective young controls (Fig. 5a). A similar age-dependent increase in Akt-phosphorylation was observed in renal tissue from WT and Sod1-deficient animals however these differences did not achieve significance (Fig. 5b).
Fig. 5.
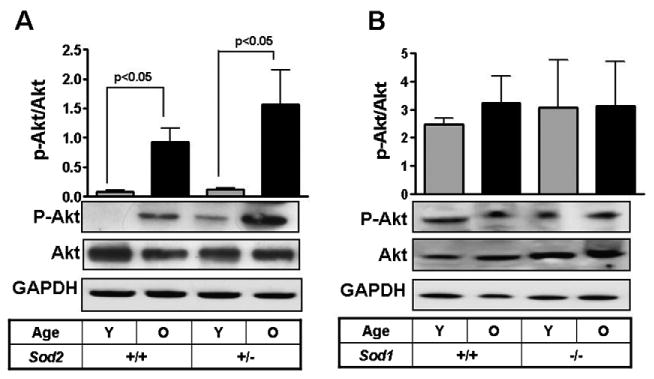
Akt phosphorylation levels in tissues from Sod1−/− and Sod2+/− mice. Akt phosphorylation was determined in heart tissues from WT and Sod2+/− mice (A) and kidney tissues from WT and Sod1−/− mice. Values reported as relative densitometric intensity and represented as mean +/− SE. n>/= 4 for each group.
3.5. Loss of collagen deposition is enhanced in renal samples from SOD1−/− mice
We next evaluated set out to determine whether the increases in MMP-13 abundance in the Sod1−/− mice were associated with decreases in collagen deposition using Trichrome staining. Quantitative analysis of total collagen levels in renal tissues suggests that a trend for decreased collagen is observed in the old Sod1−/− when compared to WT mice. The decrease in collagen levels is visually evident and reaches significance when focus is placed on the Bowman's capsule of the glomeruli (Fig. 6a and b).
Fig. 6.
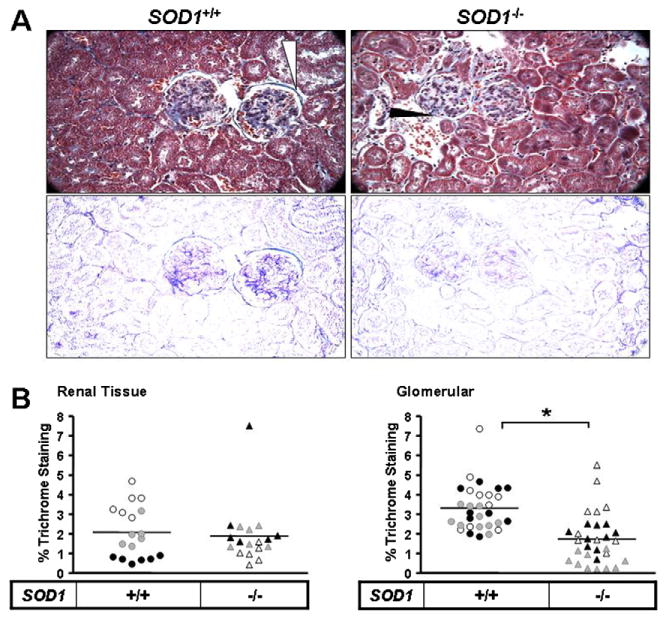
Elevated MMP-13 in aged Sod1−/− mice is associated with decreased renal collagen deposition. Collagen level in kidney tissues from aged WT and SOD1−/− mice was determined by trichrome staining (A) followed by relative quantification of the stain (B). (A) top panel shows trichrome staining of kidney tissues and corresponding bottom panel is the extracted blue stain indicative of collagen. White arrows mark areas of intense trichrome staining and black arrows to decreased staining. The arrows point to the region of the Bowman's capsule which has collagen present. (B) quantification of trichrome in kidney tissue and within glomeruli was normalized to total red stain and area, respectively. The estimated collagen levels in old SOD1−/− kidney tissue was normalized to old WT. Values are reported as relative densitometric intensity and represented as mean ± SE. n ≥ 4 for each group.
4. Discussion
In the present study using tissues from two different mouse models of oxidative stress, namely, the Sod2+/− and Sod1−/− mice and their age-matched WT controls, we demonstrate that the level of MMP-13 immunoreactive protein increased with age in all tissues studied. Renal MMP-13 levels were particularly sensitive to the level of oxidative stress in the old Sod1−/−(Fig. 2A). The heart and kidney tissues from the old Sod2+/− and WT animals showed a significant increase in the level of MMP-13 over their respective young controls (Fig. 1). However, no statistically significant differences in MMP-13 expression were observed between the old Sod2+/− and WT Though both the Sod2+/− and the Sod1−/− mice reportedly show increases in levels of oxidative stress in tissues (Muller et al., 2006; Van Remmen et al., 2003), it is interesting that only the kidneys from the old Sod1−/− mice showed an oxidant-stress dependent increase in MMP-13 expression though a trend exists in the old Sod2+/− animals. In addition, the skeletal muscles from the old Sod1−/− mice showed a significant increase in MMP-13 levels over their age-matched controls. It is interesting to note here that even at a comparatively early age (12–14 months), the old Sod1−/− mice show a significant increase in MMP-13 levels in their skeletal muscles compared to their age-matched WT controls (Fig. 2c) suggesting that oxidative stress also exacerbates age-dependent MMP-13 expression in this tissue.
The Sod1−/− mice have a shortened lifespan compared to their WT controls while a single functional Sod2 allele is sufficient to maintain a normal lifespan (Muller et al., 2006; Van Remmen et al., 2003). In addition, loss of single Sod2 allele leads to oxidative damage only to the DNA, while homozygous loss of Sod1 damages all major biomolecules (Muller et al., 2007). Oxidative stress is manifested in terms of increased incidence of cancer with no other differences in other ageing markers in the Sod2+/− while the Sod1−/− show more severe pathological outcomes including increased cataract development (Olofsson et al., 2005), early hearing loss (Keithley et al., 2005), skeletal muscle atrophy and increased incidence of cancer (Muller et al., 2006). Thus, the Sod1 dismuting activity is critical for maintenance of redox homeostasis in cells and tissues. There is evidence to show that loss of Sod1 leads to significant damage to the mitochondria even though Sod2 levels in the organelle are intact (Aquilano et al., 2006). Sod1 deficiency is associated with decreases in mitochondrial membrane potential and ATP synthesis, both of which are critical for maintenance of mitochondrial integrity. Overall, these observations suggest that the Sod1−/− mice are likely exposed to high levels of oxidative damage and may explain the more robust increase in the expression of MMP-13, relative to animals with a partial deficiency in Sod2 activity.
In spite of the differences seen between the two mouse models, both displayed an age-dependent increase in MMP-13, which appear to be exacerbated by oxidative stress. The age-associated increase in MMP-13 was associated with an increase in phosphorylation of MAPKs – Erk, JNK and p38 (Fig. 3) all of which contribute to regulating MMP expression (Wu et al., 2004). Furthermore, MAPK phosphorylation was increased by Sod deficiency in a tissue-specific manner (Fig. 4). We have previously established that JNK plays an important role in the redox-responsiveness of the human MMP-1 promoter (Nelson et al., 2006). Analysis of JNK abundance revealed that JNK2 was prominently upregulated in the aged Sod1-deficient mice. Work by Firestein and coworkers defined the importance of both JNK1 and JNK2 in human fibroblast-like synoviocytes and in a murine model of rheumatoid arthritis (Han et al., 2001). In both cases the JNK inhibitor blocked hMMP-1 or mMMP-13 expression in vitro and in vivo, respectively. In their model system, JNK2 is the dominant JNK protein and is likely the most physiologically relevant as it binds c-Jun with greater avidity than the other JNKs. In addition, the IL-1 dependent expression of mMMP-13 is more severely impaired in JNK2- than in JNK1-deficient MEFs. It is possible that this age-dependent enhancement of JNK2 expression in the Sod1-deficient animals may participate in the increased expression of MMP-1 in the renal tissue.
The PI3K pathway is also known to regulate MMP expression (Lechuga et al., 2004). Phosphorylation of Akt, a downstream target of PI3K, showed similar patterns in the two mouse models. While the Sod2+/− and WT mice showed significant age-dependent increase in Akt phosphorylation (Fig. 5a) this differences was not as striking when comparing the Sod1−/− mice and their aged matched controls. The less striking age-dependent increase in Pi-Akt in the Sod1−/− relative to Sod2+/− is likely attributed to distinct chronological age of the two cohorts (Sod1−/−, 16–18 months vs Sod2+/−, 26 months).
A high level of interstitial collagenase in humans and rodents is associated with pathologies in diverse organs. Our studies were restricted to liver, kidney, heart and skeletal muscle tissues in either one or both murine models. These organs have been shown to be prone to MMP-dependent age related pathologies and our findings suggest that aberrant collagenase production may exacerbate the disease conditions in sensitized/susceptible individuals.
Kidneys lose function in an age-dependent manner that involves loss of renal mass and increased fibrosis both of which involve augmented MMP activity (Ahmed et al., 2007). We have demonstrated that MMP-13 levels in kidneys seem to be particularly sensitive to increased oxidative stress (Fig. 2). High level of MMP-1 expression appears to be involved in renal fibrosis (Catania et al., 2007), a major cause of dialysis and kidney transplants, as well as in initiating glomerular remodeling in progressive kidney scarring (Denzinger et al., 2007). Our findings suggest that collagen deposition appears to dissipate in the Bowmans capsule. This cup-like structure houses the glomerular vessels and forms the filtration barrier with basement membrane (BM). This region is composed of laminin and type IV collagen is crucial for filtration. MMPs degrade extracellular matrix components such collagen, gelatin, fibronectin and laminins and it is possible that uncontrolled expression of MMPs in the aged and SOD deficient mice might affect filtration impairing renal function. This is clearly evident in Alport syndrome where mutations impacting collagen synthesis hamper production and assembly of collagen and composition of basement membranes. Associated with this disorder is a decline in renal function typically leading to renal failure. MMPs are also involved in other kidney pathologies such as acute kidney injury, chronic allograft nephropathy, diabetic nephropathy and polycystic kidney disease (Hirata et al., 2004). Apart from fibrosis MMPs also play an important role in the progression of renal cell carcinoma that appears to have a higher tumor grade and poor survival with age in humans (Nikkari et al., 1995) and is dependent on the MMP-1 and MMP-3 haplotype (Brown et al., 1995).
MMPs are also known to play an important role in several cardiac-associated pathologies. High levels of MMP-1 (Pasterkamp et al., 2000) along with MMP-2 (Felkin et al., 2006), MMP-9 (Kato et al., 2005) are seen in atherosclerotic plaques. Collagenase levels increase in heart failure patients while the levels of their inhibitors remain unchanged (Muller et al., 2006). Other conditions such as coronary artery disease show increases in serum MMP-1 and MMP-9 levels (Matsumura et al., 2005).
One of the phenotypes of the Sod1−/− mice is their loss of skeletal muscle tissue (Muller et al., 2006). MMP-13 profiles in the skeletal muscle of WT mice show age-dependent increases (Fig. 3). MMPs are associated with various muscle-related dystrophies and muscle degeneration(Carmeli et al., 2004). Whether increased MMP-13 in skeletal muscles contributes to sarcopenia in aged Sod1−/− animals remains to be determined.
MMPs are also important in other pathologies such as cancer and arthritis (Tasci et al., 2008). High levels of MMP-13 have been reported in rat and murine models of arthritis (Woo et al., 2007) Aberrant MMP-1 expression has been linked with bladder cancer (Zhu et al., 2001) and colorectal cancer (Zhu et al., 2001). MMP-1 is thought to contribute to tumor initiation and development (Lee et al., 2005) promoting a tissue microenvironment that is permissive to tumor growth. MMPs can act on more than just the components of the ECM, including growth factors, cytokines and chemokines. MMP-dependent cleavage of these factors has also been shown to activate and increase their bioavailability (Churg et al., 2003). Cardiac hypertrophy in response to increased IGF signaling has also been linked to increased MMP-1 and MMP-2 activity during β-adrenergic stimulation that is blocked by MMP inhibition (Pardo and Selman, 2005). MMPs can also cleave and release membrane bound TNF-α making it available for signaling as observed in cigarette-smoke induced inflammation (Churg et al., 2003). This latter scenario may increase the pathology of lung emphysema associated with smoking, which is also exacerbated with age. MMP-1 can also cleave and activate other MMPs serving to aggravate ECM degradation process. Thus, high endogenous levels of MMP-13 in aged murine tissues and that of the different collagenase in humans might serve to make the tissue microenvironment more susceptible to damage, decrease the threshold for disease induction and increase the pathology of diseases.
To summarize, the current work demonstrates that MMP-13 increases with age in murine tissues and can be used as a biomarker for ageing and potentially oxidative insult. It is important to emphasize that the fold increase in age-dependent induction of MMP-13, ERK1/2, p38, JNK and Akt remains relatively constant between the SOD genotypes studied. However, the amplitude of the signals driving their induction is redox-sensitive. Thus, increases in MMP-13 expression with age are likely responsible for aberrant extracellular matrix degradation and may be exacerbated by conditions that augment oxidant production.
Acknowledgments
This work was supported by Public Health Service grant AG031067 from the National Institute of Aging and in part, by Philip Morris USA Inc. and Philip Morris International.
References
- Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007;71:755–763. doi: 10.1038/sj.ki.5002108. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Vigilanza P, Rotilio G, Ciriolo MR. Mitochondrial damage due to SOD1 deficiency in SH-SY5Y neuroblastoma cells: a rationale for the redundancy of SOD1. FASEB J. 2006;20:1683–1685. doi: 10.1096/fj.05-5225fje. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic Biol Med. 1997;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- Burlaka AP, Sidorik EP, Ganusevich II, Lestchenko YM, Burlaka AA, Osinsky SP. High formation of superoxide anion and nitric oxide, and matrix metalloproteinases activity in vascular wall of rectal carcinoma vessels. Exp Oncol. 2006;28:323–325. [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis 109. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Cand F, Verdetti J. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Free Radic Biol Med. 1989;7:59–63. doi: 10.1016/0891-5849(89)90101-9. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol. 2007;292:F905–F911. doi: 10.1152/ajprenal.00421.2006. [DOI] [PubMed] [Google Scholar]
- Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-{alpha} release 2040. Am J Respir Crit Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- Denzinger S, Otto W, Burger M, Hammerschmied C, Junker K, Hartmann A, Wieland WF, Walter B. Sporadic renal cell carcinoma in young and elderly patients: are there different clinicopathological features and disease specific survival rates? World J Surg Oncol. 2007;5:16. doi: 10.1186/1477-7819-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson RL, Van RH, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Felkin LE, Birks EJ, George R, Wong S, Khaghani A, Yacoub MH, Barton PJ. A quantitative gene expression profile of matrix metalloproteinases (MMPS) and their inhibitors (TIMPS) in the myocardium of patients with deteriorating heart failure requiring left ventricular assist device support. J Heart Lung Transplant. 2006;25:1413–1419. doi: 10.1016/j.healun.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, Wang X, Rosenberg GA, Lo EH, Moskowitz MA. Cortical spreading depression activates and upregulates MMP-9. J Clin Invest. 2004;113:1447–1455. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. C-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood–brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okayama N, Naito K, Inoue R, Yoshihiro S, Matsuyama H, Suehiro Y, Hamanaka Y, Hinoda Y. Association of a haplotype of matrix metalloproteinase (MMP)-1 and MMP-3 polymorphisms with renal cell carcinoma. Carcinogenesis. 2004;25:2379–2384. doi: 10.1093/carcin/bgh254. [DOI] [PubMed] [Google Scholar]
- Hornebeck W. Down-regulation of tissue inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol (Paris) 2003;51:569–573. doi: 10.1016/j.patbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Huang TT, Yasunami M, Carlson EJ, Gillespie AM, Reaume AG, Hoffman EK, Chan PH, Scott RW, Epstein CJ. Superoxide-mediated cytotoxicity in superoxide dismutase-deficient fetal fibroblasts 1098. Arch Biochem Biophys. 1997;344:424–432. doi: 10.1006/abbi.1997.0237. [DOI] [PubMed] [Google Scholar]
- Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D'Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema 108. Am J Respir Crit Care Med. 2001;163:786–791. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- Ito A, Sato T, Iga T, Mori Y. Tumor necrosis factor bifunctionally regulates matrix metalloproteinases and tissue inhibitor of metalloproteinases (TIMP) production by human fibroblasts. FEBS Lett. 1990;269:93–95. doi: 10.1016/0014-5793(90)81127-a. [DOI] [PubMed] [Google Scholar]
- Kato R, Momiyama Y, Ohmori R, Taniguchi H, Nakamura H, Ohsuzu F. Levels of matrix metalloproteinase-1 in patients with and without coronary artery disease and relation to complex and noncomplex coronary plaques. Am J Cardiol. 2005;95:90–92. doi: 10.1016/j.amjcard.2004.08.066. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Millis AJ, Baglioni C. Expression of interleukin 1-inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA. 1992;89:4683–4687. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechuga CG, Hernandez-Nazara ZH, Rosales JA, Morris ER, Rincon AR, Rivas-Estilla AM, Esteban-Gamboa A, Rojkind M. TGF-{beta}1 modulates matrix metalloproteinase-13 expression in hepatic stellate cells by complex mechanisms involving p38MAPK, PI3-kinase, AKT, and p70S6k. Am J Physiol Gastrointest Liver Physiol. 2004;287:G974–G987. doi: 10.1152/ajpgi.00264.2003. [DOI] [PubMed] [Google Scholar]
- Lee RT, Schoen FJ, Loree HM, Lark MW, Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arterioscler Thromb Vasc Biol. 1996;16:1070–1073. doi: 10.1161/01.atv.16.8.1070. [DOI] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors 2164. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Zhong D, Saito F, Arai K, Adachi K, Kawai H, Higuchi I, Nishino I, Shimizu T. Proteolysis of beta-dystroglycan in muscular diseases. Neuromuscul Disord. 2005;15:336–341. doi: 10.1016/j.nmd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van RH. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Csete M, Faulkner JA, Van RH. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nelson KK, Subbaram S, Connor KM, Dasgupta J, Ha XF, Meng TC, Tonks NK, Melendez JA. Redox-dependent matrix metalloproteinase-1 expression is regulated by JNK through Ets and AP-1 promoter motifs. J Biol Chem. 2006;281:14100–14110. doi: 10.1074/jbc.M601820200. [DOI] [PubMed] [Google Scholar]
- Nikkari ST, O'Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, Clowes AW. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis102. Circulation. 1995;92:1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- Nomura T, Ishii A, Oishi Y, Kohma H, Hara K. Tissue inhibitors of metalloproteinases level and collagenase activity in gingival crevicular fluid: the relevance to periodontal diseases. Oral Dis. 1998;4:231–240. doi: 10.1111/j.1601-0825.1998.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Olofsson EM, Marklund SL, Karlsson K, Brannstrom T, Behndig A. In vitro glucose-induced cataract in copper-zinc superoxide dismutase null mice. Exp Eye Res. 2005;81:639–646. doi: 10.1016/j.exer.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–288. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Schoneveld AH, Hijnen DJ, de Kleijn DP, Teepen H, van der Wal AC, Borst C. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000;150:245–253. doi: 10.1016/s0021-9150(99)00371-8. [DOI] [PubMed] [Google Scholar]
- Polte T, Tyrrell RM. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression 2192. Free Radic Biol Med. 2004;36:1566–1574. doi: 10.1016/j.freeradbiomed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Shah SV, Baricos WH, Basci A. Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase(s) by reactive oxygen metabolites. J Clin Invest. 1987;79:25–31. doi: 10.1172/JCI112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum JK, Melendez JA, Jeffrey JJ. Serotonin-induced MMP-13 production is mediated via phospholipase C, protein kinase C, and ERK1/2 in rat uterine smooth muscle cells. J Biol Chem. 2002;277:42830–42840. doi: 10.1074/jbc.M205094200. [DOI] [PubMed] [Google Scholar]
- Tasci AI, Tugcu V, Ozbek E, Ozbay B, Simsek A, Koksal V. A single-nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances bladder cancer susceptibility. BJU Int. 2008;101:503–507. doi: 10.1111/j.1464-410X.2007.07315.x. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M, Park K, Nam J, Kim JC. Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2007;22:1064–1070. doi: 10.1111/j.1440-1746.2006.04424.x. [DOI] [PubMed] [Google Scholar]
- Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90:1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- Zaw KK, Yokoyama Y, Abe M, Ishikawa O. Catalase restores the altered mRNA expression of collagen and matrix metalloproteinases by dermal fibroblasts exposed to reactive oxygen species. Eur J Dermatol. 2006;16:375–379. [PubMed] [Google Scholar]
- Zhu Y, Spitz MR, Lei L, Mills GB, Wu X. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances lung cancer susceptibility1638. Cancer Res. 2001;61:7825–7829. [PubMed] [Google Scholar]


