Abstract
Lysyl oxidase (LOX) catalyzes cross-linking of elastin and collagen, which is essential for structural integrity and function of bone tissue. The present study examined the role of Lox gene deficiency for the osteoblast phenotype in primary calvarial osteoblasts from E18.5 Lox knockout (Lox-/-) and wild type (wt) (C57 BL/6) mice. Next to Lox gene depletion, mRNA expression of Lox isoforms, LOXL1-4, was significantly down-regulated in Lox-/- bone tissue. A significant decrease of DNA synthesis of Lox-/- osteoblasts compared to wt was found. Early stages of osteoblastic apoptosis studied by Annexin-V binding as well as later stages of DNA fragmentation were not affected. However, mineral nodule formation and osteoblastic differentiation were markedly decreased, as revealed by significant down-regulation of osteoblastic markers, type I collagen, BSP and Runx2/Cbfa1.
Keywords: Lysyl oxidase, LOXL1-4, Knockout, Osteoblast
Introduction
Type I collagen is the principal constituent of extracellular bone matrix and a crucial determinant for mechanical properties of bone tissue [1, 2]. Posttranslational collagen modifications result in the formation of a mature functional matrix, which is essential for subsequent matrix mineralization [3-6]. Lysyl oxidase (protein-lysine 6-oxidase; LOX) is a copper-dependent enzyme that initiates cross-linking of collagen and elastin by catalyzing oxidative deamination of ε-amino groups of lysine and hydroxylysine residues [3, 7]. Several LOX isoforms, lysyl oxidase-like proteins 1-4 (LOXL1-4) have been identified [8-13] and amine oxidase activities of some of them have been demonstrated [14, 15]. In bone tissue, pyridinolines and deoxypyridinolines are the primary cross-links of mature type I collagen which provide mechanical integrity, rigidity, and strength [16, 17] and diminished LOX enzyme activity results in an increased risk of bone deformities and fractures [18, 19].
Cells secrete 50 kDa pro-LOX which is then processed by procollagen C-proteinases in the extracellular region, resulting in 32 kDa mature LOX enzyme and its 18 kDa LOX propeptide [20]. LOX has been detected in the intracellular space [21-23] and it has been suggested to regulate gene transcription and to play a dominant role in stabilizing a normal cell phenotype [24-28]. In osteoblastic cells (MC3T3-E1), we have shown that LOX seems to be specifically regulated in the course of osteoblast cell differentiation and this regulation is required for normal collagen deposition [29]. A stage-dependent distribution of LOX in differentiating osteoblasts with co-localization with the nuclear region as well as with the tubular network was found, which could indicate a role of LOX in the regulation of osteoblast development [21].
A lack of Lox gene expression leads to perinatal lethality in mice [30, 31]. Lox knockout mice (Lox-/-) develop to term but die soon thereafter just before or at birth [30, 31]. Due to defective collagen and elastin cross-linkage, they suffer from severe cardiovascular and pulmonary defects [30-32]. In the present study, we examined the effects of Lox gene deficiency on the skeletal phenotype and osteoblast development.
Material and methods
Histological analysis
For Alcian Blue and Alizarin Red staining, embryonic day (E) 18.5 Lox-/- mice as well as wt mice (C57 BL/6) were removed from the uterus, fixed and stored in 70 % ethanol. Prior to staining, skin and eyes were removed and calcified tissues were stained with Alcian Blue (0.3 % Alcian blue 8GX (EMD Chemicals, Gibbstown, NJ, USA), 70 % ethanol) and Alizarin Red solution (0.1 % Alizarin Red S (Wako Chemicals, Richmond, VA, USA), 95 % ethanol, 1 volume glacial acetic acid, 17 volumes 70 % ethanol) for 3 days. Samples were placed in 1 % KOH for 24-72 h and then stored in 70 % glycerol.
Moreover, three-dimensional histological reconstruction of two heads of each genotype was performed. Samples were fixed and embedded paraffin sections were cut at 10 μm-thick serial sections using a rotary microtome (Model 2065 Microtome; Reichert-Jung, Heidelberg, Germany) in frontal plane. Hematoxylin-eosin sections were evaluated and every 8th section was photographed and scanned images were aligned. Calcified tissues using the contours of well characterized landmarks (i.e. skull base structures) were reconstructed by computer software (Analysis Software; SIS, Münster, Germany) [33].
Measurement of collagen fibril diameter
Tissues were fixed in Karnowsky solution (1% glutaraldehyde, 1% tannin in 0.2 M phosphate buffer, pH 7.4) and then post-fixed with 1% osmium tetraoxide in 0.1 M phosphate buffer. The samples were rinsed, dehydrated and embedded in epon/araldite502 resin (Ted Pella, Redding, CA, USA). Sections of 30-50 nm were stained with uranyl acetate and lead citrate and the images were observed on a CM-12 transmission electron microscope (Philips Electron Optics, Eindhoven, The Netherlands). Images were recorded at × 35,000 on SO-163 electron image film (Eastman Kodak, Rochester, NY). 500 fibril diameters were measured in randomly chosen areas using Analysis Software (SIS, Münster, Germany).
Primary calvarial osteoblast cultures
Lox-/- and wt calvariae were digested (0.2 % collagenase), minced and cultured in growth medium containing of α-MEM supplemented with 10 % FCS, 100 U/ml penicillin, 100 μg/ml streptomycin and nonessential amino acids in 6-well plates as has been previously established [34, 35]. In the present study, cells from each calvaria were cultured separately, because the genotype of each embryo was determined by Southern blotting at a later timepoint. First and second cell passages were used for the experiments.
BrdU incorporation
Primary osteoblasts were plated on 96-well plates and cultivated for up to 48 h at 37 °C in growth medium. DNA synthesis was assessed in monolayer cultures by colorimetric immunoassay (BrdU Roche, Basel, Switzerland) at 405 nm. The assay is based on measuring BrdU (5-Bromo-2′-deoxyuridine) incorporation following 2 h labeling into newly synthesized DNA of replicating cells, by ELISA.
Cell apoptosis
Rapid binding of annexin V to phosphatidyl serine was used for the early identification of cells undergoing apoptosis, as described previously [36]. Cells, plated on 24-well plates were serum-starved for 24 h and then grown in the presence and absence of 1 μM staurosporine for 6 h, 16 h, and 24 h at 37 °C. Then, cells were incubated with FITC-labeled annexin V (1 μg/ml) and PI (2 μg/ml) for 15 min at 15-25 °C and analyzed by fluorescence microscopy. In addition, a photometric ELISA was applied for detection of cytoplasmatic histone-associated DNA fragments (mono- and oligonucleosomes) in apoptotic osteoblastic cells (Cell Death Detection ELISA, Roche, Basel, Switzerland) [37, 38]. Osteoblasts were cultivated in the presence and absence of 1 μM staurosporine for 6 h, 16 h and 24 h at 37 °C. Then, cells were washed, lysed for 30 min and centrifuged at 15000 rpm for 10 min. The supernatant was transferred into a streptavidin-precoated microtiter plate and incubated with the immunoreagent (anti-histone biotin, anti-DNA peroxidase) for 2 h. After washing, substrate solution was added and absorbance was determined at 405 nm.
Also, lactate dehydrogenase (LDH) release, as a marker of cell necrosis, was determined in the supernatants by colorimetric Cytotoxicity Dectection Kit (Roche, Basel, Switzerland).
Mineral nodule formation
Osteoblast differentiation was induced by growth medium supplementation with 5 mM β-glycerophosphate and 50 μg/ml ascorbic acid for up to 21 d. Mineralized cultures were fixed and stained with 2 % Alizarin Red (pH 4.0) for 20 min. For quantification, incorporated Alizarin Red stain was eluted with 10 % cetylpyridiniumchloride and optical density was measured at 562 nm using a spectrophotometer.
Quantitative real-time PCR
Total cellular RNA from calvariae of E 18.5 murine embryos was isolated (RNeasy Mini Kit, Qiagen, Hilden, Germany) according to the manufacturer's protocol and real-time PCR was performed using sequence specific primers and the ABI Prism 7000 Sequence detection system (Applied Biosystems, Lincoln, CA, USA). Specific primers for LOX (# Mm00495386_ml), LOXL1 (# Mm01145734_ml), LOXL2 (# Mm00804740_ml), LOXL3 (# Mm00442953_ml), LOXL4 (# Mm00446385_ml), for bone differentiation markers bone sialoprotein (BSP) (#Mm00492555_ml), COL1A1 (# Mm 00801666_g1) and Runx2/Cbfa1 (# Mm00501578_ml) and β-2 microglobulin (# Mm00437762_m1) were Taqman probes and were purchased from Applied Biosystems (Lincoln, CA, USA). β-2 microglobulin was used as internal control. The mRNA expression relative to β-2 microglobulin was determined and the fold changes were calculated using the wt values as calibrator by means of 2 -ΔΔCT method [39].
Statistical methods
Statistical analyses were performed using a statistical software package (SPSS for Windows, version 14.0, Chicago, IL, USA) Comparisons between data of Lox-/- and wt were made using an unpaired Student's t-test assuming equal variances, and P values < 0.05 were considered statistically significant.
Results
Skeletal phenotype
Alcian blue and Alizarin Red staining of the Lox-/- compared to the wt mice demonstrated normal morphology of calcified structures (Fig. 1). Three-dimensional reconstructions (Fig. 2) indicate normal development of nasal, alveolar as well as teeth-related structures in Lox-/- mice. However, handling of Lox-/- samples compared to wt revealed fragile tissues and high sensitivity to KOH maceration.
Figure 1.
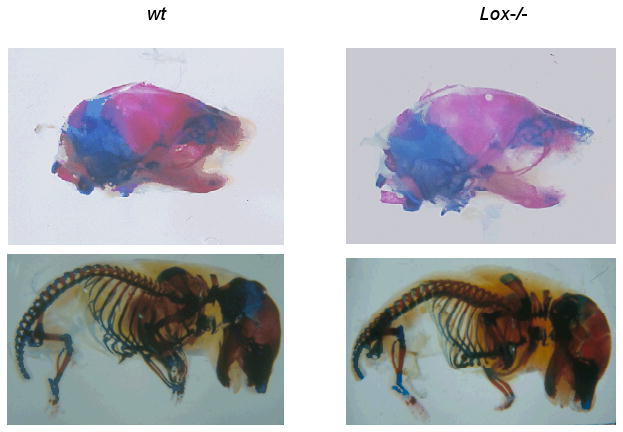
Lateral views of E 18.5 calcified tissues of Lox-/- and wt mice stained with Alizarin Red and Alcian Blue. Experiments were performed three times. Representative images of one experiment are shown.
Figure 2.
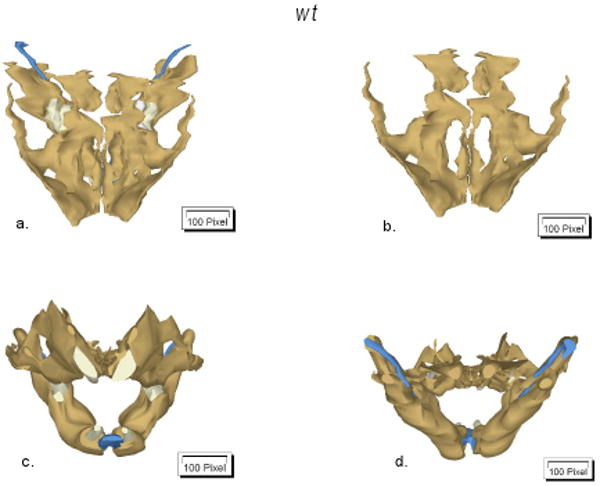
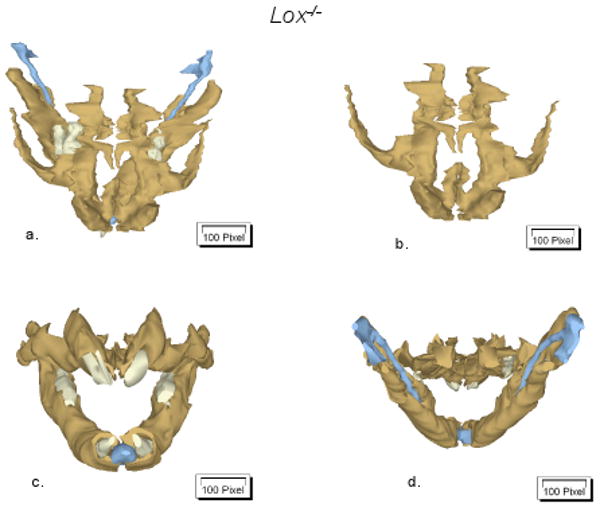
Three-dimensional reconstructions of of Lox-/- and wt alveolar as well as of teeth-related structures. Reconstructions were performed two times for each genotype. Representative images (coronal (a-b), frontal (c) and dorsal (d) views) of one experiment are shown. Bone tissue is presented in brown, while Meckel's cartilage and teeth are given in blue and white colors, respectively.
Decreased collagen fibril diameter
Data in Figure 3 demonstrate decreased collagen fibril diameters in Lox-/- compared to wt bone tissues. Quantitative analyses showed that the mean fibril diameter significantly (p < 0.001) decreased from 34.06 ± 4.18 nm in wt to 31.01 ± 3.78 nm in Lox-/-.
Figure 3.
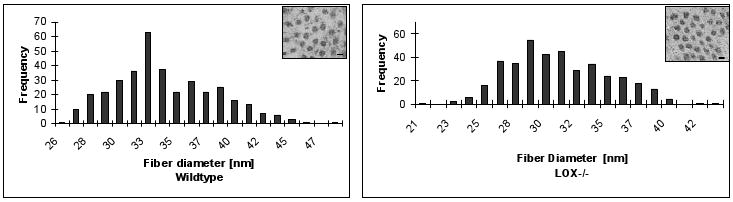
Electronmicroscopic analysis of collagen fibril diameters of craniofacial bone tissues. For each genotype, 500 fibril diameters were measured in randomly chosen areas. Representative images are shown for Lox-/- and wt. (bar = 30 nm; magnification = × 35,000)
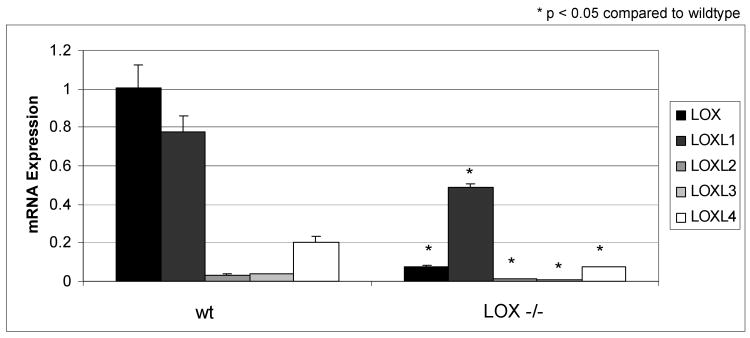
LOX mRNA expression
To establish that LOX gene expression is diminished in Lox-/- calvarial bone tissue and to analyze mRNA expression of LOX isoforms (LOXL1-4) quantitative real-time PCR was performed. Figure 4 shows a significantly (p < 0.05) diminished mRNA expression of LOX and it's isoforms in Lox-/- compared to wt.
Figure 4.
Real-time RT-PCR analysis of LOX and its isoforms, LOXL1-4, from Lox-/- and wt mice. Data are presented as mean ± SD obtained from three measurents of pooled RNA samples. Asterisks indicate significant differences between Lox-/- and wt mice (p<0.05; unpaired student's t-test assuming equal variances).
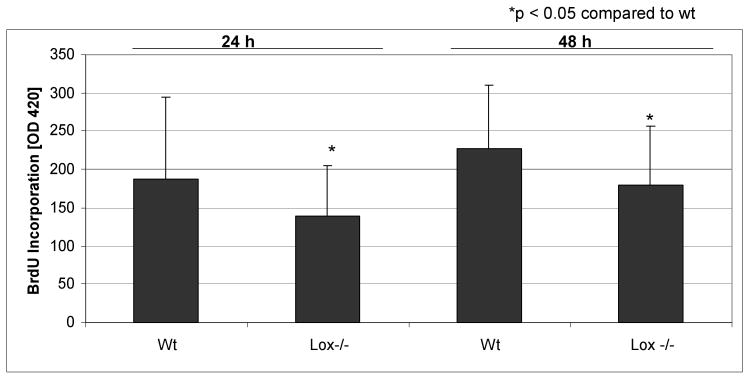
BrdU incorporation
Primary osteoblasts from Lox-/- and wt calvariae were cultivated for up to 48 h. Figure 5 demonstrates a statistically significant (p < 0.05) decrease of BrdU incorporation in Lox-/- osteoblasts compared to wt.
Figure 5.
BrdU incorporation in primary Lox-/- and wt osteoblastic cells after 24 h and 48 h measured by ELISA at 405 nm. Data are presented as means ± SD of three experiments (n = 6 cultures/genotype). Asterisks indicate significant differences between Lox-/- and wt cells (p<0.05; unpaired student's t-test assuming equal variances).
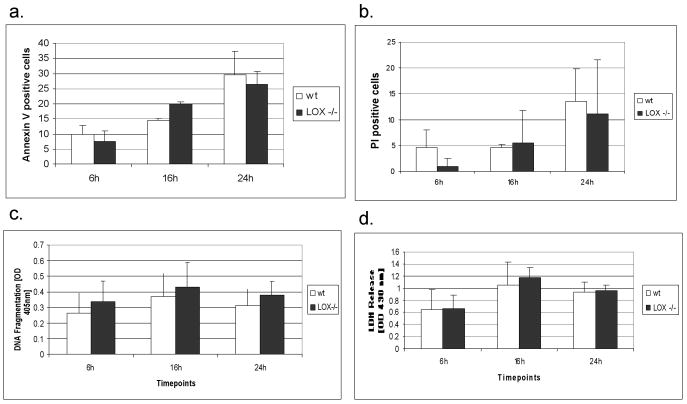
Apoptosis
Evaluation of cell apoptosis in Lox-/- compared to wt are given in Figure 6. At 6 h, 16 h and 24 h, no differences in annexin-V binding on outward-facing phosphatidylserine or PI binding (a,b) as well as in the detection of cytoplasmatic histone-associated DNA fragments were found (c). Also, in the presence of 1 μM staurosporine, as potent inducer of cell apoptosis, no differences in annexin-V binding and DNA fragmentation were noted between Lox-/- and wt osteoblasts (data not shown). To exclude non-specific annexin V binding and DNA fragmentation we measured LDH release in the supernatants, as a marker of cell necrosis (d). No differences were found between Lox-/- and wt osteoblasts at any time point studied.
Figure 6.
Annexin-V positive (a), propidium iodine (PI) positive (b) cell count in primary Lox-/- and wt osteoblastic cells after 6 h, 16 h and 24 h measured by fluorescence microscopy. Measurement of DNA fragmentation (c) in primary Lox-/- and wt osteoblastic cells after 6 h, 16 h and 24 h by ELISA at 405 nm. Spectrophotometric measurement of LDH release (d) in primary Lox-/- and wt osteoblastic cells after 6 h, 16 h and 24 h at 490 nm. Data are presented as means ± SD of three experiments (n = 3 cultures/genotype).
Analysis of mineral deposition
We next examined whether decreased cell proliferation in Lox-/- cells affects later stages of osteoblast differentiation such as mineral nodule deposition. Significantly (p < 0.05) less Alizarin Red stain was eluted from primary Lox-/- osteoblast cultures compared to wt, indicating less mineralized nodule formation in Lox-/- cells at day 14 as well as at day 21 (Table 1).
Table 1.
Means and standard deviations of optical density (OD) of 3 experiments (n = 3 cultures/genotype). Asterisks indicate significant differences between Lox-/- and wt cells (Alizarin Red eluat of differentiated osteoblasts; p < 0.05; unpaired student's t-test assuming equal variances).
| Genotype | Day 14 OD 562 nm [mean ± SD] |
Day 21 OD 562 nm [mean ± SD] |
|---|---|---|
| Wt | 1.156 ± 0.107 | 1.736 ± 0.134 |
| Lox-/- | 0.474 ± 0.066* | 0.765 ± 0.087* |
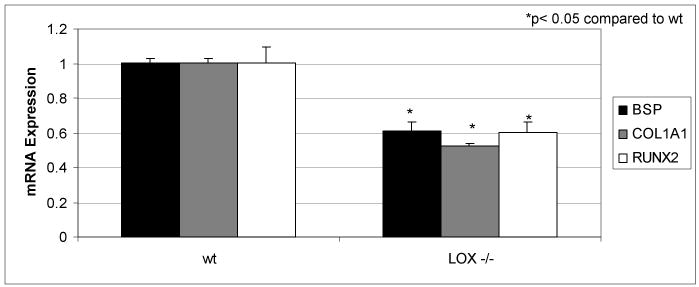
Analysis of osteoblast differentiation markers
Osteoblasts undergo different phases of differentiation [40]. In accordance with decreased mineralized nodule formation observed in Lox-/- osteoblasts, figure 7 shows a statistically significant (p < 0.05) decrease of COL1A1 as well as of BSP and Runx2/Cbfa1mRNA expression compared to wt.
Figure 7.
Real-time RT-PCR analysis of bone markers, COL1A1, bone sialoprotein (BSP) and Runx2/Cbfa1from Lox-/- and wt mice. Data are presented as mean ± SD obtained from three measurents of pooled RNA samples. Asterisks indicate significant differences between Lox-/- and wt cells (p<0.05; unpaired student's t-test assuming equal variances).
Discussion
Extracellular posttranslational modifications of fibrillar type I-III collagens by LOX are crucial for collagen cross-linkage and for the accumulation of a functional collagen matrix in bone tissue [3, 5, 7]. In the present study, besides normal morphology of calcified structures, significant changes in collagen fibril formation as well as altered osteoblast differentiation were found in Lox-/- compared to wt mice.
A significantly diminished truncated and non-functional LOX mRNA expression was demonstrated in Lox-/- samples. In other collagen producing cell types such as mouse fibroblasts and vascular smooth muscle cells isolated from Lox-/- mice, lysyl oxidation itself has been reduced approximately by 80 % [32] and the overall amount of immature collagen cross-links have been reported to be reduced by 40 % in Lox-/- embryos [31], thus indicating that LOX's role in cross-linking is essential and may be also be important in osteoblasts. Also, amine oxidase activities have been demonstrated for other LOX isoforms [14, 15], thus indicating that multiple LOX isoforms may be involved in cross-linkage. The present data show that LOX and LOXL1 are clearly the prominent forms at the RNA level in bone tissue. In contrary, LOXL2 and LOXL3 showed only minor expression, which may suggest that they are less relevant in bone metabolism. In MC3T3-E1 cells, it was shown that LOX, LOXL1 and 4 were regulated in the course of osteoblasts differentiation whereas constituent expression of LOXL3 and no expression of LOXL2 were found [41]. It has been suggested that LOX isoforms may partly compensate for the lack of LOX [30], which may have contributed to the fact that normal bone morphology was found in Lox-/- bone tissues by histological means. However, in the present study, in the absence of Lox, other genes including LOX isoforms, LOXL1-4, were significantly down-regulated as well, which may suggest regulatory interactions between different isoforms in bone tissue. Whether effects on osteoblast development seen in the present study are primarily due to Lox deficiency or to its isoforms, or a combination of both, is still unknown. Moreover, observed effects of Lox depletion on osteoblasts could also potentially depend on both changes in LOX enzyme activity, as well as on altered functions of LOX propeptide, as recently shown in other cell types [24, 27, 28].
The formation of covalent cross-links by enzymatic LOX activity are required for the formation of mature and insoluble collagen [3, 5, 7]. In the present study, significantly decreased collagen fiber diameters have been measured in Lox-/- tissues, indicating impaired collagen matrix accumulation. A functional collagenous bone matrix may be critical for cell-cell and cell-matrix interactions affecting cell differentiation. Previous studies suggest that LOX plays an important role in osteoblastic differentiation [21, 29, 42, 43]. The present study demonstrates for the first time that Lox gene deficiency affects the osteoblastic phenotype. DNA synthesis was significantly decreased in Lox-/- osteoblasts compared to wt cells. From our previous data, it is known that LOX can be localized in the intracellular space and LOX has been suggested to regulate gene transcription [21, 24, 25, 29]. LOX binds to intracellular cell components, in particular to the microtubular network [21], which is considered a prerequisite for development of a proper mitotic spindle apparatus and normal cell cycle progression [44]. Further studies will examine involved transcriptional control of observed effects of Lox depletion on cell devision. In addition, the balance of osteoblast proliferation and apoptosis determines the size of the osteoblast population [45]. In the present study, decreased cell proliferation was accompanied by normal regulation of apoptosis in Lox-/- osteoblasts, suggesting an overall decrease of the size of osteoblast population.
Osteoblasts pass through phenotypic changes with distinct patterns of gene expression as they differentiate [40]. The present data indicate that Lox gene does not only influence early but also later stages of osteoblast differentiation as well, as decreased mineral nodule formation in Lox-/- cultures was noted. In addition, to gain insights into molecular mechanisms associated with this inhibition, we determined the expression levels of osteoblast transcription factors and phenotypic markers of various stages of osteoblast differentiation. Our data show that early markers of osteoblast differentiation such as type I collagen as well as the marker of fully differentiated osteoblasts, BSP, were significantly down-regulated in bone tissue lacking Lox gene. Additionally, Runx2/cbfa1, a master transcription factor for the osteoblast lineage, which positively controls the expression of type I collagen and BSP [46], was significantly decreased. Altogether, inhibition of differentiation could be a consequence of inhibited cell proliferation. Besides, Lox depletion seems to directly affect osteoblast differentiation, as shown by down-regulation of the expression of osteoblastic differentiation markers, and thus can not completely be explained by diminished cell density.
Further studies are warranted to examine the interaction between enzymatic collagen cross-linkage and cellular effects as well as its effects on the formation of functional bone tissue. Based on the present data, we suspect that LOX promotes bone development through mechanisms in addition to its well established role in collagen cross-linking, and further studies are in progress to address novel mechanisms of action in normal developing osteoblast cultures.
Acknowledgments
We thank B. Danielowski and V. Kanitz for the technical support in the laboratory. This study has been supported by a grant DE140066 to P.T., grants 202469 from the Health Science Council and the S. Juselius Foundation to J. M., a research stipend from DFG GK-325-00 to P.M. and N.H. as well as by a short term research fellowship from EMBO, from COST, a habilitation stipend from the Charité - Universitätsmedizin Berlin and by a research grant from DFG PI 420/3 to N.P.
References
- 1.Boskey AL. Matrix protein and mineralization: An overview. Conn Tissue Res. 1996;35:375–363. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- 2.Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–2123. [Google Scholar]
- 3.Kagan H, Trackman P. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 4.Prockop D, Kivirikko K. Collagens: molecular biology, diseases, and potentials for therapy. Ann Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi M. Collagen: The major matrix molecule in mineralized tissues. In: JJB A, Garner S, editors. Calcium and phosphorus in health and disease. CRC Press; New York: 1996. pp. 127–141. [Google Scholar]
- 6.Knott L, Bailey A. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 7.Kagan H. Biology and Regulation of Extracellular Matrix: A Series. In: Mecham RP, editor. Regulation of Matrix Accumulation. Vol. 1. Orlando: Academic Press; 1986. pp. 321–398. [Google Scholar]
- 8.Mäki JM, Kivirikko KI. Cloning and characterization of a fourth human lysyl oxidase isoenzyme. J Biochem. 2001;355:381–387. doi: 10.1042/0264-6021:3550381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mäki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20:493–496. doi: 10.1016/s0945-053x(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon K, Modi WS, Contente S, Friedman RM. A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24-q25. J Biol Chem. 1993;268:18435–18437. [PubMed] [Google Scholar]
- 11.Ito H, Akiyama H, Iguchi H, Iyama K, Miyamoto M, Ohsawa K, et al. Molecular cloning and biological activity of a novel lysyl oxidase-related gene expressed in cartilage. J Biol Chem. 2001;276:24023–24029. doi: 10.1074/jbc.M100861200. [DOI] [PubMed] [Google Scholar]
- 12.Jourdan-Le Saux C, Le Saux O, Donlon T, Boyd CD, Csiszar K. The human lysyl oxidase-related gene (LOXL2) maps between markers D8S280 and D8S278 on chromosome 8p21.2-p21.3. Genomics. 1998;51:305–307. doi: 10.1006/geno.1998.5356. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Dai J, Tang R, Zhao W, Zhou Z, Wang W, et al. Cloning and characterization of a human lysyl oxidase-like 3 gene (hLOXL3) Matrix Biol. 2001;20:153–157. doi: 10.1016/s0945-053x(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 14.Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, et al. Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem. 2001;276:48944–48949. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Kim SS, Jung ST, Park JY, Yoon HW, Ko J, et al. Expression and purification of enzymatically active forms of the human lysyl oxidase-like protein 4. J Biol Chem. 2003;278:52071–52074. doi: 10.1074/jbc.M308856200. [DOI] [PubMed] [Google Scholar]
- 16.Knott L, Whitehead C, Fleming R, Bailey A. Biochemical changes in the collagenous matrix of osteoporotic avian bone. J Biochem. 1995;310:1045–1051. doi: 10.1042/bj3101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyre DR, Dickson IR, Van Ness K. Collagen crosslinking in human bone and articular cartilage: age-related changes in the content of mature hydroxypyridinium residues. J Biochem. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiger BJ, Steenbock H, Parsons HT. Lathyrism in the rat. J Nutri. 1933;6:427–442. doi: 10.1111/j.1753-4887.1976.tb05778.x. [DOI] [PubMed] [Google Scholar]
- 19.Shim H, Harris ZL. Genetic defects in copper metabolism. J Nutr. 2003;133:1527S–1531S. doi: 10.1093/jn/133.5.1527S. [DOI] [PubMed] [Google Scholar]
- 20.Uzel M, Scott I, Babakhanlou-Chase H, Palamakumbura A, Pappano W, Hong H, et al. Multiple bone morphogenic protein-1 related mammal metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276:22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Pischon N, Palamakumbura AH, Trackman P. Intracellular distribution of the lysyl oxidase propeptide in osteoblastic cells. Am J Physiol Cell Physiol. 2007;292:2095–2102. doi: 10.1152/ajpcell.00613.2006. [DOI] [PubMed] [Google Scholar]
- 22.Nellaiappan K, Risitano A, Liu G, Nicklas G, Kagan H. Fully processed lysyl oxidase catalyst translocates from the extracellular space into nuclei of aortic smooth-muscle cells. J Biol Chem. 2000;79:576–582. doi: 10.1002/1097-4644(20001215)79:4<576::aid-jcb60>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Nellaiappan K, Strassmaier T, Graham L, Thomas K, Kagan H. Localization and activity of lysyl oxidase within nuclei of fibrogenic cells. Proc Natl Acad Sci USA. 1997;94:12817–12822. doi: 10.1073/pnas.94.24.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palamakumbura A, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein G, et al. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–40600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 25.Jeay S, Pianetti S, Kagan H, S GE. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Mol Cell Biol. 2003;23:2251–2263. doi: 10.1128/MCB.23.7.2251-2263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giampuzzi M, Oleggini R, Di Donato A. Demonstration of in vitro interaction between tumor suppressor lysyl oxidase and histones H1 and H2: definition of the regions involved. Biochim et Biophys Acta. 2003;1647:245–251. doi: 10.1016/s1570-9639(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 27.Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, et al. The tumor supressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–1112. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- 28.Wu M, Min C, Wang X, Yu Z, Kirsch K, Trackman PC, et al. Repression of BCL2 by the tumor supressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 29.Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, et al. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- 30.Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 31.Hornstra I, Birge S, Starcher B, Bailey A, Mecham R, Shapiro S. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 32.Mäki J, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radlanski RJ, Renz H, Klarkowski MC. Prenatal development of the human mandible. 3D reconstructions, morphometry and bone remodelling pattern, sizes 12-117 mm. CRL Anat Embryol. 2003;207:221–232. doi: 10.1007/s00429-003-0343-4. [DOI] [PubMed] [Google Scholar]
- 34.Bellows CG, Aubin JE, H JNM, Antosz ME. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 35.Ecarot-Charrier B, Glorieux FH, Van der Rest M, Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol. 1983;96:639–643. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hippenstiel S, Schmeck B, N'Guessan PD, Seybold J, Krull M, Preissner K, et al. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L830–838. doi: 10.1152/ajplung.00467.2001. [DOI] [PubMed] [Google Scholar]
- 37.N'Guessan PD, Vigelahn M, Bachmann S, Zabel S, Opitz B, Schmeck B, et al. The UspA1 protein of Moraxella catarrhalis induces CEACAM-1-dependent apoptosis in alveolar epithelial cells. J Infect Dis. 2007;195:1651–1660. doi: 10.1086/514820. [DOI] [PubMed] [Google Scholar]
- 38.N'Guessan PD, Schmeck B, Ayim A, Hocke AC, Brell B, Hammerschmidt S, et al. Streptococcus pneumoniae R6x induced p38 MAPK and JNK-mediated caspase-dependent apoptosis in human endothelial cells. Thromb Haemost. 2005;94:295–303. doi: 10.1160/TH04-12-0822. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schnmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-ˆˆC(T) method. Methods Enzymol. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Stein G, Lian J, Stein J, Van Wijnen J, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996;76:593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- 41.Atsawasuwan P, Mochida Y, Parisuthiman D, Yamauchi M. Expression of lysyl oxidase isoforms in MC3T3-E1 osteoblastic cells. Biochemical and Biophysical Research Communications. 2005;327:1042–1046. doi: 10.1016/j.bbrc.2004.12.119. [DOI] [PubMed] [Google Scholar]
- 42.Pischon N, Darbois LM, Palamakumbura AH, Kessler E, Trackman PC. Regulation of collagen deposition and lysyl oxidase by tumor necrosis factor-alpha in osteoblasts. J Biol Chem. 2004;279:30060–30065. doi: 10.1074/jbc.M404208200. [DOI] [PubMed] [Google Scholar]
- 43.Turecek C, Fratzl-Zelman N, Rumpler M, Buchinger B, Spitzer S, Zoehrer R, et al. Collagen cross-linking influences osteoblastic differentiation. Calcif Tissue Int. 2008;82:392–400. doi: 10.1007/s00223-008-9136-3. [DOI] [PubMed] [Google Scholar]
- 44.Jordan MA. Mechanism of action of antitumor drugs that interacts with microtubules and tubulin. Curr Med Chem Anti- Cancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 45.Hock JM, Krishnan V, Onyia JE, Bidwell JP, Milas j, Stanislaus D. Osteoblast apoptosis and bone turnover. J Bone Mineral Res. 2001;16:975–984. doi: 10.1359/jbmr.2001.16.6.975. [DOI] [PubMed] [Google Scholar]
- 46.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]