Abstract
Chondroitin sulfate proteoglycans (CSPGs) are upregulated in the central nervous system following injury. Chondroitin sulfate glycosaminoglycan (CS GAG) side chains substituted on this family of molecules contribute to the limited functional recovery following injury by restricting axonal growth and synaptic plasticity. In the current study, the effects of degrading CS GAGs with Chondroitinase ABC (Ch’ase ABC) in the injured spinal cords of adult cats were assessed. Three groups were evaluated for 5 months following T10 hemisections: lesion–only, lesion + control, and lesion + Ch’ase ABC. Intraspinal control and Ch’ase ABC treatments to the lesion site began immediately after injury and continued every other day, for a total of 15 treatments, using an injectable port system. Delivery and in vivo cleavage were verified anatomically in a subset of cats across the treatment period. Recovery of skilled locomotion (ladder, peg, and beam) was significantly accelerated, on average, by >3wks in Ch’ase ABC-treated cats compared to controls. Ch’ase ABC-treated cats also showed greater recovery of specific skilled locomotor features including intralimb movement patterns and significantly greater paw placement onto pegs. Although recovery of basic locomotion (bipedal treadmill and overground) was not accelerated, intralimb movement patterns were more normal in the Ch’ase ABC-treated cats. Qualitative assessment of serotonergic-immunoreactivity also suggested that Ch’ase ABC treatment enhanced plasticity. Finally, analyses using fluorophore-assisted carbohydrate electrophoresis (FACE) indicate CS GAG content is similar in cat and human. These findings show, for the first time, that intraspinal cleavage of CS GAGs can enhance recovery of function following spinal cord injury in large animals with sophisticated motor behaviors and axonal growth requirements similar to those encountered in humans.
Keywords: chondroitin sulfate proteoglycans, chondroitin sulfate glycosaminoglycans, behavioral recovery, plasticity, hemisection
INTRODUCTION
The inability of damaged axons to regrow following spinal cord injury (SCI) leads to permanent impairments in motor and sensory function below the level of the lesion. An injury-induced increase in chondroitin sulfate proteoglycans (CSPGs) at the lesion site is at least one factor that contributes to the growth inhibitory nature of the injured spinal cord (Lemons et al., 1999;Jones et al., 2002;Jones et al., 2003;Tang et al., 2003; reviewed by Morgenstern et al., 2002;Silver and Miller, 2004;Busch and Silver, 2007). The common feature among this large, diverse family of molecules is the presence of chondroitin sulfate glycosaminoglycans (CS GAGs), which are side chains attached to the core proteins of CSPGs.
Substantial evidence suggests that removal of CS GAGs, most commonly achieved using Chondroitinase ABC (Ch’ase ABC), substantially reduces the inhibitory nature of CSPGs during development and in vitro (Snow et al., 1990;McKeon et al., 1995;Zuo et al., 1998;Chung et al., 2000). More recently, disruption of CS GAGs with Ch’ase ABC in vivo has been shown to enhance axonal growth and behavioral recovery (Yick et al., 2000;Moon et al., 2001;Bradbury et al., 2002;Chau et al., 2003;Yick et al., 2003;Caggiano et al., 2005;Barritt et al., 2006;Houle et al., 2006), as well as synaptic plasticity in rodents (Tropea et al., 2003). These promising results have led our lab to investigate whether disruption of CS GAGs can promote functional recovery following SCI in a larger, more complex mammalian species, the cat.
The extension of potential therapeutic effects in the rat to the cat represents an important translational step. Ch’ase ABC continues to be studied, in part, because some think it may be a potential avenue to pursue for clinical studies of human SCI. One of the recommended strategies for translating preclinical-therapeutic candidates from the laboratory to clinical testing includes testing the efficacy of the therapy in multiple species (Anderson et al., 2005;Blight and Tuszynski, 2006). In addition to the cat’s remarkable locomotor capacity and its importance as a model in providing the foundation for evolving locomotor rehabilitation strategies (Lovely et al., 1986;Hodgson et al., 1994;Behrman and Harkema, 2000;Wernig et al., 2000;deLeon et al., 2001;Behrman et al., 2006), the cat presents significant scale-up barriers that must be overcome to successfully treat the human central nervous system after injury. The translational impact of these findings is important due to several factors including the cat’s sophisticated motor system and its size which presents physical challenges that are more similar to those that will be encountered in the human. To determine the effects of disrupting CS GAGs on motor recovery, the locomotor performances of three groups of cats with T10 spinal hemisections were compared: lesion-only, lesion + control, and lesion + Ch’ase ABC. The hemisection model was chosen as it permits substantial recovery, and thus enables evaluation of interventions on more skilled behaviors (Helgren and Goldberger, 1993). The control and Ch’ase ABC treatments were delivered intraspinally at the level of the lesion using subcutaneous ports with sub-dural tubing. Control or treatment injections into the ports were made every other day for one month (for a total of 15 injections). General recovery trends, as well as specific features of locomotion, were evaluated across a variety of basic and skilled locomotor tasks for five to six months. Our findings while confirming the work reported in rat models of SCI, identify novel effects of Ch’ase ABC including the accelerated onset of some locomotor recovery, as well as enhanced recovery of intralimb angular kinematic patterns and accuracy of limb trajectories, in the cat. Further, these findings suggest that Ch’ase ABC is effective in altering the extracellular matrix of the spinal cord and enhancing recovery of function across species.
MATERIALS AND METHODS
Fresh frozen, normal, adult, human spinal cord specimens were obtained from the University of Maryland’s Brain and Tissue Bank for Developmental Disorders (Baltimore, MD). In compliance with HIPAA guidelines, a “Certificate of Research on Decedents” was acquired from the University of Florida Institutional Review Board and a “Report of Subcommittee on Human Studies” was acquired from the Malcom Randall VA Medical Center for the use of this tissue.
All animal procedures were conducted in accordance with the NIH guidelines for the care and use of experimental animals and were approved by both the Malcom Randall VA Medical Center’s and University of Florida’s Institutional Animal Care and Use Committees.
This study reports on the locomotor function and recovery of 9 cats. These cats were carefully chosen from a larger group based upon their post mortem lesion morphology. This was done so that the lesion size and location of tissue involvement was very similar in all cats and would reduce the potential for lesion differences that could confound interpretation of any treatment effects on recovery. These lesions were left hemisections that completely disrupted ipsilateral gray and white matter, along with a partial interruption of the contralateral dorsal column (Fig. 1B). In addition to basic histological evaluation of the lesions with cresyl violet and myelin stains, immunohistochemistry was performed. Five additional cats also were prepared for histology. One, with a comparable lesion and survival period to the nine described above, was processed for all histology and immunohistochemistry. The four others had shorter survival periods (24 hours, 2 weeks, and 4 weeks) and were assessed for in vivo cleavage of CS GAGs with Ch’ase ABC. Normal spinal cord tissue specimens from 2 other cats, as well as 4 rats and 4 humans were used for biochemical evaluation. This tissue was stored at −80°C until used for fluorophore-assisted carbohydrate electrophoresis (FACE) analyses.
Figure 1.
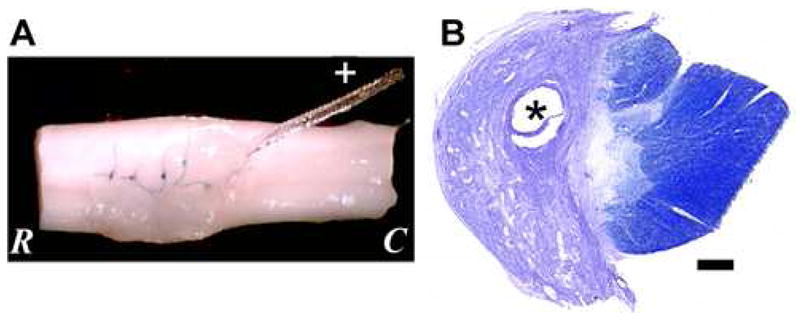
Lesion with Port Tubing. (A) Excised spinal cord from the level of the lesion. Sutures hold the dura closed, and port tubing (+) can be seen entering the lesion cavity. (B) A horizontal section at the lesion epicenter stained with cresyl violet and myelin shows a typical lesion in which the white and gray matter are disrupted on one side. The tissue is oriented with the dorsal aspect up. Scale bar: 500 μm. * indicates the hole where the port tubing was located. R: rostral, C: caudal.
All cats were purpose bred, SPF, adult, females. Cats were spayed to remove the potential for hormone-related effects on injury magnitude and prevent interruptions in behavioral training or data collection due to the postural changes associated with estrus (Sribnick et al., 2005; reviewed by Sribnick et al., 2003). Cats were trained on a daily basis and conditioned pre-injury to perform a variety of simple and skilled locomotor behaviors consistently for food rewards. Pre-injury (normal) data were recorded and used to establish performance baselines. Cats then received low thoracic spinal hemisections (~T10) and were placed into one of three groups: lesion–only (n=3), lesion + control (n=3; saline (1) and deactivated Ch’ase ABC (2)), or lesion + Ch’ase ABC (n=3) treatment. Animals placed into treatment groups had ports implanted subcutaneously and received Ch’ase ABC or control treatment into the lesion site every other day for 1 mo., for a total of 15 treatments. Post-injury, daily training resumed within days and locomotor characteristics were evaluated qualitatively and quantitatively at multiple time points for at least 5–6 mo. At the end of the behavioral studies cats were sacrificed by transcardial perfusion to assess lesion morphology and axonal growth using histological and immunohistochemical techniques, respectively.
Surgical Procedures
Spinal cord hemisection and micro-implantable port placement
Animals received Penicillin G, procaine (40,000 U/kg BW, IM) the day before, the day of, and the day after surgery. Prior to anesthesia, subcutaneous injections of atropine sulfate (0.04–0.06 mg/kg) and acetylpromazine (0.4–0.5 mg/kg) were given to control salivation and sedate the cat. Initial anesthesia was inhaled in a gaseous chamber supplying a 2–5% isoflurane and oxygen mixture. Animals were then intubated, and a surgical plane of anesthesia maintained with isoflurane (typically 2–3%). Throughout surgical procedures, heating pads were kept under animals to maintain body temperature. Temperature, blood pressure, EKG, respiration, and expired CO2 were continuously monitored and maintained within normal physiological limits; and IV fluids (Lactated Ringers, 10 ml/kg/h) were given throughout the duration of the procedures.
The spinal cord was exposed by removal of the laminae, and a left lateral hemisection (Fig. 1B) made using iridectomy scissors at ~spinal T10. Gentle suction with a pulled pipette was used to lift any fibers adhering to the dura to facilitate cutting without compromising the integrity of the dura. Immediately following hemisection, cats that received Ch’ase ABC or control treatment had gelfoam (Pharmacia & Upjohn, Inc., Peapack, NJ) soaked in either protease-free Ch’ase ABC (1 U/200 μl saline, Seikagaku Corp., Tokyo, Japan) or control solution (heat inactivated Ch’ase ABC or saline-only) placed into the lesion cavity. This gelfoam was left in the cavity for ~30 min, as the micro-implantable infusion ports (Harvard Apparatus, Holliston, MA) were put in place, and removed prior to suturing of the dura. The port body was placed within the subcutaneous space ~2½ cm lateral to the lumbar vertebral column. It was adhered to the back muscle with vetbond (Webster Veterinary Supply, Inc., Sterling, MA) and sutures. This placement permitted subcutaneous injections of Ch’ase ABC (or control) into the port. The port tubing was sutured to muscle and its distal tip placed within the lesion cavity. To maintain the position of the tubing, it was adhered to the adjacent, caudal lamina with vetbond. In addition, the dura was sutured (8-0 Prolene) so that the tubing tip was trapped under the dura at the level of the lesion cavity (Fig. 1A). The port reservoir and tubing were flushed and filled with the appropriate treatment prior to suturing of the dura. Ports were not placed into lesion-only animals, however, following hemisection the dura still was sutured. All animals had durafilm (Codman-Shurtleff, Inc., Randolph, MA) placed over the sutured dura and tucked under the cut edges of the vertebra to decrease scar tissue attachment to the dura. Gelfoam was placed on top of the durafilm, and muscle and skin were sutured in layers using 2-0 and 3-0 absorbable suture (Dexon), respectively. Anesthesia was terminated, and cats were extubated and placed in humidity and temperature controlled chambers to recover. Buprenorphine (0.01 mg/kg, SQ) was administered and given every 6–12 h (0.005–0.01 mg/kg, SQ) for ~48 h post-surgery.
Post-operative care
Procedures used to maintain the general health of the spinal cord injured cats are similar to those described in our previous studies (Howland et al., 1995a;Howland et al., 1995b). Animals were housed singly or in pairs in cages on thick beds (~12.5–20 cm) of shredded newspaper or several layers of egg crate foam post-injury. The absorbent and non-resistive nature of these beddings prevented skin breakdown, peripheral nerve compression, and pressure sores. For the first few days following injury, bladders were emptied at least three times daily using gentle manual pressure applied externally to the bladder through the abdominal wall. The health status including food intake, skin integrity, and body weight of all animals was monitored closely throughout the entire study.
Treatment administration
Control treatments were either saline-only or Ch’ase ABC that had been heat inactivated at 100°C for 15 min (referred to as “deactivated Ch’ase”). Before in vivo delivery, the presence of enzymatic activity for Ch’ase ABC and absence of enzymatic activity for control treatments was verified using one of two techniques: immunohistochemistry or fluorophore-assisted carbohydrate electrophoresis (FACE). Because the signals detected by both techniques rely on enzymatic cleavage of CS GAGs, the presence and absence of C6SPG immunoreactivity or lyase bands was used to confirm enzyme activity and inactivity, respectively.
Because Ch’ase ABC does not remain stable at body temperature for extended periods of time (Tester et al., 2007), port reservoirs were filled with a silicone substance (RTV Silicone Adhesive, NuSil Silicone Technology, Carpinteria, CA) to reduce reservoir volumes to ~25 μl. This reduction in volume guaranteed enzyme would be delivered and turned over often enough to remain active and ensure continual degradation of CS GAGs. Fifty microliters of sterile saline, deactivated Ch’ase ABC (0.25 U in 50 μl of saline), or protease-free Ch’ase ABC (0.25 U in 50 μl of saline) were injected every other day for 1 mo. The only two exceptions included the animals surviving 24 hours or 2 weeks that received treatment at the time of injury or every other day until sacrifice, respectively. The concentration delivered was the same as used in Bradbury et al. (2002). However, because the cat’s spinal cord is more than four times the size of the rat’s, the volume delivered was increased by a multiple of four. Cats were lightly anesthetized by inhalation of a 1–3% isoflurane and 1 L O2 mixture while the 50 μl were slowly injected over a 5 min period using a syringe pump (Harvard Apparatus, Holliston, MA).
Behavioral Training and Assessments
General
Prior to injury, animals were conditioned to perform bipedal treadmill locomotion (0.5 m/s) and a variety of voluntary overground tasks including traversing wide and narrow overground runways, a horizontal ladder, and a pegboard for food rewards. Following injury, training resumed within 2–3 days on the treadmill and basic overground runway. If needed, trainer assistance was initially given. Other tasks were re-integrated as soon as weight support and postural control permitted. Animals that could not independently perform the more skilled tasks were assisted in performing them until independence was achieved.
Animals were evaluated qualitatively on a daily basis; and periodically their performances were filmed for quantitative assessment of the left hindlimb, which is the limb most greatly affected by the lesion. On training days, food was provided only in the behavior room. On non-training days (weekends) animals were fed ad lib in their cages.
Locomotor tasks and quantitative assessments
Bipedal locomotion
Bipedal locomotion was typically performed on a motor driven treadmill 5 times/week at 0.5 m/s. The exception occurred immediately following injury when slower speeds were used. Animals were trained to stand with their forelimbs on a stationary platform while their hindlimbs stepped in response to the moving treadmill belt. A pureed food reward was continually provided in a food bowl suspended at the front end of the treadmill.
Overground locomotion
Cats were trained, at least 3 times/week, to traverse a 30.5 cm wide × 4.5 m long horizontal (basic overground) runway at a constant speed. Food rewards were given at each end of the runway. The choice speed for each cat was based upon the speed that appeared most comfortable in the fast walk/slow trot range for each cat. These speeds varied some between cats based upon individual disparities including physical size. Post-injury speeds chosen for quantitative analyses were closely matched to pre-injury speeds.
Ladder, pegboard, and narrow beam crossings
Cats were trained at least 2 times/week to cross a 30.5 cm × 4.5 m horizontal ladder with rungs (~2.5 cm wide spaced 15 cm apart), a 4.5 m horizontal pegboard with alternating pegs (pegs with 3.8 cm × 3.8 cm square surfaces spaced 15 cm and 20 cm apart with regards to width and length, respectively, along the runway), and a 5 cm × 4.5 m (narrow) beam. These runways were used to evaluate control of precise hindimb placement. Crossing times and speeds were calculated and the most common speeds chosen within and across time points. These speeds were invariably the speeds at which the animal performed the best. Within the chosen speeds, left hindlimb accuracy was evaluated on the ladder and pegboard, and classified into one of two categories--a hit onto the ladder rung/peg or a miss. To be categorized as a hit, the hindlimb had to be placed effectively and maintained without slipping off. On very rare occasions, an animal’s trajectory resulted in some initial contact immediately followed by loss of contact (brushed or slipped off the runway). In these extremely unusual instances, the steps were categorized as misses. If a cat never recovered the ability to cross one of these skilled runways, its recovery onset for that task was considered 150 days (the end of the post-injury behavioral analyses).
3-D angular kinematics
Left hindlimb performance on the treadmill, basic overground, and narrow beam were evaluated using 3-D angular kinematic analyses. Reflective spheres (8 mm) were placed on the shaved skin overlying 4 hindlimb bony landmarks (iliac crest, greater trochanter, lateral malleolus, and the base of the fifth metatarsal). A fifth reflective marker was placed overlying the fibula just above the lateral malleolus. This marker defined a unit vector along the shaft of the fibula, which in combination with the length of the fibula, permitted the automatic calculation and identification of the knee joint using Motus software (Vicon Peak, Englewood, CO). Using the Peak Performance Analysis System (Vicon Peak, Englewood, CO), joint angles for the left hip, knee, and ankle were calculated throughout the step cycle for ten steps for each form of locomotion at each time point reported. Only steps from bouts consisting of 7 or more contiguous step cycles performed with the cat’s head consistently at the food bowl were assessed during locomotion on the treadmill. During both treadmill and basic overground runway locomotion, the first and last steps of each bout or crossing sequence were excluded from analyses to remove acceleration and deceleration effects.
Lesion Morphology: Histological and Immunohistochemical Techniques
Perfusions
Between 5 and 7 mo post-injury, cats were deeply anesthetized with an overdose of sodium pentobarbital (>40 mg/kg, IP) and then injected intravenously with 1 cc of heparin (1000 U) followed 20 min later by an intravenous injection of 1% sodium nitrite (1 cc). Immediately after injection of sodium nitrite, cats were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Spinal cords were removed, blocked, and cryoprotected in 30% sucrose in 4% paraformaldehyde. Tissue was frozen and sectioned serially at 25 μm on a cryostat. One out of every ten sections was stained with cresyl violet (cresyl violet with acetate, Sigma-Aldrich, St. Louis, MO) and myelin stains (Eriochrome Cyanine R; Fluka, New York, NY) to examine basic lesion morphology. Some of the remaining sections were placed in buffer and processed for immunohistochemistry.
Basic histology: cresyl violet and myelin staining
Sections were mounted onto chrom alum and poly-L-lysine coated slides (chromium potassium sulfate and poly-L-lysine, Sigma-Aldrich, St. Louis, MO; gelatin, Fisher Scientific, Hampton, NH) and fully dried. Tissue was then rinsed in distilled water, dehydrated through increasing alcohol concentrations, and placed into xylene before being rehydrated through decreasing alcohol concentrations into distilled water. Sections were then stained for myelin (0.16% Eriochrome Cyanine R, 0.36% FeCl, 0.9% HCl, and 0.4% H2SO4) for 10 min, washed thoroughly, differentiated in 1% ammonium hydroxide for 1 min, and washed again. Sections next were stained with 0.5% cresyl violet acetate, rinsed with 70% ethanol, differentiated in 1% glacial acetic acid in 95% ethanol, dehydrated in 95% and 100% ethanol, and placed into xylene and coverslipped using DPX (Fluka).
Immunohistochemistry
Immunohistochemical staining was conducted for four reasons: 1) to verify the enzymatic activity of Ch’ase ABC and inactivity of heated Ch’ase ABC prior to use as an active or control treatment, respectively, in cats, 2) to verify Ch’ase delivery and CS cleavage in vivo after Ch’ase ABC treatment, 3) to identify CSPG expression in areas of the lesion 5+ mo after injury, and 4) to assess a descending supraspinal system that may show anatomical plasticity. Sections were processed using the monoclonal C6SPG (1:1000, MP Biomedicals, Irvine, CA) and polyclonal anti-serotonin (1:20,000, Diasorin, Stillwater, MN) antibodies. The C6SPG antibody labels the sugar stubs left on the core protein of chondroitin sulfate proteoglycan (CSPG) following enzymatic digestion with Ch’ase ABC (Seikagaku Corp., Tokyo, Japan). Briefly, the procedure was as follows: the tissue sections were first rinsed with Tris-buffered saline (TBS, pH 7.2) and then incubated with Ch’ase ABC (1 U/2 ml TBS containing 3% fresh NaCl, pH 8.0) at 37°C for 3 h. Following this 3 h incubation, the tissue was rinsed with 1% serum in TBS containing 0.4% triton (1% S-TBS-T, pH 7.2), blocked with TBS-T containing 10% serum for 30 min. at room temperature, and incubated with the primary antibodies overnight at 4°C in 1% S-TBS-T. The next day, the tissue sections were rinsed well with 1% S-TBS-T before and after an 1 h incubation with Alexa Fluoro 488 (1:400, Molecular Probes, Eugene, OR) or 594 (1:1000, Molecular Probes) secondaries and Hoescht (1:2000, Sigma-Aldrich). The sections were then rinsed with 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) and then 0.1 M PB (pH 7.4). Sections used to examine in vivo cleavage by Ch’ase ABC were processed without the addition of Ch’ase ABC during the immunohistochemical procedure. Positive and negative controls were processed simultaneously, and sections were coverslipped using the ProLong Anti-Fade Kit (Molecular Probes).
Density Measurements
Densities of serotonergic positive-axons were calculated for the lesioned (left) side of the spinal cord from tissue cross sections. Multiple sections were stained for serotonin and qualitatively assessed. Density measurements were calculated from the section with the greatest amount of staining in each animal within the lesion. These sections were taken from ~2000 μm into the rostral aspect of each lesion. Using the KS400 Zeiss image analysis system (version 3.0, Germany), the left side of the spinal cord (lesion) was outlined using the dorsal columns, central canal, ventral medial funiculi, and contralateral gray matter as landmarks to identify midline. The amount of immunoreactivity within the outlined area then was quantified.
Fluorophore-Assisted Carbohydrate Electrophoresis
Fluorophore-assisted carbohydrate electrophoresis (FACE) was used to 1) determine the variability in CS GAG composition from spinal cord tissue across several species and 2) confirm enzymatic activity of Ch’ase ABC and heat inactivation of deactivated Ch’ase ABC prior to use in cats. FACE methods were modified from those previously published (Gilbert et al., 2005;Tester et al., 2007). To evaluate CS GAG composition in the uninjured spinal cord across species, unfixed tissue samples were digested with 1 ml proteinase K (Invitrogen, Carlsbad, CA, 1 mg/1 ml 0.1 M ammonium acetate)/20 mg tissue (wet weight) at 60°C, overnight. The following day, samples were heated at 90°C for 10 min. and centrifuged to pellet debris. A double ethanol precipitation was then performed by adding 1 ml of −20°C absolute ethanol, 2 times to the original, 500 μl supernatant of samples and letting samples sit overnight at −20°C each time. After each precipitation, samples were centrifuged and the supernatant was removed. Once both precipitations had been performed, the remaining pellet was evaporated to completion. For those samples used to verify activity and inactivity of Ch’ase ABC, spinal cord tissue was not used. Instead, 5 μg of purified CS from bovine trachea (1 mg/ml dH2O) were evaporated to completion. Next, 100 μl 100 mM ammonium acetate and 1 μl of Ch’ase ABC (2 U/200 μl dH2O) was added to all samples, and samples were incubated at 37°C for 6 hr. After digestion with Ch’ase ABC, samples were again dried under vacuum and then incubated overnight (~16 hr) at 37°C with 10 μl sodium cyanide borohydride (2.5 mg/40 μl dH2O, Sigma, St. Louis, MO) and 10 μl of the fluorescent tag, 2-aminoacridone, hydrochloride (Molecular Probes, 25 mg/8.1 ml 3:17 acetic acid:DMSO). On the fifth, and final, day 40 μl of 25% glycerol was added to stop the reaction, and samples were loaded and separated on 30% polyacrylamide gels at 250 V for 3–4 h using a tris borate running buffer. Gels were imaged using a Biorad Gel Doc and the Quantity One imaging and analysis system. Band intensities from the different lyase products directly relate to the amount of various CS GAGs present in spinal cord tissue and allow us to compare the CS GAGs across species. Additionally, the presence or absence of bands, or lyase products, from CS samples digested with Ch’ase ABC or deactivated Ch’ase ABC confirms enzyme activity and inactivity, respectively.
Statistical analyses
Using SPSS software (Chicago, IL), one-way, between-subjects ANOVAs were conducted to determine if onset of recovery differed between the three groups for basic and skilled locomotion. Using an α level of .05, post-hoc t-tests were conducted to isolate more precisely any differences between the groups. Mixed (time × treatment) two-factor ANOVAs also were conducted to determine the effects of time and Ch’ase ABC treatment on specific features of locomotion used to assess behavioral recovery. Bonferroni-corrected post-hoc t-tests were conducted to isolate differences revealed by the ANOVAs. For time, post-hoc comparisons were made between 1) pre-injury and 20+ weeks and 2) 2 weeks and 20+ weeks. These comparisons were chosen to determine whether 1) features of locomotion at the most chronic time point were significantly different from normal and 2) the amount of recovery occurring between acute and chronic time points post-injury was significantly different. In addition, post-hoc comparisons were done to compare all treatment groups and determine whether Ch’ase ABC treatment had a significantly different effect than control treatments.
Using an α level of .05, t-tests were conducted to determine if serotonergic staining in the lesion differed between the control and Ch’ase ABC groups.
RESULTS
CS GAG Similarity Between Human And Cat
Chondroitinase ABC was used to cleave CS GAGs in spinal cord tissue samples from non-injured rat, cat, and human. The lyase products resulting from cleavage were assessed using FACE (Fig. 3). The Δ4S band density was similar in all three species; however, the Δ6S band was notably weaker in the rat compared to the cat and human. Although the Δ0S band could be seen in samples from all species, it was present in the smallest quantities (not shown). Thus, the cat and human are more similar to each other, in that they have two predominant disaccharides (Δ4S and Δ6S), compared to the rat in which Δ4S disaccharide is dominant.
Figure 3.

CS GAGs in Human, Cat, and Rat Spinal Cords. CS GAG disaccharide standards, Δ6S and Δ4S (lane 1), and purified bovine tracheal CS (lane 5) were used as controls. In human (lane 2) and cat (lane 3) spinal cord tissue, two predominant lyases, Δ6S and Δ4S, are apparent. A single dominant lyase, Δ4S, is present in the rat (lane 4).
Ch’ase ABC Cleavage In Vivo
To verify that Ch’ABC delivered in vivo was able to penetrate spinal cord tissue and cleave CS GAGs, tissue sections throughout the lesion/delivery site and adjacent segments were assessed using an antibody that attaches only after cleavage. Assessment of tissue sections after a single treatment, as well as after 2 weeks and 4 weeks of treatment showed that Ch’ase ABC was effectively delivered and capable of cleaving CS GAGs in the cat. Staining was seen at the lesion edges 24 hours after a single exposure to Ch’ase ABC at the time of injury. The area of staining was greatly expanded at 2 weeks, such that it was apparent around the lesion and in adjacent gray and white matter. Staining was primarily isolated to the lesion side and extended at least 2 spinal segments (the maximum distance assessed). A similar staining distribution was seen after 4 weeks of treatment (Fig. 2). Comparison with an animal not treated with Ch’ase ABC showed that staining was correlated with the delivery of Ch’ase ABC.
Figure 2.
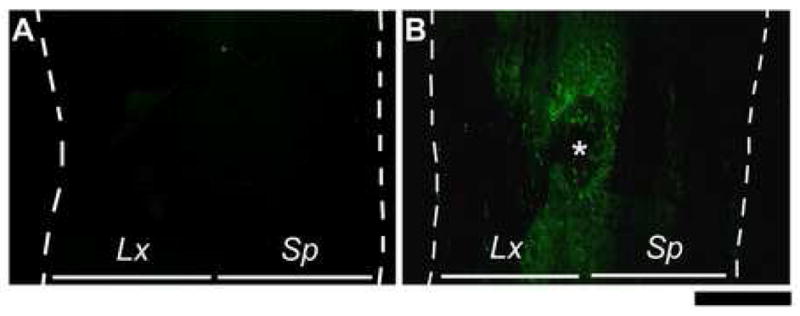
Chondroitinase ABC Cleavage In Vivo. Longitudinal (horizontal) tissue sections were incubated with the C6SPG antibody. (A) Tissue section from an animal that received no treatment shows no antibody binding. (B) Tissue section from an animal treated with Ch’ase ABC for 4 weeks in vivo shows C6SPG-immunoreactivity, indicating effective delivery of Ch’ase ABC and its cleavage of CS GAGs. Scale bar: 1000 μm. Dashed lines indicate tissue edges. * indicates site of port tubing in treated animal. Lx: lesioned-side, Sp: spared-side.
Rate of Behavioral Recovery
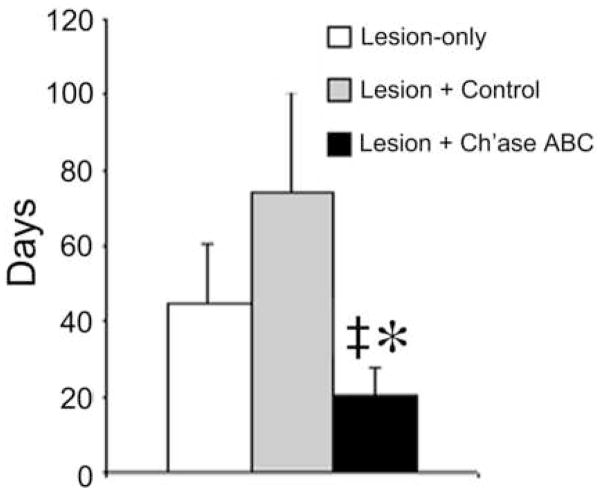
General health was not compromised for any of the cats following spinal cord injury. Food intake, skin integrity, and body weight all were maintained and normal for the duration of the study. The primary effect of the lesion was on the control of the hindlimb ipsilateral to the lesion (the left hindlimb). Behavioral data assessing recovery (rate and amount) is reported for three experimental conditions: lesion-only (no port placement, n=3), lesion + control (port placement with control treatment; saline, n=1 and deactivated Ch’ase ABC diluted in saline, n=2); and lesion + Ch’ase ABC (port placement with Ch’ase ABC diluted in saline, n=3). Behavioral recovery of the control animals treated with saline or deactivated Ch’ase ABC was similar for all three animals; and therefore, the data for these animals was combined and is presented as lesion + control data. For animals in all groups, the left hindlimb was quickly re-integrated during bipedal treadmill and basic overground locomotion. Although some cats began to use the left hindlimb within days of injury, all cats had actively integrated the left hindlimb during both tasks by the second week post-injury. The ability to produce primarily plantar steps (versus stepping on the dorsum of the paw) during both tasks occurred during the second and third weeks of recovery. These results are consistent with other reports of recovery following hemisection (Helgren and Goldberger, 1993;Basso et al., 1994;Kuhtz-Buschbeck et al., 1996), and no differences in the average onset of recovery for these two tasks or plantar stepping were seen between the three groups of cats. Recovery of skilled locomotor tasks (ladder, pegboard, and narrow beam crossings) requiring greater balance and limb accuracy occurred more slowly than either bipedal or basic overground locomotion, and did not begin until at least the second week post-injury. The ability to cross the ladder occurred the earliest, followed by crossing of the narrow beam and pegboard. The average onset of recovery was significantly accelerated in the cats treated with Ch’ase ABC compared to cats with lesions-only (t(15) = 2.407, p =.029) and cats receiving control treatment (t(15)=2.506, p = .024) (Fig. 4). Moreover, all cats treated with Ch’ase ABC regained the ability to accomplish all three skilled tasks while some control cats did not. One lesion + control animal never regained the ability to traverse the narrow beam, while two lesion + control animals never regained the ability to independently traverse the pegboard. Finally, while all Ch’ase ABC treated cats integrated their left hindlimbs during these tasks, some control cats crossed these runways using only three limbs (i.e. without using the left hindlimb). These findings suggest that Ch’ase ABC accelerates and enhances recovery. To determine if Ch’ase ABC treatment had additional effects, we also assessed specific features of locomotion (below).
Figure 4.
Recovery Onset for Skilled Locomotor Tasks. The recovery onset of skilled locomotor behaviors was significantly earlier for Ch’ase ABC-treated animals, compared to lesion-only (*) and lesion + control treatment (‡) groups. Error bars denote SEM.
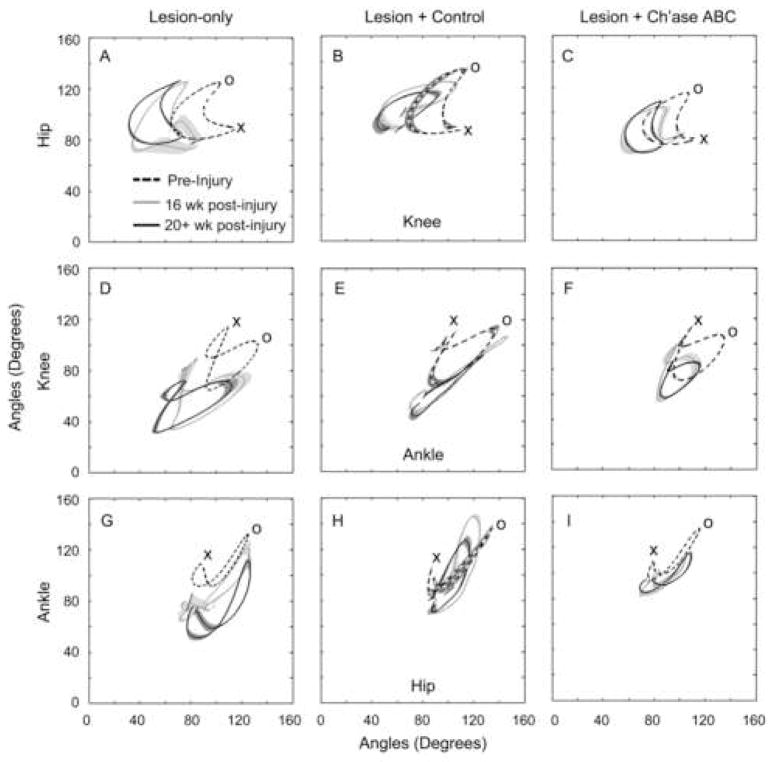
Intralimb Movement Patterns
Intralimb movement patterns were evaluated by monitoring the hip, knee, and ankle joint angles used throughout the step cycle during bipedal treadmill locomotion, as well as on crossings of the basic overground runway and narrow beam. These data are presented as plotted forms in which two joint angles are graphed against each other (see inset in Fig. 5). For these analyses, it is critical to compare pre- and post-injury performance within cats as each cat, like humans, may have unique movement patterns. The most common post-injury change seen was a general increase in knee flexion on all three locomotor tasks in both control groups (Fig. 5 and Fig. 6). The Ch’ase ABC group, however, typically did not show this abnormal increase in flexion during bipedal treadmill or basic overground locomotion. Examples of these kinematic differences between pre- and post-injury time points and groups are shown for the hip and knee in Fig. 5. The intralimb movement patterns of Ch’ase ABC treated cats at 16 weeks post-injury, when recovery has plateaued, are very similar to pre-injury patterns, which is in contrast to the control groups. This similarity can be appreciated as an overlap of the plotted forms for pre-injury (dashed) and 16 weeks post-injury (gray) joint angles (Fig. 5C,F). During crossing of the narrow beam, increases in both knee and ankle flexion were observed in all groups (Fig. 6). On this skilled task, intralimb dynamics did not recover to the same degree as on the basic forms of locomotion. However, the performance of the Ch’ase ABC treated cats is superior in several ways. As indicated above, all Ch’ase ABC treated cats recovered the ability to use their left limb while independently traversing the narrow beam, while some controls did not. The controls that did not use their limb (example shown in Fig. 6B,E,H) showed a general flexion-extension cycling pattern without a stance phase or the characteristic points of transition between stance and swing. Although controls that used their left hindlimbs showed a more normal limb dynamic (Fig. 6A,D,H), the range of their general limb movements were larger than those seen pre-injury and in the Ch’ase ABC treated group (Fig. 6C,F,I), suggesting that they had less limb control overall.
Figure 5.
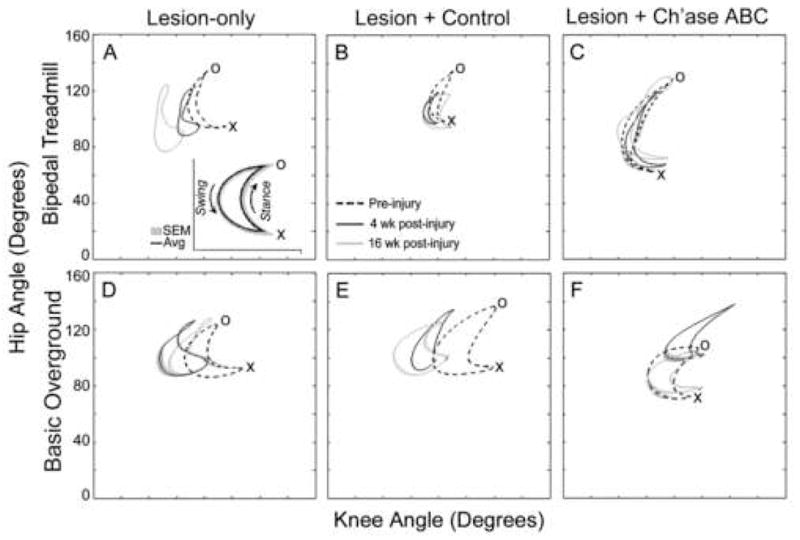
Angle-Angle Graphs – Treadmill and Basic Overground Locomotion. Using 3-D angular kinematic data, one joint angle (i.e. hip, y-axis) is graphed against another joint angle (i.e. knee, x-axis) over the course of the step cycle (inset) to permit rapid comparison of movement at two joints across time. An averaged graph is shown for a representative cat from each group for bipedal treadmill (A,B,C) and basic overground (D,E,F) locomotion. These graphs show the averages of ten left hindlimb step cycles for each cat pre-injury (dashed), 4 weeks post-injury (black), and 16 weeks post-injury (gray). In animals treated with Ch’ase ABC, intralimb movement patterns at 16 weeks post-injury (gray) were very similar to those seen pre-injury (dashed) during both bipedal treadmill (C) and basic overground (F). X denotes the transition from swing to stance and O denotes the transition from stance to swing. Decreasing values for the knee (movement of forms towards the y-axis) or hip (movement of forms toward the x-axis) indicate increasing flexion. Increasing values (movement of forms away from the y- or x-axis) reflect increasing extension for the knee or hip joint, respectively. Shading denotes SEM.
Figure 6.
Angle-Angle Graphs – Narrow Beam. Averages of left hindlimb step cycles for pre-injury (dashed), 16 weeks post-injury (gray), and 20+ weeks post-injury black) for the highest functioning animal in each treatment group are shown. The Ch’ase ABC treated animal had intralimb movement patterns at 16 and 20+ weeks post-injury that were more similar to pre-injury (C,F,I) compared to the lesion-only (A,D,G) or lesion + control (B,E,H) animals. X denotes the transition from swing to stance and O denotes the transition from stance to swing for pre-injury curves. See the Figure 4 legend for interpretation of increasing and decreasing values. Shading denotes SEM.
Hindlimb Accuracy
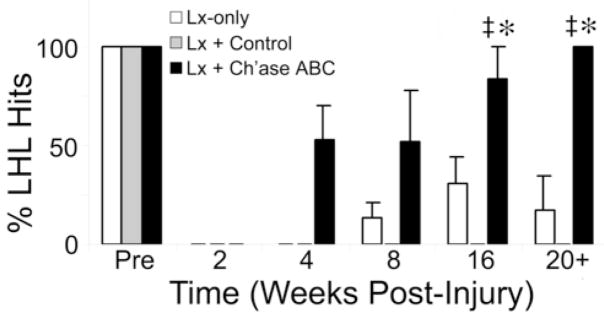
Left hindlimb control was further evaluated by assessing hindlimb placement onto ladder rungs and pegs during crossings on the horizontal ladder and pegboard. Although the mean number of step cycles in which the left hindlimb was placed and maintained on the ladder rung was greater at all time points, except 8 weeks, for the Ch’ase ABC treated group compared to controls, the mixed-factor did not reveal these treatment differences to be significant. The time factor, however, was significant (F(1.73,10.37) = 20.94, p = 0.000 ε = 0.346, Greenhouse-Geisser correction (ε) was applied for violation of the assumptions of sphericity for comparison involving 2 or more degrees of freedom). Post-hoc comparisons isolated the differences between the 2 week and 20+ week time points, suggesting a significant amount of recovery occurred over time, independent of treatment. In contrast, performance on the pegboard (Fig. 7) was significantly different across both time (F(2.75,16.51) = 42.48, p = 0.000, ε = 0.550 and treatment (F(2,6) = 23.41, p = 0.001), Greenhouse-Geisser correction (ε) was applied for violation of the assumptions of sphericity for comparison involving 2 or more degrees of freedom). Differences in the average performance of all cats collectively, across time, were statistically significant. Pair wise comparisons detected significance between pre-injury and 20+ weeks (p = 0.001) and 2 and 20+ weeks (p = 0.008). This suggests that although hindlimb placement improves significantly over time after injury, the average collective performance post-injury is still significantly different from pre-injury performance. Pairwise comparisons for treatment effects detected significant improvement in hindlimb accuracy for Ch’ase ABC treated animals in comparison to control animals (control treatment, p = 0.002 and lesion-only, p = 0.007), with no statistical difference found between control groups. By 4 weeks post-injury, cats in the Ch’ase ABC treated group were placing and maintaining their left hindlimbs on the pegs 50% of the time. This is a period when no cats in the control groups could even cross the pegboard (Fig. 7). The performance of Ch’ase ABC treated animals continued to improve so that by 20+ weeks after injury, hindlimb placement onto pegs occurred 100% of the time compared to an average of <25% by the best control group. The superior performance of the Ch’ase ABC group was accomplished using a strategy not typically seen prior to injury. Normal cats typically keep their left limbs on the pegs on the left side of the board and their right limbs on the right pegs. In contrast, the Ch’ase ABC treated cats consistently placed their left hindlimbs on the right pegs. Moreover, they placed their left hindlimb on the right peg just before or immediately after the right hindlimb vacated the peg. In addition, it is important to note that animals treated with Ch’ase ABC crossed both the ladder and peg board at speeds equivalent to or faster than animals in control groups (data not shown). Thus, slowed (more cautious) crossing speeds did not account for this group’s ability to place and maintain the left hindlimb on a peg.
Figure 7.
Hindlimb Precision on the Pegboard. The percentages of left hindlimb hits were determined from each animal’s 3 best crossings and averaged within treatment groups. Animals treated with Ch’ase ABC recovered more quickly and were significantly better at placing their hindlimbs onto pegs than the lesion-only (*) and lesion + control treatment (‡) groups. Error bars denote SEM.
Lesion Characterization
Serial sections throughout the lesion from each cat 20+ weeks post-injury were stained using cresyl violet and myelin stains to determine the cross sectional extent of each animal’s lesion. All animals included in the study showed comparable disruption and preservation of the gray and white matter at the lesion epicenter (Fig. 1B). To further characterize the lesion area, antibodies against CSPGs and serotonin were used.
Regardless of treatment group, the scar area was positive for CS GAGs in all cats at the terminal time point (20+ weeks, Fig. 8A,B). In comparison to the robust immunoreactivity on the injured side, the spared side of each spinal cord showed a relatively normal distribution of CS GAGs (not shown). This includes perineuronal staining throughout the gray matter, as well as the typical honeycomb staining throughout the white matter (Pindzola et al., 1993;Davies et al., 1999;Lemons et al., 1999). To assess if any digested CS GAGs remained from the in vivo applications of Ch’ase ABC 4 months earlier, the levels of C6SPG immunoreactivity were compared in sections with and without incubation in Ch’ase ABC in vitro. Attachment of the C6SPG antibody requires cleavage of the CS GAGs with Ch’ase ABC. Without incubation in vitro, no immunoreactivity was seen indicating that all CSPGs with CS GAGs digested by in vivo Ch’ase ABC delivery during the first post-injury month had been removed.
Figure 8.
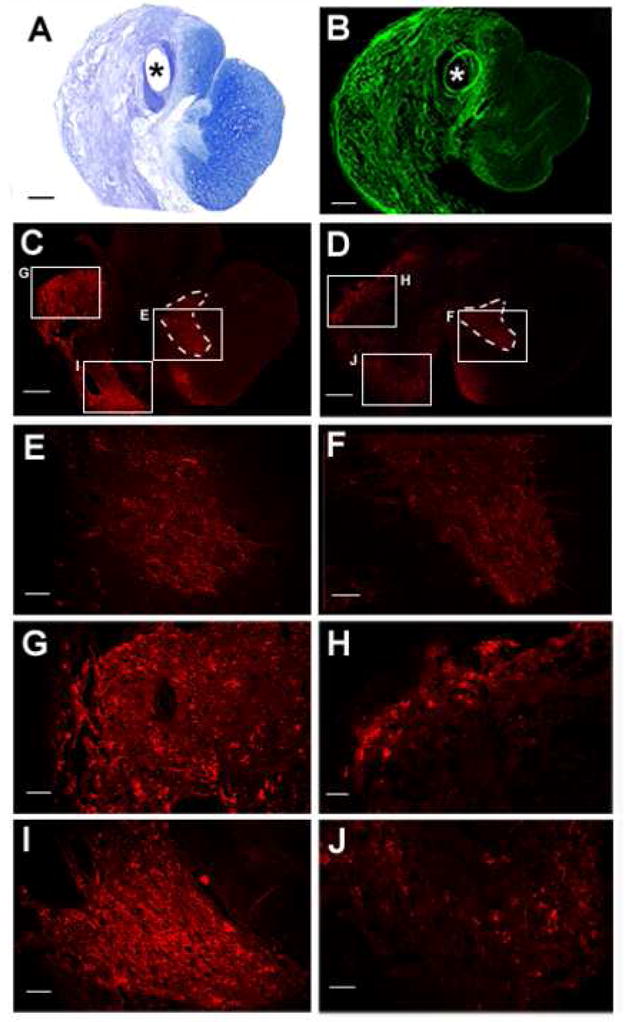
Increases in Serotonergic-Immunoreactivity with Ch’ase ABC Treatment. Tissue cross sections stained with cresyl violet and myelin (A) and the C6SPG antibody (B) depict areas of scarring 20+ weeks post-injury. Serotonergic positive-axons are seen in the rostral part of the lesion (C–J). Tissue sections from Ch’ase ABC-treated (C, magnifications in E,G,I) and control (D, magnifications in F,H,J) animals that showed the greatest amount of serotonergic immunostaining are shown. Serotonin is typically abundant within the gray matter of the cat spinal cord, as seen in the ventral horn (E and F) on the uninjured side of both animals. On the injured side of the spinal cord serotonergic positive axons in CSPG positive areas of the scar can easily be seen in animals from each group at higher magnifications of the lesion in areas previously occupied by the dorsal lateral (G,H) and ventral medial (I,J) white matter. The axonal profiles were considerably larger and denser in the Ch’ase ABC-treated cat (G,I) compared to the control cat (H,J). Sections in A, B, C, and D are oriented the same with the dorsal aspect up. Scale bar for A–D: 500 μm and scale bar for E–J: 100 μm. * indicates the hole where the port tubing was located. Dashed lines outline intact gray matter. Lx: lesioned-side, Sp: spared-side.
Axonal growth was assessed in the lesion areas using an antibody against serotonin. Serotonergic-immunoreactive axons were seen within the lesion areas in animals from all groups (Fig. 8). Some axons were seen in regions of the scar in locations that would have previously been occupied by white matter that is not normally populated by serotonergic axons. These locations suggest this axonal growth was new. While serotonergic-immunoreactivity in the rostral lesion area was seen to some degree in all animals, immunoreactive profiles qualitatively appeared more numerous in animals treated with Ch’ase ABC. Limited quantitative assessment of fiber densities (~2000 microns into the rostral lesion) showed a trend (p=0.08) towards a significant difference between controls (lesion-only and lesion + control) and Ch’ase ABC treated animals (Fig. 8).
DISCUSSION
The current study shows that degradation of CS GAGs after spinal cord injury in the cat can enhance behavioral recovery and promote axonal growth. Although all groups of cats recovered substantial locomotor function, the performance of the cats treated with intraspinal Ch’ase ABC was superior in multiple ways. During both bipedal treadmill and basic overground locomotion, the kinematic patterns of the Ch’ase ABC treated cats were more similar than either control group to those seen prior to injury. Although the ability to perform bipedal treadmill and basic overground locomotion recovered within a similar time frame for all groups, recovery on skilled forms of locomotion was significantly accelerated for the cats treated with Ch’ase ABC compared to the control groups. In addition, Ch’ase ABC treated cats integrated their left hindimbs during these skilled locomotor tasks and placed their left hindlimbs on the pegboard significantly more than either control group. These significant differences across groups with a small “n” underscore the robustness of Ch’ase ABC effects. In addition to the novelty of the specific behavioral recovery, this is the first demonstration that Ch’ase ABC can enhance recovery following spinal cord injury in a species other than the rodent.
Translational Impact
The cat offers a variety of benefits as a translational model. The larger size of the cat spinal cord compared to a rodent’s spinal cord presents physical challenges that are more similar to those that will be encountered in human spinal cord injury. These include the greater distance requirements for axonal regeneration and/or sprouting to effectively contribute to functional recovery as well as similar volume and area treatment considerations.
Data presented in this paper suggest that the CS GAGs in the cat spinal cord are more similar to the human than the rat (Fig. 3), underscoring the importance of the cat as a translational model for determining the effects of Ch’ase ABC for use in the human. The commercially available Ch’ase ABC, used in this study and others, is produced by Proteus vulgaris and contains varying mixtures of the endolytic (ABC I) and exolytic (ABC II) lyases (Hamai et al., 1997). Although both isoforms act on the same substrates (Derby and Pintar, 1978;Hamai et al., 1997;Calabro et al., 2000;Plaas et al., 2001;Calabro et al., 2001), they act on them at significantly different rates (Hamai et al., 1997). There are no mammalian chondroitinase equivalents (Ryan et al., 1994), and therefore special care must be taken in matching the activities of the chondroitinase(s) used with the CS substrates in a particular species in order to enhance the therapeutic effects for that species and determine the translational impact. It also is important to note that although no chondroitinases have been identified in the human genome, several hyaluronidase-like enzymes bearing chondroitinase-like properties have been discovered (Csoka et al., 2001). Thus, depending upon the mechanism by which Ch’ase ABC works in vivo to produce desired behavioral and/or anatomical outcomes, these hyaluronidase-like enzymes may provide one future alternative to chondroitinases of bacterial origin.
Timing of Treatment and Recovery
For the first month post-injury, Ch’ase ABC was delivered every other day using a subcutaneous injectable port system. Based upon our recent findings (Tester et al., 2007), as well as those of others (Curinga et al., 2007) showing that Ch’ase ABC thermostability is short-lived at body temperature, the choice and design of this system were important to ensure delivery of active enzyme. The effects of Ch’ase ABC in this study were determined using a battery of locomotor tasks of increasing complexity to test the function of different levels of the neural axis post-injury. This battery allowed collection of data that showed dramatic differences in the rate of recovery resulting from endogenous repair (seen in controls) versus Ch’ase ABC intervention. Although Ch’ase ABC treatment did not substantially alter the onset of recovery for basic forms of locomotion, which occurred during the first week following injury, it significantly accelerated recovery of the skilled forms of locomotion during the first month post-injury. These skilled forms of locomotion recovered much later, or not at all, in lesion-only and control treated cats. The pairing of this accelerated and consistent recovery within the period of Ch’ase ABC delivery suggests that the enzyme had some relatively immediate effects. The continued improvement in recovery past the treatment window, however suggests that Ch’ase ABC promoted prolonged plasticity. Whether the same degree of short- and long-term recovery could be obtained using a shorter treatment period remains to be determined.
Pathways Involved in Ch’ase ABC-Mediated Recovery
Coordinated hindlimb walking on a treadmill can be accomplished using a combination of intraspinal networks in the lumbar spinal cord, peripheral afferents, and motoneurons with no supraspinal input (Grillner and Zangger, 1987;Guiliani and Smith, 1987;Howland et al., 1995). Although basic voluntary forms of locomotion require supraspinal input to intraspinal networks (known as spinal pattern generators for locomotion) to initiate stepping, it is the skilled tasks requiring greater balance and control of limb trajectory that demand the greatest supraspinal contributions. Work in the cat has clearly shown that both the cortico- and rubro-spinal tracts are recruited during visually guided tasks requiring voluntary gait modifications (Widajewicz et al., 1994;Armstrong and Marple-Horvat, 1996;Drew et al., 1996;Drew et al., 2002). This suggests that the greater recovery of accurate limb placement on the pegboard by cats treated with Ch’ase ABC is likely to be mediated directly or indirectly by one or both of these two pathways. The unique strategy used by Ch’ase ABC treated cats in placing their left hindlimbs onto the pegs, however, suggests alternative circuitry also is involved. Recent work by other laboratories has documented that several forms of alternative circuitry can occur following SCI (Bareyre et al., 2004;Blagoveshchenskii et al., 2005), opening the possibility that Ch’ase ABC may affect novel or spared pathways. Studies to assess these specific pathways have been initiated.
Locomotor Training Effects
All of the cats in these experiments were extensively trained throughout the study. Thus, the effects of Ch’ase ABC treatment were combined with a “physical therapy” treatment. This was done to maximize the potential for cats to perform well and identify the effects of treatment without some of the typical confounds associated with non-use of the limbs in studies of spinal cord injury including varying degrees of muscle weakness, joint integrity, changes in range of motion, reluctance to use an affected limb, and motivational issues. This training paradigm also mimics what is likely to occur in the most progressive clinical settings based upon currently ongoing clinical trails evaluating the use of treadmill and overground locomotor training (Hodgson et al., 1994;Behrman and Harkema, 2000;Wernig et al., 2000;Behrman et al.; 2006;Dobkin et al., 2006). These current clinical studies are based upon fundamental studies in the cat showing that training and the type of training can profoundly impact recovery of locomotion (Lovely et al., 1986;Barbeau and Rossignol, 1987;de Leon et al., 1999). In addition to the physical conditioning and practice that occurs with training, studies also have shown that extensive exercise stimulates expression of a variety of growth factors and receptors, thereby likely enhancing plasticity within the spinal cord (Gomez-Pinilla et al., 2002;Skup et al., 2002;Ying et al., 2003;Hutchinson et al., 2004;Ying et al., 2006). Although it is doubtful, it is not known if the same functional recovery would have been achieved with Ch’ase ABC application-only. However, it is clear that training-alone was inferior to the effects of training plus Ch’ase ABC treatment. It will be important in future studies to begin to understand the potential interactions between these two potential therapies and if the timing of either or both is critical.
Conclusions
The cat provides barriers that aptly challenge many of the demands set forth by human spinal cord injury. The data from this study demonstrate that administration of Ch’ase ABC in spinal cord injured cats may enhance axonal growth and significantly improve locomotor recovery. Most impressive in this model, is the increased rate and extent at which the ipsilateral hindlimb is effectively integrated by Ch’ase ABC treated cats during skilled behaviors requiring balance and accuracy. These improvements in recovery of function suggest that Ch’ase ABC effects changes which result in robust and prolonged plasticity. They also indicate that Ch’ase ABC, or some other intervention through which CS GAGs can be disrupted, is likely to play important roles in our understanding of neural repair and the future treatment of spinal cord injury.
Acknowledgments
This research was supported by NIH NINDS RO1 NS050699-01 and T32 HD043730, the Daniel Heumann Fund, the Department of Veteran’s Affairs, and the State of Florida Brain and Spinal Cord Injury Rehabilitation Trust Fund. Human tissue specimens were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland (Baltimore, MD). We thank a variety of students and technicians including Stephanie Jefferson, Adele Blum, Sarah Sumner, David Kirby, Eric Neeley, Jimmy Lapnawan, Matt Gardner, Othel O’Steen, and Wilbur O’Steen who assisted with data collection. In addition, we thank Drs. Anna Plaas and Ryan Gilbert, as well as Dr. Brian Howland for their invaluable input with the FACE technique and statistical analyses, respectively. Finally, we would like to thank Dr. Todd White for his assistance with quantifying fiber density.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DK, Beattie M, Blesch A, Bresnahan J, Bunge M, Dietrich D, Dietz V, Dobkin B, Fawcett J, Fehlings M, Fischer I, Grossman R, Guest J, Hagg T, Hall ED, Houle J, Kleitman N, McDonald J, Murray M, Privat A, Reier P, Steeves J, Steward O, Tetzlaff W, Tuszynski MH, Waxman SG, Whittemore S, Wolpaw J, Young W, Zheng B. Recommended guidelines for studies of human subjects with spinal cord injury. Spinal Cord. 2005;43:453–458. doi: 10.1038/sj.sc.3101746. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Marple-Horvat DE. Role of the cerebellum and motor cortex in the regulation of visually controlled locomotion. Can J Physiol Pharmacol. 1996;4:443–455. [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davie M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase abc promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Murray M, Goldberger ME. Differential recovery of bipedal and overground locomotion following complete spinal cord hemisection in cats. Restorative Neurology and Neuroscience. 1994;7:95–110. doi: 10.3233/RNN-1994-7205. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Behrman A, Harkema S. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskii ED, Pettersson LG, Perfil’ev SN. Control of fine movements mediated by propriospinal neurons. Neurosci and Behav Physiol. 2005;35:299–304. [PubMed] [Google Scholar]
- Blight AR, Tuszynski MH. Clinical trials in spinal cord injury. J Neurotrauma. 2006;23:586–593. doi: 10.1089/neu.2006.23.586. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Cafferty WBJ, Yang S, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI Improves Locomotion and Bladder Function following Contusion Injury of the Rat Spinal Cord. J Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. doi: 10.1093/glycob/10.3.283. [DOI] [PubMed] [Google Scholar]
- Calabro A, Midura R, Wang A, West L, Plaas A, Hascall VC. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthritis Cartilage. 2001;9(Suppl A):S16–S22. doi: 10.1053/joca.2001.0439. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Reinnervation of the mammalian spinal cord after neonatal dorsal root crush. J Neurocytol. 1988;17:335–350. doi: 10.1007/BF01187856. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Dalsgaard CJ, Molander C. Regrowth of lesioned dorsal root nerve fibers into the spinal cord of neonatal rats. Neurosci Lett. 1987;74:14–18. doi: 10.1016/0304-3940(87)90043-7. [DOI] [PubMed] [Google Scholar]
- Chau CH, Shum DKY, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2003;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Chung KY, Taylor JS, Shum DK, Chan SO. Axon routing at the optic chiasm after enzymatic removal of chondroitin sulfate in mouse embryos. Development. 2000;127:2673–2683. doi: 10.1242/dev.127.12.2673. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Curinga GM, Snow DM, Mashburn C, Kohler K, Thobaben R, Caggiano AO, Smith GM. Mammalian-produced chondroitinase AC mitigates axon inhibition by chondroitin sulfate proteoglycans. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04530.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;14:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J Neurophysiol. 1999;81:85–94. doi: 10.1152/jn.1999.81.1.85. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Roy RR, Edgerton VR. Is the recovery of stepping following spinal cord injury mediated by modifying existing neural pathways or by generating new pathways? A perspective. Phys Ther. 2001;81:1904–1911. [PubMed] [Google Scholar]
- Derby MA, Pintar JE. The histochemical specificity of Streptomyces hyaluronidase and chondroitinase ABC. Histochem J. 1978;10:529–547. doi: 10.1007/BF01003135. [DOI] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Kably B, Lavoie S. Role of the motor cortex in the control of visually triggered gait modifications. Can J Physiol Pharmacol. 1996;74:426–442. [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during lcomotion in the cat. Brain Res Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, Scott M. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;4:484–93. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol (Lond) 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pearson KG. Restoring walking after spinal cord injury. Prog Neurobiol. 2004;73:107–126. doi: 10.1016/j.pneurobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central genration of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Guiliani CA, Smith JL. Stepping behaviors in chronic spinal cats with one hindlimb deafferented. J Neurosci. 1987;7:2537–2546. [PMC free article] [PubMed] [Google Scholar]
- Hall ED. Neuroprotective actions of glucocorticoid and nonglucocorticoid steroids in acute neuronal injury. Cell Mol Neurobiol. 1993;13:415–432. doi: 10.1007/BF00711581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamai A, Hashimoto N, Mochizuki H, Kato F, Makiguchi Y, Horie K, Suzuki S. Two distinct chondroitin sulfate ABC lyases. An endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J Biol Chem. 1997;272:9123–9130. doi: 10.1074/jbc.272.14.9123. [DOI] [PubMed] [Google Scholar]
- Harkema S. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Helgren ME, Goldberger ME. The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways. Exp Neurol. 1993;123:17–34. doi: 10.1006/exnr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Med Sci Sports Exerc. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptors and the neuromodulatory control of spinal cord function. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. CRC Press; Boca Raton: 2001. pp. 47–87. [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Tessler A, Goldberger ME. Development of locomotor behavior in the spinal kitten. Exp Neurol. 1995a;135:108–122. doi: 10.1006/exnr.1995.1071. [DOI] [PubMed] [Google Scholar]
- Howland DR, Bregman BS, Tessler A, Goldberger ME. Transplants enhance locomotion in neonatal kittens whose spinal cords are transected: a behavioral and anatomical study. Exp Neurol. 1995b;135:123–145. doi: 10.1006/exnr.1995.1072. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Inman DM, Steward O. Ascending sensory, but not other long-tract axons, regenerate into the connective tissue matrix that forms at the site of a spinal cord injury in mice. J Comp Neurol. 2003;462:431–449. doi: 10.1002/cne.10768. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Matsuda M, Watanabe E, Maeda N, Oohira A. Two types of brain chondroitin sulfate proteoglycan: their distribution and possible functions in the rat embryo. Neurosci Res. 1998;31:273–282. doi: 10.1016/s0168-0102(98)00047-9. [DOI] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Boczek-Funcke A, Mautes A, Nacimiento W, Weinhardt C. Recovery of locomotion after spinal cord hemisection: an X-ray study of the cat hindlimb. Exp Neurol. 1996;137:212–224. doi: 10.1006/exnr.1996.0020. [DOI] [PubMed] [Google Scholar]
- Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1187–1189. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Plaas AH, West L, Midura RJ, Hascall VC. Disaccharide composition of hyaluronan and chondroitin/dermatan sulfate. Analysis with fluorophore-assisted carbohydrate electrophoresis. Methods Mol Biol. 2001;171:117–128. doi: 10.1385/1-59259-209-0:117. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Khandke KM, Tilley BC, Lotvin JA. Cloning and expression of the chondroitinase I and II genes from Proteus vulgaris. WO 94/25567 Pat. 1994
- Skup M, Dwornik A, Macias M, Sulejczak D, Wiater M, Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Snow DM, Letourneau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG) J Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann NY Acad Sci. 2003;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. [DOI] [PubMed] [Google Scholar]
- Tang W, Davies JM, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase abc’s ability to cleave CS GAGs. J Neurosci Res. 2007;85:1110–1118. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J Neurosci. 2003;23:7034–7044. doi: 10.1523/JNEUROSCI.23-18-07034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Nanassy A, Muller S. Laufband (LB) therapy in spinal cord lesioned persons. Prog Brain Res. 2000;128:89–97. doi: 10.1016/S0079-6123(00)28009-6. [DOI] [PubMed] [Google Scholar]
- Widajewicz W, Kably B, Drew T. Motor cortical activity during voluntary gait modifications in the cat. II Cells related to the hindlimbs. J Neurophysiol. 1994;72:2070–2089. doi: 10.1152/jn.1994.72.5.2070. [DOI] [PubMed] [Google Scholar]
- Wilson MT, Snow DM. Chondroitin sulfate proteoglycan expression pattern in hippocampal development: potential regulation of axon tract formation. J Comp Neurol. 2000;424:532–546. doi: 10.1002/1096-9861(20000828)424:3<532::aid-cne10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Yick LW, Cheung PT, So KF, Wu W. Axonal regeneration of Clarke’s neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol. 2003;182:160–168. doi: 10.1016/s0014-4886(02)00052-3. [DOI] [PubMed] [Google Scholar]
- Yick LW, Wu W, So KF, Yip HK, Shum DK. Chondroitinase ABC promotes axonal regeneration of Clarke’s neurons after spinal cord injury. Neuroreport. 2000;11:1063–1067. doi: 10.1097/00001756-200004070-00032. [DOI] [PubMed] [Google Scholar]
- Yick LW, So KF, Cheung PT, Wu WT. Lithium chloride reinforces the regeneration-promoting effect of chondroitinase ABC on rubrospinal neurons after spinal cord injury. J Neurotrauma. 2004;21:932–943. doi: 10.1089/neu.2004.21.932. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2006;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Young W. Methylprednisolone treatment of acute spinal cord injury: an introduction. J Neurotrauma Suppl. 1991;1:S43–46. [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite- promoting potential of spinal cord tissue. Exp Neurol. 1998;154:654–662. doi: 10.1006/exnr.1998.6951. [DOI] [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]