Abstract
Persons with aphasia vary greatly with regard to clinical profile; yet, they all share one common feature—anomia—an impairment in naming common objects. Previous research has demonstrated that particular naming errors are associated with specific left hemisphere lesions. However, we know very little about the cortical activity in the preserved brain areas that is associated with aphasic speech errors. Utilizing functional magnetic resonance imaging (fMRI), we show for the first time that specific speech errors are associated with common cortical activity in different types and severities of aphasia. Specifically, productions of phonemic errors recruited the left posterior perilesional occipital and temporal lobe areas. A similar pattern of activity was associated with semantic errors, albeit in the right hemisphere. This study does not discount variability in cortical activity following left hemisphere stroke; rather, it highlights commonalities in brain modulation in a population of patients with a common diagnosis but vastly different clinical profiles. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: stroke, anomia, speech errors, paraphasia, recovery, right hemisphere, neuroimaging, fMRI
INTRODUCTION
“One group of symptoms is not a direct result of damage to a part of the brain. It is the expression of the struggle of the changed organism to cope with the defect, and to meet the demands of a milieu with which it is no longer equipped to deal.”
—Goldstein, 1942, p 69.
Although substantial evidence suggests that aphasic deficits directly reflect the neuroanatomical damage to the language system, it is important to consider that aphasic errors may also reflect the system's attempt to compensate for its damaged components. Goldstein [ 1942] was among the first to advocate that disordered language behaviors were not merely a reflection of neurological damage, but the “struggle of the organism with the defect.” This “struggle” is reflective of the fact that neurological damage does not occur to a static system, but rather to a dynamic one that is plastic, active, and capable of reorganization. Thus, language processing in aphasia is not simply a reflection of a damaged language system, but also is a manifestation of neurocognitive compensation. Therefore, the study of aphasia not only provides evidence to clarify the brain‐language relationship in normal persons but also provides a window into understanding how the brain attempts to restore a damaged cognitive system.
In most cases of aphasia, an individual's language profile will change over time following stroke, and chronic deficits often differ greatly from the acute profile. Certainly, much of the initial change in the acute aphasic profile results from neurophysiological factors, such as the reduction of edema [Fazzini et al., 1986; Pizzamiglio et al., 2001], resolution of diaschisis [Seitz et al., 1999], reperfusion of ischemic tissue [Fridriksson et al., 2002; Hillis et al., 2006b], and clearing of glutamate [Kwakkel et al., 2004]. However, neural reorganization also plays an important role in the recovery of language functions, especially in the later phases of stroke. Treatment‐induced language improvement and recovery in the subacute and chronic stages theoretically relies on the premise of reorganization of the structure/function relationships and the establishment of new pathways [Hillis and Heidler, 2002]. Because a portion of the language network has been destroyed, the remaining language areas and other nonlinguistic areas must function in the absence of the damaged area. Cortical reorganization in language recovery allows cortical compensation for some of the functions lost and may include recruitment of areas that were not previously involved in language or redistribution of functions within the remaining language areas.
A handful of studies have failed to reveal a relationship between cortical activity and language recovery in aphasia, although others have found either positive or negative correlations in specific cortical areas of interest [Cardebat et al., 2003; de Boissezon et al., 2005; Heiss et al., 1999]. Several possible explanations may account for the discrepancies in these findings. First, it is possible that findings may be influenced greatly by the time post‐stroke at which the study occurs. In a longitudinal study of persons with aphasia, Saur et al. [ 2006] found a dynamic pattern of reorganization that occurred in three stages as patients moved from the acute to chronic phase. In the acute phase, cortical activation in the left hemisphere language areas was greatly reduced. In the subacute phase, a regression analysis revealed that improved language function was associated with increased activity in the right hemisphere homologues of Broca's area and the supplementary motor area. However, in the chronic phase, further language improvements were associated with an increase or “reshift” of cortical activity to perilesional left hemisphere areas. Therefore, the increased right hemisphere activity associated with language improvement shortly after stroke did not persist into the chronic phase. In fact, there is some evidence to suggest that right hemisphere recruitment of Broca's homologue may reflect transcollosal disinhibition that may not reflect recovery [Abo et al., 2004; Xu et al., 2004] and possibly may be maladaptive for long‐term recovery [Blank et al., 2003; Fernandez et al., 2004; Naeser et al., 2004; Price and Crinion, 2005; Rosen et al., 2000]. However, this notion was challenged by Raboyeau et al. [ 2008], who found increased activity in the right posterior inferior frontal lobe and the right insula associated with successful language treatment outcome in a group of 10 persons with aphasia. That is, greater activity in the frontal right hemisphere was predictive of treatment success, suggesting that the right homologue of Broca's area is important for aphasia recovery.
Lesion analyses following stroke have proven helpful in understanding general brain‐language relationships. However, they yield little insight into productive or counterproductive neural compensations that occur in chronic aphasia. Lesion analyses can reveal what is damaged, but not what is occurring in the intact regions that remain and dynamically adapt to the damage within the system. Thus, to understand how the brain is actively attempting to compensate for affected language areas, it is crucial to look at the functional changes in cortical activation in the presence of a damaged system.
The purpose of this study was to investigate common brain activity associated with correct picture naming as well as naming errors in a group of aphasic patients. Just as correct naming tends to recruit relatively consistent brain areas across normal participants, there may also be commonalities in neural recruitment associated with naming errors in aphasia. Although aphasic patients vary considerably based on various factors, such as lesion location and extent, it is possible that their errors are rooted in the same functional anatomy. Therefore, this research sought to answer whether patients with different types and severities of aphasia recruit similar cortical areas when they produce semantic or phonemic paraphasias. For comparison, brain activation associated with correct naming in aphasia was contrasted with data from 10 normal control participants. In addition, the relationship between right hemisphere activity and naming ability was explored. Namely, the intensity of neural activity in distinct cortical areas recruited for correct naming was measured and correlated with the number of correct naming attempts by each participant.
MATERIALS AND METHODS
Participants
Eleven persons (six males) with chronic stroke‐induced aphasia were included in this study (Table I). The mean age was 58.8 years (SD = 14.7) with a range of 45 years. All participants were at least 10 months postonset, and all but one (P4) were retired at the time of the study. To explore commonalities in brain activation associated with naming in aphasia, persons with a wide range of aphasia severity were tested. Aphasia assessment employing the Western Aphasia Battery [WAB; Kertesz, 1982] revealed a spectrum of language impairment—five participants presented with nonfluent aphasia and six presented with fluent aphasia. Fluency ratings on the WAB (maximum score possible is 10) ranged from 2 by P1 and P3 (single words, often paraphasias, effortful, and hesitant) to 9 by P11 (mostly complete sentences with some word finding difficulty). Similarly, the composite auditory comprehension score on the WAB (maximum score possible is 10) showed a wide range. The lowest auditory comprehension score (5.85) was received by P2. In contrast, the highest auditory comprehension score (9.95) was received by P11, who demonstrated very mild aphasia characterized by only intermittent word finding difficulty. In addition to the WAB, all participants were administered the Boston Naming Test to further characterize their anomia [BNT; Kaplan et al., 1983]. Although all participants had incurred a stroke in the left middle cerebral artery circulation, the size and site of damage varied among the participants. Lesion size varied from a minimum of 3.04 cc (P11) to a maximum of 342.2 cc (P2) (Table I). The greatest overlap among lesion locations included the posterior portion of Brodmann's areas (BA) 22 and 42, where six of the 11 participants had damage (see Fig. 1).
Table I.
Biographical information and testing results for each participant
| P | Biographical information | Test results | Lesion description | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Postonseta | Occupationb | WAB: Fluencyc | WAB: Aud. compc | WAB: AQd | BNTe | Size (cc) | Lesion location | |
| 1 | F | 33 | 17 | Accountant | 2 | 6.90 | 31.8 Broca's | 0 | 34.95 | White matter damage deep to BA 6 and 44 and the anterior and middle insula. The arcuate fasciculus is completely severed and the lateral portion of the putamen is involved. |
| 2 | M | 63 | 101 | Minister | 4 | 5.85 | 47.1 Broca's | 3 | 342.2 | Entire MCA distribution and portions of the anterior medial frontal lobe with basal ganglia involvement. |
| 3 | M | 78 | 47 | Surveyor | 2 | 8.10 | 47.6 TMA | 2 | 23.48 | Posterior middle and superior temporal lobe including BA 37, 22, and 42. Basal ganglia and thalamus involved. |
| 4 | F | 43 | 49 | House cleaner | 4 | 8.05 | 50.7 Broca's | 13 | 56.76 | Complete destruction of Broca's area (BA 44 and 45) and middle and inferior portions of BA 6; involvement of middle and inferior parietal lobe (BA 40) and superior temporal lobe (BA 22 and 42); BA 4 is intact. |
| 5 | M | 58 | 43 | Teacher | 4 | 9.70 | 71.6 Broca's | 8 | 87.42 | Complete destruction of BA 44, 45, anterior portion of BA 38 including the middle and anterior insula; BA 1, 2, 3, and 4 are intact. |
| 6 | F | 41 | 39 | Factory worker | 6 | 8.00 | 74.4 Anomic | 38 | 145.9 | Mostly posterior damage including the middle and superior temporal lobe (BA 22 and 42) as well as middle and inferior parietal lobes (BA 40). |
| 7 | M | 74 | 18 | Adjustor | 8 | 8.85 | 81.9 Anomic | 31 | 19.35 | Middle and superior temporal lobe involving portions of BA 37, 21, 22, and 39. |
| 8 | F | 71 | 10 | School assistant | 8 | 8.95 | 83.9 Anomic | 38 | 18.4 | Temporal lobe damage including BA 21 and 22. White matter damage deep in the middle and superior parietal lobe. |
| 9 | M | 51 | 47 | Cook | 7 | 8.25 | 84.3 Anomic | 41 | 23.57 | BA 22, 42, 39 and posterior portion of BA 38; inferior and middle parietal lobe (BA 40). |
| 10 | F | 71 | 18 | Secretary | 8 | 9.50 | 89.4 Anomic | 38 | 8.9 | BA 22 partially involved; complete destruction of the medial BA 6. |
| 11 | M | 52 | 25 | Truck driver | 9 | 9.95 | 91.5 Anomic | 45 | 3.04 | White matter damage underlying the superior portion of BA 44 and 6. |
The time postonset of stroke is measured in months.
All participants are now retired with the exception of P4.
Maximum score of 10.
Maximum score of 100.
Maximum score of 60.
Figure 1.
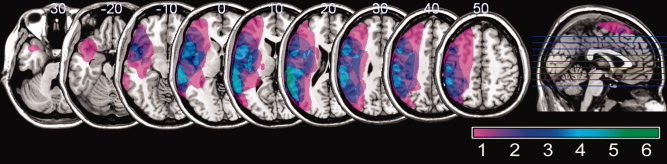
Lesion overlay map showing the distribution of brain damage for the study sample overlaid on a standard brain template. The color scale shows the degree of overlap among brain lesions in different participants. Note that the range of the scale (1–7) depicts the greatest overlap in brain lesions (7) for the group of 11 participants. The sagittal image on the right illustrates the location of the axial slices on the left.
To examine whether brain activity associated with correct naming by persons with aphasia is comparable to what is seen in normal participants, functional magnetic resonance imaging (fMRI) data from 10 normal individuals (six females) included in an earlier study [Fridriksson et al., 2007] were used. The mean age range for these participants was 58.3 years with a range of 42 years. These participants were scanned using the same fMRI paradigm that was used in this study.
Behavioral Measures
The behavioral task consisted of naming pictures of high‐frequency common nouns [Frances and Kucera, 1982]. During the 20‐min fMRI run, 80 colored pictures were presented for 2 s each on a back‐projected mirror located on top of the head coil. To establish a comparative fMRI baseline, 40 abstract color pictures were presented at random among the 80 real object pictures. Participants were instructed to name every picture aloud and to say nothing when the abstract pictures were presented. We have successfully used this paradigm in several previous fMRI studies [Fridriksson et al., 2006a, b, 2007]. A nonferrous microphone placed 1–3 cm from participants' mouth was used to record naming attempts, which were recorded with sufficient clarity for scoring and later scored off‐line using the scoring criteria of the Philadelphia Naming Test [PNT; Roach et al., 1996]. Naming responses were categorized as follows: (1) correct naming, (2) phonemic paraphasia, (3) semantic paraphasia, (4) mixed paraphasia, (5) unrelated response, (6) neologism, and (7) nonresponse.
Image Acquisition
For the purpose of lesion analyses and the anatomical reference for statistical activation maps, all participants underwent high‐resolution T1‐MRI using a TFE sequence, yielding a 1‐mm isotropic image—FOV = 256 mm × 256 mm, 160 sagittal slices, 15° flip angle, TR = 9.5 ms, TE = 5.7 ms. The sparse fMRI data collection utilized the following parameters: TR = 10.0 s; TA = 2.0 s; TE = 30 ms, in‐plane resolution 3.25 mm × 3.25 mm. A total of 32 axial slices (3.25 mm thick) covering the supratentorial brain were collected 120 times each. Oblique axial planes parallel to the AC (anterior commissure)–PC (posterior commissure) line were utilized for image orientation. The mismatch between TR and TA allowed for an 8 s period of scanner silence following the collection of each whole brain volume. This time was utilized for picture presentation and audio recording of naming attempts. To improve modeling of the hemodynamic response, the time between picture presentations was randomized with a mean interstimulus interval (ISI) of 6 s and a range of 3–9 s. No pictures were presented during the last 2 s before each TA.
Statistical Analyses
Brain lesions were demarcated on T1‐MRI using MRIcro [Rorden and Brett, 2000] and collapsed to make the lesion overlay map seen in Figure 1. The fMRI analysis utilized the FMRIB Software Library [FSL; Smith et al., 2004]. The first‐level analysis was carried out using FMRI Expert Analysis Tool (FEAT) Version 5.4, part of FSL. The following prestatistics processing was applied: motion correction [Jenkinson et al., 2002]; nonbrain removal [Smith, 2002]; spatial smoothing using a Gaussian kernel of FWHM 8 mm; mean‐based intensity normalization of all volumes by the same factor; and highpass temporal filtering (Gaussian‐weighted LSF straight line fitting, with sigma = 60.0 s). The time‐series statistical analysis employed general linear modeling (GLM) [Woolrich et al., 2001] and a Gamma function. A timing vector was created for correct naming and baseline (viewing abstract pictures) as well as specific naming errors for each participant. Thus, brain activity associated with correct naming and a specific error type over baseline was analyzed separately in the first‐level analysis. Z (Gaussianised T/F) statistic images were thresholded using the default values in FSL, where a cluster was determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05 [Worsley et al., 1992]. Data for each person were analyzed and registered to their native space and later coregistered in Montreal Neurological Institute (MNI) space using FMRIB's Linear Image Registration Tool [FLIRT; Jenkinson and Smith, 2001; Jenkinson et al., 2002]. To improve coregistration of damaged brains, individual binary lesion masks were applied to the high‐resolution reference images as well as the EPI input data.
To examine similarities in brain activation associated with naming in individuals with aphasia, a higher‐level group analysis was carried out using a two‐stage local analysis of mixed effects [Beckmann et al., 2003]. As can be seen in Figure 1, lesion size and location varied significantly from one aphasic participant to another. Because the focus of this study was on similarities in brain activation, rather than differences, the lesion map seen in Figure 1 was used in the higher‐level analysis as an exclusionary mask to investigate mean activity among all participants. That is, only voxels representing areas that were preserved in all individuals were included in the higher‐level analyses. Otherwise, it would have been possible to find mean brain activation in an area that was damaged in one or more participants—a result which only would have pertained to a subset of the study sample. Similar to the first‐level analysis, the Z (Gaussianised T/F) statistic images were generated using a cluster threshold of Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05 [Worsley et al., 1992]. Mean statistical maps generated from the participants' data were rendered in MNI space using cost functions.
Brain activity during correct naming was estimated by combining the first‐level statistical maps associated with correct naming greater than baseline for each participant. To assess activity related to the production of paraphasias, higher‐level contrasts (t‐tests) were created by comparing statistical maps associated with correct naming to those associated with phonemic and semantic paraphasias. For example, to compare activity associated with correct naming and phonemic paraphasias, the following contrasts were created: “correct naming > baseline” greater than “phonemic paraphasias > baseline” and vice versa. Similar contrasts were used to compare cortical activity associated with correct naming and semantic paraphasias.
As expected, the participants with aphasia varied greatly with regard to the number of correctly named items and error types. As the present study focused on similarities in brain activity, it was important to factor out the variance associated with naming few/many pictures as well as the number of different kinds of errors by each participant. Without controlling for this variance, some participants would have contributed more greatly to the higher‐level analyses than others. Accordingly, nuisance variables (factors of no interest) representing the number of a given response (correct naming, phonemic paraphasia, or semantic paraphasias) by each participant were included in the higher‐level analyses. An example of how a nuisance variable is utilized in FSL can be found at [http://www.fmrib.ox.ac.uk/fsl/feat5/detail.html#SingleGroupAveragewithAdditionalCovariate].
A “between‐group” t‐test was performed to compare brain activation associated with correct naming (over baseline) in the participants with aphasia and their normal counterparts. As in the previous higher‐level analyses, the overall lesion map was used to exclude voxels that represented areas that were damaged in one or more participants.
To study the contribution of different brain areas to correct naming performance by the aphasic participants, each voxel cluster associated with correct naming in the higher‐level statistical map was utilized as a volume of interest (VOI). Percent signal change associated with correct naming in each cluster was calculated on case‐by‐case basis (i.e., for each aphasic participant) and correlated (Spearman) with the number of correct naming attempts during the fMRI task. The rationale behind examining intensity of brain activation as a predictor of correct naming performance was as following: Although a mean statistical map can represent cortical activity associated with executing a given task or a response type, it does not reveal whether increased or decreased activation in an “activated” area is related to successful completion of the task at hand. With regard to this study, the VOI analysis was performed to examine whether cortical areas activated during correct naming contributed differentially (i.e., showed increased/decreased activation) to task success. The following list includes the general anatomical location and size of the five right hemisphere clusters that comprised the statistical map associated with correct naming: (1) the posterior inferior frontal lobe and anterior insula—portions of BA 44, BA 45, and BA 47—size = 3.41 cc; (2) the motor and premotor cortex—portions of the lateral inferior BA 4 and BA 6—size = 5.01 cc; (3) the supplementary motor area—the superior and medial BA 6—size = 2.50 cc; (4) the parietal lobe—primarily portions of BA 40 but also part of BA 2—size = 3.82 cc; (5) the temporal lobe—portions of BA 21, BA 22, and BA 38—size = 16.39 cc.
RESULTS
Task Performance
As expected, performance on the naming task varied greatly among the participants (Table II). Correct naming ranged from 1/80 to 66/80, and most participants made errors categorized as phonemic or semantic paraphasias. The distribution of errors across the other categories varied, but very few mixed paraphasias and unrelated responses were recorded. The number of correct naming attempts was significantly higher (Mann–Whitney U; P = 0.004) for the fluent participants (P6–P11) compared with the nonfluent participants (P1–P5). No differences were detected between the two groups for the number of specific paraphasia types (phonemic errors, P = 0.329; semantic errors, P = 0.790). These results suggest that the participants with fluent aphasia named more pictures correctly, but both groups produced a similar percentage of phonemic and semantic errors irrespective of their ability to correctly name pictures.
Table II.
Number of correct naming attempts and errors by each participant
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Correct | 1 | 30 | 2 | 23 | 21 | 51 | 43 | 66 | 46 | 42 | 55 |
| Semantic | 2 | 19 | 6 | 5 | 4 | 7 | 11 | 6 | 4 | 7 | 8 |
| Phonemic | 12 | 5 | 1 | 10 | 38 | 18 | 8 | 6 | 23 | 12 | 6 |
| Mixed | 7 | 4 | 0 | 4 | 6 | 0 | 0 | 0 | 4 | 1 | 0 |
| Unrelated | 1 | 7 | 35 | 1 | 3 | 0 | 2 | 1 | 0 | 1 | 3 |
| Neologism | 29 | 2 | 15 | 0 | 5 | 0 | 2 | 0 | 1 | 4 | 0 |
| Nonresponse | 28 | 13 | 21 | 37 | 3 | 4 | 14 | 1 | 2 | 13 | 8 |
| Total | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
Brain Activity
The fMRI analysis revealed widespread cortical activation in the right hemisphere associated with correct naming compared to the baseline condition (viewing abstract pictures) across the participants with aphasia. Brain activity associated with correct naming compared to baseline was revealed in the right hemisphere homologues of the classical language areas (Wernicke's and Broca's areas) in addition to the right motor cortex, the superior‐medial frontal lobe, and the parietal lobe (Fig. 2; Table III). Local maxima (voxels with the highest values in a given cluster of activity) were recorded in the right homologue of Broca's area (BA 45), precentral gyrus (BA 4 and BA 6), supplementary motor area (BA 6), and supramarginal gyrus (BA 40) as well as the middle and superior temporal lobe (BA 21 and BA 22) and temporal pole (BA 48). A between‐groups t‐test failed to show a statistically significant difference in cortical activation associated with correct naming in the aphasic group compared with 10 normal control participants.
Figure 2.
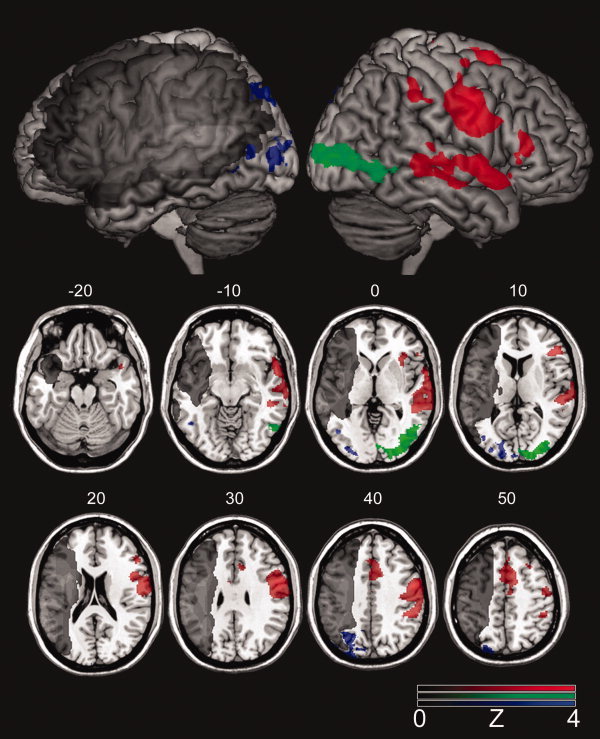
Brain activity associated with “correct naming > errors” (red‐color scale) as well as for the contrasts “phonemic errors > correct naming” (blue‐color scale) and “semantic errors > correct naming” (green‐color scale). The gradient of the color scale represents Z‐scores compared with baseline. The lesion overlay map is shown in grayscale.
Table III.
Standard coordinates for local maxima
| Z a | x | y | z | Hemisphere | Location | BAb |
|---|---|---|---|---|---|---|
| Correct naming | ||||||
| 4.07 | 58 | 0 | 40 | Right | Precentral gyrus | 6 |
| 3.72 | 57 | 1 | 32 | Right | Precentral gyrus | 4 |
| 3.68 | 6 | 8 | 68 | Right | Superior frontal gyrus | 6 |
| 3.57 | 64 | 6 | −3 | Right | Temporal pole | 38 |
| 3.28 | 57 | −31 | 0 | Right | Middle temporal gyrus | 21 |
| 3.17 | 53 | −37 | 45 | Right | Supramarginal gyrus | 40 |
| 3.13 | 49 | 33 | 9 | Right | Pars triangularis | 45 |
| 2.83 | 70 | −21 | 6 | Right | Superior temporal gyrus | 22 |
| Phonemic errors > correct naming | ||||||
| 3.31 | −20 | −86 | 42 | Left | Precuneus | 19 |
| 3.20 | −24 | −90 | 38 | Left | Cuneus | 19 |
| 2.98 | −31 | −64 | 42 | Left | Inferior parietal lobe | 7 |
| 2.97 | −22 | −82 | 46 | Left | Superior occipital lobe | 7 |
| 2.81 | −26 | −73 | 45 | Left | Superior parietal lobe | 7 |
| 2.75 | −44 | −61 | −4 | Left | Inferior temporal gyrus | 37 |
| Semantic errors > correct naming | ||||||
| 4.29 | 20 | −98 | 6 | Right | Middle occipital gyrus | 18 |
| 4.24 | 18 | −96 | 10 | Right | Cuneus | 18 |
| 4.21 | 40 | −80 | 4 | Right | Middle occipital gyrus | 19 |
| 4.14 | 32 | −86 | 6 | Right | Middle occipital gyrus | 18 |
| 4.10 | 58 | −62 | −6 | Right | Inferior temporal gyrus | 37 |
Standard coordinates for local maxima where cortical activity was associated with “correct naming > baseline” (top), as well as for the contrasts “phonemic errors > correct naming” (middle) and “semantic errors > correct naming” (bottom). Note that in the first‐level analysis, errors and correct naming were compared with baseline (viewing of abstract pictures).
Highest Z‐value for a voxel within a given cluster of activation.
BA = Brodmann's area.
Compared to neural recruitment associated with correct naming, greater cortical activity was noted in distinct cortical areas during the production of phonemic and semantic paraphasias (Fig. 2; Table III). It is important to emphasize that the difference in brain activity among the conditions was examined by contrasting “correct naming > baseline” with “phonemic paraphasias > baseline” and “semantic paraphasias > baseline.” The production of phonemic paraphasias recruited the left occipital, parietal, and posterior inferior temporal lobe. Local maxima were revealed in the cuneus and precuneus (BA 19), along with the posterior and superior parietal lobe (BA 7) and the inferior temporal gyrus (BA 37) (Fig. 2; Table III). A similar pattern of activity was found for the contrast “semantic paraphasias (>baseline) > correct naming (>baseline),” albeit in the right hemisphere. Local maxima were found in the middle occipital gyrus and cuneus (BA 18 and BA 19), as well as the posterior inferior temporal gyrus (BA 37). Greater activity associated with correct naming over semantic or phonemic paraphasias was not revealed.
Volumes of Interest
There was a clear relationship between the number of correctly named pictures on the fMRI task and percent BOLD signal change in the right posterior inferior frontal lobe (portions of BA 44, BA 45, and BA 47) (see Fig. 3). More specifically, greater naming success was associated with higher percent signal change in this area. This was not the case for the other VOIs. However, an inspection of the data suggested that P3 was a clear outlier (greater than two standard deviations from the regression line) in the temporal lobe and the supplementary motor area. The data from P3 were subsequently removed from the dataset, and the Spearman correlation coefficients were recalculated. This second analysis suggested that, along with the posterior inferior frontal lobe, less severe aphasia was associated with increased signal change in the motor/premotor cortex and the right temporal lobe (see Fig. 3). One interpretation of these findings would suggest that increased activity was simply related to the size of the left hemisphere lesions. To examine this issue, partial correlation coefficients were calculated among the percent signal change in the VOIs and the number of correctly named pictures on the fMRI task while factoring out lesion size in each participant. Once the variance associated with this factor was removed, the relationship between percent signal change and correct naming only reached statistical significance in the posterior inferior frontal lobe (r(10) = 0.810, P = 0.004). The outcome did not change when data from P3 were excluded from the analysis.
Figure 3.
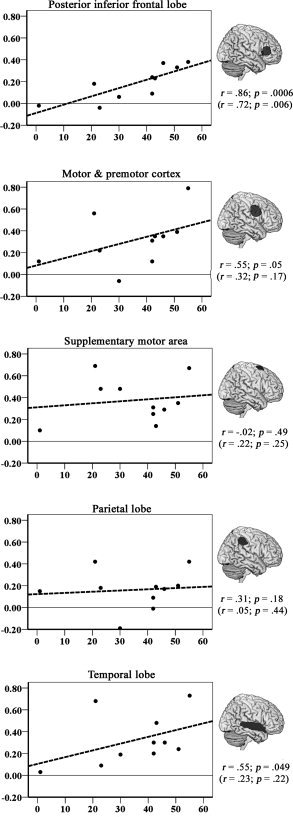
Scatter plots for the relationship between cortical activity in selective VOIs (Y‐axis; measured as the percent signal change in VOI) and naming performance (X‐axis; measured as the number of correctly named pictures on the fMRI task). The VOIs were extracted from the mean statistical map associated with correct naming (>baseline) in 11 participants and are displayed to the right of each scatter plot (rendered on a standard brain). Spearman correlation coefficients (one‐tailed) for each relationship are shown below each VOI based on data from 10 participants (excluding a single outlier, P3). The same relationship is also shown in parentheses when data from all 11 participants were included.
DISCUSSION
To better understand brain activity associated with naming errors in aphasia, this study included 11 participants with aphasia who underwent fMRI scanning while naming common objects. The specific purpose of this investigation was to highlight similarities in cortical activation associated with correct naming and productions of two types of errors on the naming task by individuals with different types and severity of aphasia. It is important to note that the voxel‐based analysis in this study did not address differences in task‐related cortical activation. Rather, it focused on determining what common areas of the brain are modulated during correct or erroneous naming. By the same token, this study did not seek to highlight differences in cortical activity associated with object naming in persons with aphasia—a task that is inherently difficult for most aphasic patients. In addition, the relationship between naming performance and neural activity in the right hemisphere was explored.
A higher‐level fMRI analysis was used to generate a single activation map that showed distinct areas of activity for correct naming in addition to phonemic and semantic paraphasias. When correct naming was compared with baseline (viewing abstract pictures), increased cortical activation was primarily observed in the right hemisphere homologues of the classical language areas as well as in the right supplementary motor area and parietal lobe. Differences in cortical activation associated with correct naming were not revealed between the aphasic and normal participants (note that the between‐groups t‐test only included voxels representing areas that were preserved in all of the aphasic participants). Based on this data, this finding suggests that the intensity and loci of activation associated with correct naming in aphasia is similar to that found in normal participants. Right hemisphere activity associated with correct naming is commonly reported in studies of picture naming in normal participants [Christoffels et al., 2007; Fridriksson et al., 2006a, b; Mechelli et al., 2007]. Nevertheless, the common right hemisphere activity does not discount the role of the intact left hemisphere in correct naming. The group analysis does not reflect individual differences in brain activation among our participants. Yet, it underscores the purpose of this study, which was to examine similarities, rather than differences, in brain activity associated with language processing in patients with aphasia. As we discuss later, our data, as well as others', suggest that the right hemisphere not only plays an important role in successful language processing in persons with aphasia, but it also plays a crucial role in aphasia recovery.
Compared with correct naming, distinct areas of neural activity were associated with the production of phonemic and semantic paraphasias across persons with different aphasia types and levels of severity. The production of phonemic paraphasias recruited the left cuneus and precuneus (BA 7 and BA 19) along with the posterior inferior temporal lobe (BA 37). Each of these would be considered perilesional areas in persons with inferior parietal lobe damage. The left inferior parietal lobe has traditionally been associated with phonological processing [Baddeley, 2003]. Moreover, there is evidence to suggest that conduction aphasia—characterized by impaired repetition and frequent phonemic paraphasias—is associated with gray matter damage to the inferior parietal lobe [Bartha and Benke, 2003; Geldmacher et al., 2007; Hickok et al., 2000; Mazzocchi and Vignolo, 1979; Quigg et al., 2006]. Therefore, it is possible that the increased activity in the perilesional areas of the damaged parietal lobe reflects impaired phonological processing.
Although precuneus activity has been linked to phonological processing by normal participants [Voets et al., 2006], improved auditory comprehension in patients with aphasia [Musso et al., 1999], and improved naming in two patients with nonfluent aphasia [Fridriksson et al., 2007], these results link increased activity in the left precuneus with the production of phonemic paraphasias. In light of this seemingly paradoxical evidence, it is important to consider the nature of phonemic paraphasias. Although they certainly are not correct responses, phonemic paraphasias are, nevertheless, close approximation of the target, particularly when compared with neologisms. Nickels and Howard [ 1995] suggested that phonemic paraphasias represent breakdowns in phonological processing along a continuum where neologisms would be considered the most severe example of impaired phonological processing, and single phoneme substitutions would represent the mildest form. For most participants in this study, the precuneus and cuneus would be considered perilesional areas. Although increased activation in the precuneus may provide support for language processing in some cases, it may be insufficient in other cases, resulting in phonemic paraphasias. The lack of connection to other areas associated with phonological processing, as well as the integrity of these cortical areas, is likely a factor in determining the success of increased precuneus involvement.
In a series of studies, Hillis et al. [ 2002, a, b, 2005, 2006b] have provided strong evidence suggesting that the critical lesion location that causes impaired lexical retrieval in stroke is in the left BA 37. That is, those patients with left BA 37 involvement were more likely to have difficulty retrieving the target word on naming tasks compared with those whose left BA 37 was spared. BA 37 is a large cortical area that includes part of the posterior/lateral and ventral inferior temporal gyrus as well as the fusiform gyrus. In this study, the spared portion of the left BA 37 showed greater activity during the production of phonemic paraphasias compared with correct naming. We suggest that this pattern of activity represents an impaired lexical‐phonological network resulting in incorrect pairing of phonemes with correct lexical retrieval. Given that phonemic paraphasias are seen in most aphasia types, it is not surprising that common areas were recruited among our study sample. Nevertheless, this finding certainly does not preclude individual differences in brain modulation associated with phonemic paraphasias, but rather, highlights the fact that similar phonological errors during speech production recruit some of the same brain areas in patients with different types and severities of aphasia.
Somewhat similar to the left hemisphere activation pattern associated with phonemic errors, the productions of semantic paraphasias recruited the right middle occipital gyrus, the cuneus, and BA 37. Semantic paraphasias can occur from disruptions in selecting the defining features or in associating those specific features with a lexical representation [DeLeon et al., 2007]. Because semantic paraphasias are conceptually related to the target, access to at least partial semantic information is assumed. However, incomplete or underspecified semantic distinction or impaired lexical access can result in incorrect selection of a semantically related lexical representation. Hickok and Poeppel [ 2007] suggest that lexical retrieval relies on the bilateral posterior inferior temporal lobes with important computational differences among the two hemispheres. Similarly, the cerebral hemispheres contribute to semantic processing in different ways where the right hemisphere is thought to have broad overlapping semantic maps; in contrast, the semantic fields in the left hemisphere have more definite anatomical boundaries [Jung‐Beeman, 2005]. Thus, the increased activity in the right hemisphere associated with the production of semantic paraphasias may reflect reliance on lexical‐semantic related processing in the presence of inadequate semantic specification.
Although the nature of right hemisphere semantic processing has been debated, it is clear that semantic‐related tasks recruit the right hemisphere in normal participants and that patients with right posterior inferior temporal lobe damage make distinct semantic errors [Vandenbulcke et al., 2006]. The greatest lesion overlap among the participants in this study was in the left posterior superior temporal lobe. Hillis et al. [ 2006a] found that damage to this area is a strong predictor of semantic errors in acute stroke patients. Although lesion‐symptom mapping highlights the location of brain damage related to a specific impairment, this study provides complimentary evidence suggesting that semantic paraphasias reflect increased right hemisphere activity compared with correct naming. Suggesting that semantic errors arise from right hemisphere activity is certainly not new, Coltheart [ 1980, 2000] hypothesized that deep dyslexia—an acquired reading disorder where patients make frequent semantic errors during reading—reflects right hemisphere processing rather than reliance on the damaged left hemisphere. It is important to point out, however, that this study did not include a detailed examination of the underlying semantic impairment in each patient. That is, it is quite possible that the semantic errors generated by different participants reflected different levels of semantic impairment, in which some participants may have produced semantic errors in all modalities, whereas others might have made semantic errors only in overt speech. If more detailed behavioral testing would be completed, this would have allowed us to group participants in the higher‐level fMRI analysis based on their underlying semantic impairment. However, given that only 11 total participants were included in this study, statistical power would have decreased significantly by dividing the study sample into smaller groups.
In the VOI analysis, the right hemisphere VOI that included portions of the anterior insula and the homologue of Broca's area showed increased activation related to the number of correctly named items by each participant. Specifically, those who could name more items on the naming task also had greater percent BOLD signal change in this area—even when lesion size was factored out. Such a finding suggests that regardless of the extent of brain damage, intensity of brain activity (as measured by fMRI) in the right homologue of Broca's area and the anterior insula is strongly related to naming task success. In contrast to some previous findings [Blank et al., 2003; Naeser et al., 2004], the nonfluent patients in this study did not show abnormally high brain activity in the right frontal lobe compared with the fluent patients. In fact, the nonfluent (and more severe) patients consistently had lower percent signal change associated with correct naming in the right homologue of Broca's area compared with their fluent counterparts. It is important to note, however, that the analyses in the aforementioned studies included data associated with all naming attempts rather than only successful naming.
Recently, a study by Raboyeau et al. [ 2008] found that right hemisphere activity is important for overt picture naming in aphasia. Including 10 stroke patients with left inferior frontal lobe and/or anterior insular damage and 10 normal control participants, this study revealed that increased regional cerebral blood flow in the right inferior frontal gyrus (BA 45/46/47) and the right insula correlates with improved naming performance following language‐based treatment in the participants with aphasia. Moreover, a similar anatomical pattern of increased blood flow associated with learning new words was found in the normal control participants. On the basis of these results, Raboyeau et al. [ 2008] concluded that increased activity in the right frontal lobe in aphasia is not merely the consequence of damaged homologues in the left hemisphere but, rather, is a reflection of increased reliance on the right hemisphere to support aphasia recovery. Evidence regarding the importance of the right inferior frontal lobe in aphasia recovery also comes from research utilizing transcranial magnetic stimulation (TMS). Winhuisen et al. [ 2007] found that TMS over the right posterior inferior frontal lobe impairs language processing in some subacute stroke patients with aphasia. However, most of their patients seemed to be relying more on perilesional brain areas for language processing because TMS did not affect language task performance in all cases.
When data from P3 were excluded from the analysis, a statistically significant correlation was found between the number of correctly named items and percent signal change in the right motor/premotor cortex and the temporal lobe. When lesion size was factored out, these relationships did not reach significance, suggesting that the right inferior frontal activity was a far more robust predictor of naming task success in our sample. Nevertheless, these results are consistent with a number of studies that have found increased right temporal lobe activity associated with better auditory comprehension in persons with aphasia [Ansaldo et al., 2002; Crinion and Price 2005; Musso et al., 1999; Sharp et al., 2004].
CONCLUSIONS
Our findings suggest common cortical activation associated with correct naming as well as during the production of phonemic and semantic paraphasias in participants with fluent and nonfluent aphasia. The common right hemisphere network recruited for correct naming in our participants with aphasia is very similar to what has been reported for normal participants. This finding emphasizes the importance of the residual cortical language network in successful language processing in aphasia. However, it does not discount the role of the left hemisphere in recovery because greater variation in task‐modulated activation is most likely seen in the left hemisphere, reflecting different locations and extent of damage. We further emphasize that these findings compliment lesion‐symptom mapping studies that have revealed the association between localized left hemisphere damage and specific speech errors in aphasia. That is, although a given lesion can lead to the productions of particular speech errors, these errors are associated with the recruitment of some of the same cortical areas in patients with different types and severity of aphasia. We are currently collecting more data to address further questions between aphasia severity, naming errors, and brain activation in stroke.
We are not aware of any other studies that have demonstrated common cortical activation across aphasia type and severity associated with particular naming errors. Along the lines of Goldstein's postulations [ 1942], it is possible that these patterns of brain activity may not represent maladaptation to brain damage; instead, they may reflect the “struggle” of the “organism” to cope with a damaged language network.
Potential conflict of interest: None declared.
REFERENCES
- Abo M,Senoo A,Watanabe S,Miyano S,Doseki K,Sasaki N,Kobayashi K,Kikuchi Y,Yonemoto K ( 2004): Language‐related brain function during word repetition in post‐stroke aphasics. Neuroreport 15: 1891–1894. [DOI] [PubMed] [Google Scholar]
- Ansaldo AI,Arguin M,Lecours AR ( 2002): The contribution of the right cerebral hemisphere to the recovery from aphasia: A single longitudinal case study. Brain Lang 82: 206–222. [DOI] [PubMed] [Google Scholar]
- Baddeley A ( 2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 10: 829–839. [DOI] [PubMed] [Google Scholar]
- Bartha L,Benke T ( 2003): Acute conduction aphasia: An analysis of 20 cases. Brain Lang 85: 93–108. [DOI] [PubMed] [Google Scholar]
- Beckmann CF,Jenkinson M,Smith SM ( 2003): General multi‐level linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Blank SC,Bird H,Turkheimer F,Wise RJ ( 2003): Speech production after stroke: The role of the right pars opercularis. Ann Neurol 54: 310–320. [DOI] [PubMed] [Google Scholar]
- Cardebat D,Demonet JF,de Boissezon X,Marie N,Marie RM,Lambert J,Baron JC,Puel M ( 2003): Behavioral and neurofunctional changes over time in healthy and aphasic subjects: A PET language activation study. Stroke 34: 2900–2906. [DOI] [PubMed] [Google Scholar]
- Christoffels IK,Fomisano E,Schiller NO ( 2007): Neural correlates of verbal feedback processing: An fMRI study employing overt speech. Hum Brain Mapp 28: 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M ( 1980): Deep dyslexia: A right‐hemisphere hypothesis In: Coltheart M,Patterson KE,Marshall JC, editors. Deep Dyslexia. London: Routledge and Kegan Paul; pp 326–380. [Google Scholar]
- Coltheart M ( 2000): Deep dyslexia is right‐hemisphere reading. Brain Lang 71: 299–309. [DOI] [PubMed] [Google Scholar]
- Crinion J,Price CJ ( 2005): Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain 128: 2858–2871. [DOI] [PubMed] [Google Scholar]
- de Boissezon X,Demonet JF,Puel M,Marie N,Raboyeau G,Albucher JF,Chollet F,Cardebat D ( 2005): Subcortical aphasia: A longitudinal PET study. Brain 36: 1467–1473. [DOI] [PubMed] [Google Scholar]
- DeLeon J,Gottesman RF,Kleinman JT,Newhart M,Davis C,Heidler‐Gary J,Lee A,Hillis AE ( 2007): Neural regions essential for distinct cognitive processes underlying picture naming. Brain 130: 1408–1422. [DOI] [PubMed] [Google Scholar]
- Fazzini E,Bachman D,Albert ML ( 1986): Recovery of function in aphasia. J Neurolinguistics 2: 22–46. [Google Scholar]
- Fernandez B,Cardebat D,Demonet JF,Joseph PA,Mazuax JM,Barat M,Allard M ( 2004): Functional MRI follow‐up study of language processes in healthy subject and during recovery in a case of aphasia. Stroke 35: 2171–2176. [DOI] [PubMed] [Google Scholar]
- Frances WN,Kucera H ( 1982): Frequency Analysis of English Usage. Boston: Houghton Mifflin. [Google Scholar]
- Fridriksson J,Holland AL,Coull B,Plante E,Trouard T,Beeson P ( 2002): Aphasia severity: Association with cerebral perfusion and diffusion. Aphasiology 16: 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J,Morrow KL,Moser D,Baylis GC ( 2006a): Age related variability in cortical activity during language processing. J Speech Lang Hear Res 49: 690–697. [DOI] [PubMed] [Google Scholar]
- Fridriksson J,Morrow L,Moser D,Fridriksson A,Baylis G ( 2006b): Neural correlates of anomia recovery in aphasia. Neuroimage 32: 1403–1412. [DOI] [PubMed] [Google Scholar]
- Fridriksson J,Moser D,Bonilha L,Morrow‐Odom KL,Shaw H,Fridriksson A,Baylis GC,Rorden C ( 2007): Neural correlates of phonological and semantic‐based anomia treatment in aphasia. Neuropsychologia 45: 1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher DS,Quigg M,Elias WJ,Yamada K,Nagakane Y,Mizuno T,Hosomi A,Nakagawa M,Nishimura T ( 2007): MR tractography depicting damage to the arcuate fasciculus in a patient with conduction aphasia. Neurology 69: 321–322. [DOI] [PubMed] [Google Scholar]
- Goldstein K ( 1942): After Effects of Brain Injuries in War, Their Evaluation and Treatment: The Application of Pyschologic Methods in the Clinic. New York: Grune and Stratton. [Google Scholar]
- Heiss WD,Kessler J,Thiel A,Ghaemi M,Karbe H ( 1999): Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 45: 430–438. [DOI] [PubMed] [Google Scholar]
- Hickok G,Poeppel D ( 2007): The cortical organization of speech processing. Nat Rev Neurosci 8: 393–402. [DOI] [PubMed] [Google Scholar]
- Hickok G,Erhard P,Kassubek J,Helms‐Tillery AK,Naeve‐Velguth S,Strupp JP,Strick PL,Ugurbil K ( 2000): A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: Implications for the explanation of conduction aphasia. Neurosci Lett 287: 156–160. [DOI] [PubMed] [Google Scholar]
- Hillis A,Heidler J ( 2002): Mechanisms of early aphasia recovery. Aphasiology 16: 885–895. [Google Scholar]
- Hillis AE,Kane A,Tuffiash E,Ulatowski JA,Barker P,Beauchamp N,Wityk RJ ( 2002a): Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Lang 79: 495–510. [DOI] [PubMed] [Google Scholar]
- Hillis AE,Tuffiash E,Wityk RJ,Barker PB ( 2002b): Regions of neural dysfunction associated with impaired naming of actions and objects in acute stroke. Cogn Neuropsychol 19: 523–534. [DOI] [PubMed] [Google Scholar]
- Hillis AE,Newhart M,Heidler J,Barker PB,Herskovits E,Degaonkar M ( 2005): The roles of the “visual word form area” in reading. Neuroimage 24: 548–559. [DOI] [PubMed] [Google Scholar]
- Hillis AE,Chaudhry P,Davis C,Kleinman JT,Newhart M,Heidler‐Gary J ( 2006a): Where (in the brain) do semantic errors come from? Brain Lang 99: 73–74. [Google Scholar]
- Hillis AE,Kleinman JT,Newhart M,Heidler‐Gary J,Gottesman R,Barker PB,Aldrich E,Llinas R,Wityk R,Chaudhry P ( 2006b): Restoring cerebral blood flow reveals neural regions critical for naming. J Cogn Neurosci 26: 8069–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M,Smith SM ( 2001): A global optimization method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M,Bannister M,Smith S ( 2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Jung‐Beeman M ( 2005): Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9: 512–518. [DOI] [PubMed] [Google Scholar]
- Kaplan E,Goodglass H,Weintrab S ( 1983): The Boston Naming Test. Philadelphia: Lea and Febiger. [Google Scholar]
- Kertesz A ( 1982): Western Aphasia Battery. New York: Grune and Stratton. [Google Scholar]
- Kwakkel G,Boudewijn K,Lindeman E ( 2004): Understanding the pattern of functional recovery after stroke: Facts and theories. Restor Neurol Neurosci 22: 281–299. [PubMed] [Google Scholar]
- Mazzocchi F,Vignolo LA ( 1979): Localization of lesions in aphasia: Clinical CT scan correlation in stroke patients. Cortex 15: 627–653. [DOI] [PubMed] [Google Scholar]
- Mechelli A,Josephs O,Lambon Ralph MA,McClelland JL,Price CJ ( 2007): Dissociating stimulus‐driven semantic and phonological effects during reading and naming. Hum Brain Mapp 28: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M,Weiller C,Kiebel S,Müller S,Bulau P,Rijntjes M ( 1999): Training‐induced brain plasticity in aphasia. Brain 122: 1781–1790. [DOI] [PubMed] [Google Scholar]
- Naeser MA,Martin PI,Baker EH,Hodge SM,Sczerzenie SE,Nichols M,Palumbo CL,Goodglass H,Wingfield A,Samaraweera R,Harris G,Baird A,Renshaw P,Yurgelun‐Todd D ( 2004): Overt propositional speech in chronic non‐fluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage 22: 29–41. [DOI] [PubMed] [Google Scholar]
- Nickels L,Howard D ( 1995): Phonological errors in aphasic naming: Comprehension, monitoring, and lexicality. Cortex 31: 209–237. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L,Galati G,Committeri G ( 2001): The contribution of functional neuroimaging to recovery after brain damage: A review. Cortex 37: 11–31. [DOI] [PubMed] [Google Scholar]
- Price CJ,Crinion J ( 2005): The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol 18: 429–434. [DOI] [PubMed] [Google Scholar]
- Quigg M,Geldmacher DS,Elias WJ ( 2006): Conduction aphasia as a function of the dominant posterior perisylvian cortex. Report of two cases. J Neurosurg 104: 845–848. [DOI] [PubMed] [Google Scholar]
- Raboyeau G,De Boissezon X,Marie N,Balduyck S,Puel M,Bézy C,Démonet JF,Cardebat D ( 2008): Right hemisphere activation in recovery from aphasia: Lesion effect or function recruitment? Neurology 70: 290–298. [DOI] [PubMed] [Google Scholar]
- Roach A,Schwartz MF,Martin N,Grewal RS,Brecher A ( 1996): Philadelphia Naming Test: Scoring and rationale. Aphasiology 24: 121–133. [Google Scholar]
- Rorden C,Brett M ( 2000): Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Rosen HJ,Petersen SE,Linenweber MR,Snyder AZ,White DA,Chapman L,Dromerick AW,Fiez JA,Corbetta MD ( 2000): Neural correlates of recovery from aphasia after damage to left inferior cortex. Neurology 55: 1883–1894. [DOI] [PubMed] [Google Scholar]
- Saur D,Lange R,Baumgaertner A,Schraknepper V,Willmes K,Rijntjes M,Weiller C ( 2006): Dynamics of language reorganization after stroke. Brain 129: 1371–1384. [DOI] [PubMed] [Google Scholar]
- Seitz RJ,Azari NP,Knorr U,Binkofski F,Herzog H,Freund HJ ( 1999): The role of diaschisis in stroke recovery. Stroke 30: 1844–1850. [DOI] [PubMed] [Google Scholar]
- Sharp DJ,Scott SK,Wise RJ ( 2004): Retrieving meaning after temporal lobe infraction: The role of the basal language area. Ann Neurol 56: 836–846. [DOI] [PubMed] [Google Scholar]
- Smith S ( 2002). Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM,Jenkinson M,Woolrich MW,Beckmann CF,Behrens TE,Johansen‐Berg H,Bannister PR,De Luca M,Drobnjak I,Flitney DE,Niazy RK,Saunders J,Vickers J,Zhang Y,De Stefano N,Brady JM,Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Vandenbulcke M,Peeters R,Fannes K,Vandenberghe R ( 2006): Knowledge of visual attributes in the right hemisphere. Nat Neurosci 9: 964–970. [DOI] [PubMed] [Google Scholar]
- Voets NL,Adcock JE,Flitney DE,Behrens TE,Hart Y,Stacey R,Carpenter K,Matthews PM ( 2006): Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain 129: 754–766. [DOI] [PubMed] [Google Scholar]
- Winhuisen L,Thiel A,Schumacher B,Kessler J,Rudolf J,Haupt W,Heiss W ( 2007): The right inferior frontal gyrus and poststroke aphasia: A follow‐up investigation. Stroke 38: 1286–1292. [DOI] [PubMed] [Google Scholar]
- Woolrich MW,Ripley BD,Brady JM,Smith SM ( 2001): Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ,Evans AC,Marrett S,Neelin P ( 1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]
- Xu XJ,Zhang MM,Shang DS,Wang QD,Luo BY,Weng XC ( 2004): Cortical language activation in aphasia: A functional MRI study. Chin Med J (Engl) 117: 1011–1016. [PubMed] [Google Scholar]


