Abstract
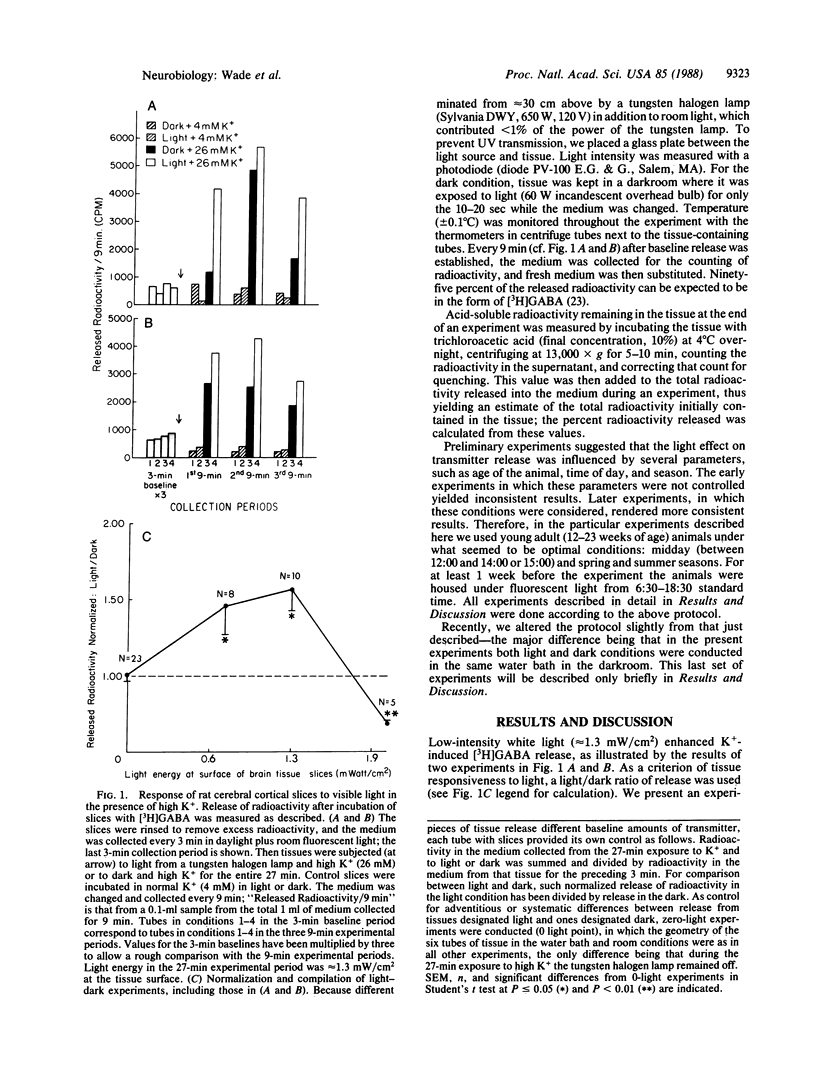
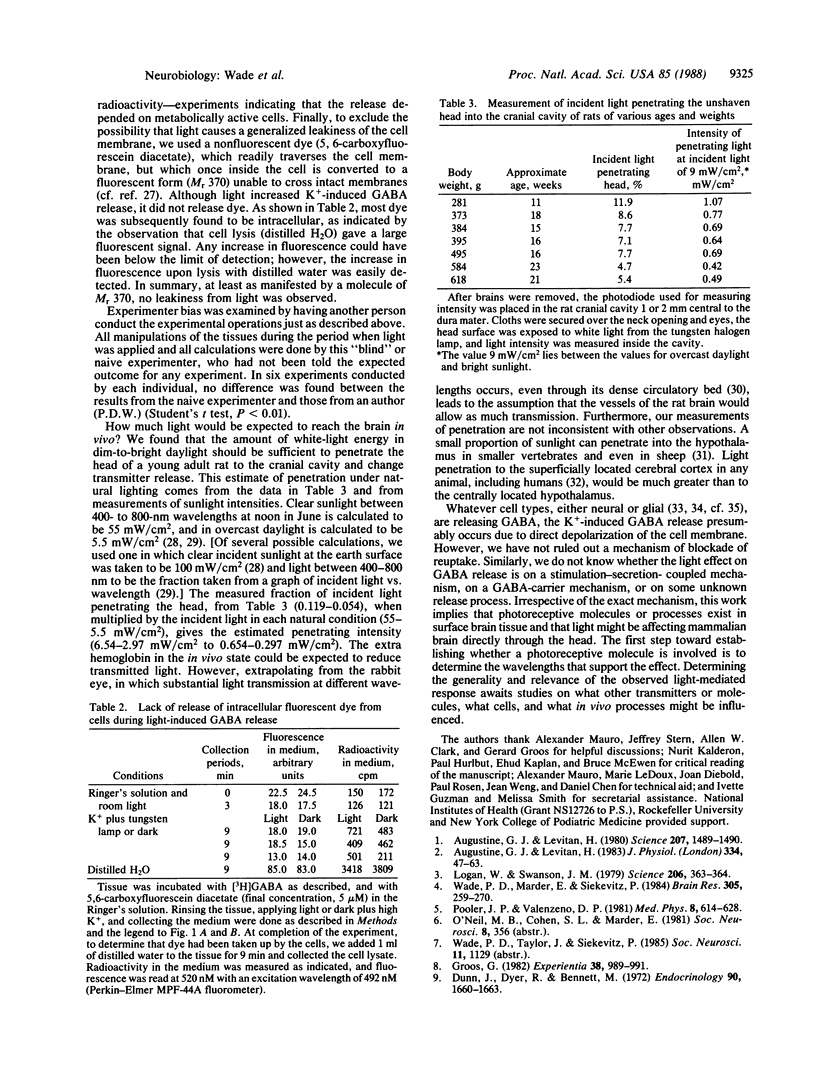
Low levels of visible light directed onto slices of rat cerebral cortical tissue enhanced net potassium-induced release of the neurotransmitter gamma-aminobutyric acid (GABA) from these brain slices. At higher light intensity, net potassium-induced release was suppressed. These effects were apparently not from increased temperature. The amount of light enhancing this neurotransmitter release is approximately equal to the amount of light that can penetrate the head and reach the brain at the intensities of sunlight; this was determined by measuring the light entering the rat head through fur, scalp, skull, and dura mater and considering several natural lighting conditions. These results suggest that ambient light may be sufficient to alter the release of transmitters from mammalian cerebral cortex in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Jr, Levitan H. Neurotransmitter release from a vertebrate neuromuscular synapse affected by a food dye. Science. 1980 Mar 28;207(4438):1489–1490. doi: 10.1126/science.6244619. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Levitan H. Neurotransmitter release and nerve terminal morphology at the frog neuromuscular junction affected by the dye Erythrosin B. J Physiol. 1983 Jan;334:47–63. doi: 10.1113/jphysiol.1983.sp014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Botz D., Chang D. B., Mahaffey D. Temperature-sensitive aspects of evoked and spontaneous transmitter release at the frog neuromuscular junction. J Physiol. 1978 Jun;279:253–273. doi: 10.1113/jphysiol.1978.sp012343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J., Dryer R., Bennett M. Diurnal variation in plasma corticosterone following long term exposure to continuous illumination. Endocrinology. 1972 Jun;90(6):1660–1663. doi: 10.1210/endo-90-6-1660. [DOI] [PubMed] [Google Scholar]
- Fork R. L. Laser stimulation of nerve cells in Aplysia. Science. 1971 Mar 5;171(3974):907–908. doi: 10.1126/science.171.3974.907. [DOI] [PubMed] [Google Scholar]
- Goodall H., Johnson M. H. Use of carboxyfluorescein diacetate to study formation of permeable channels between mouse blastomeres. Nature. 1982 Feb 11;295(5849):524–526. doi: 10.1038/295524a0. [DOI] [PubMed] [Google Scholar]
- Groos G. A., van der Kooy D. Functional absence of brain photoreceptors mediating entrainment of circadian rhythms in the adult rat. Experientia. 1981 Jan 15;37(1):71–72. doi: 10.1007/BF01965576. [DOI] [PubMed] [Google Scholar]
- Groos G. The comparative physiology of extraocular photoreception. Experientia. 1982 Sep 15;38(9):989–991. doi: 10.1007/BF01955340. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Moore R. Y. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979 Oct 5;174(2):245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- LISK R. D., KANNWISCHER L. R. LIGHT: EVIDENCE FOR ITS DIRECT EFFECT ON HYPOTHALAMIC NEURONS. Science. 1964 Oct 9;146(3641):272–273. doi: 10.1126/science.146.3641.272. [DOI] [PubMed] [Google Scholar]
- Logan W. J., Swanson J. M. Erythrosin B inhibition of neurotransmitter accumulation by rat brain homogenate. Science. 1979 Oct 19;206(4416):363–364. doi: 10.1126/science.39341. [DOI] [PubMed] [Google Scholar]
- Obata K., Takeda K. Release of gamma-aminobutyric acid into the fourth ventricle induced by stimulation of the cat's cerebellum. J Neurochem. 1969 Jul;16(7):1043–1047. doi: 10.1111/j.1471-4159.1969.tb05948.x. [DOI] [PubMed] [Google Scholar]
- Peregrin J. The influence of the intraocular blood content on the relative spectral sensitivity curves. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1974;17(3):263–270. [PubMed] [Google Scholar]
- Pooler J. P., Valenzeno D. P. Dye-sensitized photodynamic inactivation of cells. Med Phys. 1981 Sep-Oct;8(5):614–628. doi: 10.1118/1.595020. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Sarthy P. V. Release of [3H]gamma-aminobutyric acid from glial (Müller) cells of the rat retina: effects of K+, veratridine, and ethylenediamine. J Neurosci. 1983 Dec;3(12):2494–2503. doi: 10.1523/JNEUROSCI.03-12-02494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Young A. B., Bennett J. P., Mulder A. H. Synaptic biochemistry of amino acids. Fed Proc. 1973 Oct;32(10):2039–2047. [PubMed] [Google Scholar]
- Tzeng M. C., Cohen R. S., Siekevitz P. Release of neurotransmitters and depletion of synaptic vesicles in cerebral cortex slices by alpha-latrotoxin from black widow spider venom. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4016–4020. doi: 10.1073/pnas.75.8.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H., Groos G. Vertebrate circadian rhythms: retinal and extraretinal photoreception. Experientia. 1982 Sep 15;38(9):1013–1021. doi: 10.1007/BF01955345. [DOI] [PubMed] [Google Scholar]
- VANBRUNT E. E., SHEPHERD M. D., WALL J. R., GANONG W. F., CLEGG M. T. PENETRATION OF LIGHT INTO THE BRAIN OF MAMMALS. Ann N Y Acad Sci. 1964 Sep 10;117:217–227. doi: 10.1111/j.1749-6632.1964.tb48177.x. [DOI] [PubMed] [Google Scholar]
- Van Gelder N. M. The effect of aminooxyacetic acid on the metabolism of gamma-aminobutyric acid in brain. Biochem Pharmacol. 1966 May;15(5):533–539. doi: 10.1016/0006-2952(66)90019-0. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Mester E., Tisza S., Mester A. Acetylcholine releasing effect of laser irradiation on Auerbach's plexus in guinea-pig ileum. J Neural Transm. 1977;40(4):305–308. doi: 10.1007/BF01257022. [DOI] [PubMed] [Google Scholar]
- Wade P. D., Fritz L. C., Siekevitz P. The effect of diamide on transmitter release and on synaptic vesicle population at vertebrate synapses. Brain Res. 1981 Nov 30;225(2):357–372. doi: 10.1016/0006-8993(81)90842-8. [DOI] [PubMed] [Google Scholar]
- Wade P. D., Marder E., Siekevitz P. Characterization of transmitter release as a response of vertebrate neural tissue to erythrosin B. Brain Res. 1984 Jul 9;305(2):259–270. doi: 10.1016/0006-8993(84)90432-3. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Akhanjee L. K. Laser-induced somatosensory evoked potentials: evidence of photosensitivity in peripheral nerves. Brain Res. 1985 Oct 7;344(2):281–285. doi: 10.1016/0006-8993(85)90805-4. [DOI] [PubMed] [Google Scholar]
- Walker J. B. Temporary suppression of clonus in humans by brief photostimulation. Brain Res. 1985 Aug 5;340(1):109–113. doi: 10.1016/0006-8993(85)90779-6. [DOI] [PubMed] [Google Scholar]
- Wan S., Parrish J. A., Anderson R. R., Madden M. Transmittance of nonionizing radiation in human tissues. Photochem Photobiol. 1981 Dec;34(6):679–681. doi: 10.1111/j.1751-1097.1981.tb09063.x. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Ponnudurai R., Katz J., Pott C. B., Chilcoat R., Uncini A., Rapoport S., Wade P., Mauro A. Failure to confirm report of light-evoked response of peripheral nerve to low power helium-neon laser light stimulus. Brain Res. 1987 Jan 20;401(2):407–408. doi: 10.1016/0006-8993(87)91430-2. [DOI] [PubMed] [Google Scholar]


