Abstract
Background:
Lacunar stroke is common, but the etiology of the small vessel abnormality is unknown. Retinal vessels share ontogeny, size, and physiologic characteristics with cerebral small vessels, and retinopathy is associated with stroke. We compared retinal microvessel appearance as a surrogate for cerebral small vessels in patients with lacunar and large artery cortical ischemic stroke.
Methods:
We prospectively recruited patients with lacunar ischemic stroke and cortical stroke controls. We took digital retinal photographs of each eye. We assessed central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) diameters and arteriovenous ratios (AVRs) using semiautomated computer software methods and quantified arteriovenous nicking and focal arteriolar narrowing.
Results:
Among 212 patients (105 lacunar, 107 cortical strokes) of mean age 68 years (SD 12 years), AVR was decreased (0.76 vs 0.78, p = 0.03) and CRVE was increased (44.9 pixels/218 μm vs 42.8 pixels/208 μm, p = 0.01) in lacunar patients compared with cortical patients, but CRAE did not differ (33.2 pixels/161 μm vs 33.7 pixels/163 μm, p = 0.4). On multivariable analysis, increased CRVE was associated with lacunar stroke subtype (p = 0.03) and younger age (p < 0.001) after correcting for other vascular risk factors. Arteriovenous nicking and focal arteriolar narrowing did not differ between ischemic stroke subtypes.
Conclusions:
Retinal venules are wider and arteriovenous ratios are smaller in patients with lacunar strokes compared with those in patients with cortical strokes.
GLOSSARY
- AVN
= arteriovenous nicking;
- AVR
= arteriovenous ratio;
- CI
= confidence interval;
- CRAE
= central retinal artery equivalent;
- CRVE
= central retinal vein equivalent;
- FAN
= focal arteriolar narrowing;
- FLAIR
= fluid-attenuated inversion recovery;
- MR
= magnetic resonance;
- NA
= not applicable;
- NASCET
= North American Symptomatic Carotid Endarterectomy Trial;
- NIHSS
= NIH Stroke Scale;
- OR
= odds ratio.
Twenty-five percent of all ischemic stroke is lacunar,1 but the exact etiology remains unknown.2 Lacunar strokes are caused by disease in a single perforating artery. Large artery atheroma and cardiac embolism, the common causes of most cortical ischemic strokes, probably account for only 15% to 20% of lacunar strokes, suggesting that other mechanisms may be responsible for the majority of lacunar stroke.2 However, visualizing the cerebral small vessel morphologic changes is difficult. Postmortem findings generally reflect late-stage changes and the vessels themselves are too small to visualize in detail using current human imaging methods.
Retinal and cerebral small vessels are developmentally related, of similar size, and share physiologic characteristics.3 The retinal vascular bed can be directly visualized noninvasively with retinal photography. Retinal microvascular abnormalities are associated with stroke4,5: retinal vessel widths predict future risk of stroke6–8 and may be associated with a previous cerebral infarction9,10; arteriovenous nicking (AVN) and focal arteriolar narrowing (FAN) are associated with infarcts on magnetic resonance (MR) brain scanning.9,10 However, although some studies suggest that there are associations between retinal changes and lacunar stroke, there is little information about whether retinal appearances truly differ in ischemic stroke subtypes. This is because these studies either did not subtype ischemic stroke6 or only investigated asymptomatic lacunes seen on MR scanning, which are of uncertain relevance to clinical stroke.4,11,12
Therefore, we studied retinal arteriolar and venular widths, FAN, and AVN in patients presenting with acute lacunar and cortical ischemic stroke to test the hypothesis that the retinal small vessels would be morphologically different in patients with lacunar stroke compared with patients with large artery cortical atherothromboembolic stroke.
We prospectively recruited consecutive patients with clinical lacunar or mild cortical stroke seen at our hospital stroke service, aiming to recruit all relevant patients as consecutively as possible. We used patients with cortical stroke as controls to identify any findings specific to lacunar stroke. Normal age-matched controls or a nonstroke control group would only allow us to identify differences due to any stroke, not to stroke subtype. Furthermore, cortical stroke patients have risk factor profiles and medications similar to those of patients with lacunar stroke, thus controlling for potential confounders.
METHODS
All patients were examined by an experienced stroke physician. We assessed stroke severity with the NIH Stroke Scale (NIHSS)13 and classified the stroke clinical syndrome (lacunar or cortical) according to the Oxfordshire Community Stroke Project classification.14 We defined mild cortical stroke syndrome as a maximum clinical deficit of weakness or sensory loss in the face, arm, or leg; loss of higher cerebral dysfunction (dysphasia or neglect); weakness in more than one limb in the presence of loss of higher cerebral function (all in keeping with a partial anterior circulation stroke); or a homonymous hemianopia suggestive of occipital cortical infarct (in keeping with a cortical posterior circulation stroke).14 We defined lacunar stroke as per the classic lacunar syndromes (pure motor weakness or sensory loss or both in face and arm, arm and leg, or all three; ataxic hemiparesis; or clumsy hand dysarthria syndrome).14 We also classified stroke subtype using radiologic criteria (whether the recent infarct on MRI was cortical or lacunar) and used both the clinical and radiologic classification to assign a final stroke subtype classification.14 Where the clinical classification differed from the radiologic classification, the radiologic classification was used, because using clinical criteria alone may result in misclassification of cortical and lacunar infarcts in up to 20% of cases.15 This study was approved by the local research ethics committee, and all patients gave written informed consent.
Patients underwent usual investigations for stroke (brain imaging as below, carotid Doppler ultrasound, electrocardiogram, blood tests, and other tests if indicated). We recorded personal medical history of diabetes (physician-diagnosed history of diabetes), hypertension (physician-diagnosed history of hypertension), ischemic heart disease (physician-diagnosed history of angina, myocardial infarction, coronary angioplasty, or bypass grafting), peripheral vascular disease (physician-diagnosed history of or symptoms of intermittent claudication), cigarette smoking status, and medication use. We defined symptomatic carotid stenosis as greater than 50% measured with the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method in the relevant artery.16 We defined atrial fibrillation as either a physician-diagnosed history of atrial fibrillation or an electrocardiogram showing atrial fibrillation.
Patients had cerebral MRI at presentation, on a 1.5T Signa Horizon MR/I 1.5T HDX scanner (operating under a research collaboration with GE Medical Systems, Milwaukee, WI, operating as IGE in the UK) with 22 mT m–1 maximum strength gradients. Diagnostic MRI included axial diffusion-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR) and gradient echo sequences (details available on request).
All patients had six-field retinal photography (centered on the disk, macula, lateral macula, nasal to the disk, upper arcade, and lower arcade) of the left and right eyes, with 1% tropicamide eye drops where necessary, using a digital retinal camera (CR-DGi; Canon USA Inc., Lake Success, NY).
MRI analysis.
All MRI scans were coded for the presence, location, and size of the recent infarct and any old infarcts or hemorrhages. A recent infarct was a hyperintense area on diffusion imaging with corresponding reduced signal on apparent diffusion coefficient with or without increased signal on FLAIR or T2-weighted imaging, in a distribution compatible with an arterial territory. Lacunar infarcts were in the cerebral hemispheric white matter, basal ganglia, or brain stem and <2 cm diameter if recent (subcortical lesions >2 cm were classed as striatocapsular or cortical because they are caused by large artery disease).
Retinal image analysis.
All retinal images were analyzed blind to clinical and brain imaging features. Each color retinal image was stored within OptoMize® (Digital Healthcare, Cambridge, UK) software as tagged image file format files.
For measurement of arteriovenous ratio (AVR), central retinal artery equivalent (CRAE), and central retinal vein equivalent (CRVE), retinal images were analyzed using a custom-written validated image analysis program (within MatLab: The MathWorks, Natick, MA). Each image was processed and converted to grayscale. Left and right eye vessel widths are highly correlated,17 and we randomly chose one image centered on the optic disk from one eye from each patient. The software drew a circle delineating the optic disk. The grader identified the six largest arterioles and venules passing through a zone between half and one disk diameter away from the border of the disk and on each vessel identified two points between which the software measured the vessel profile at multiple (5–20) locations (figure) with microdensitrometry. The vessel width was calculated for each profile as the width of the intensity profiles at half the height of the intensity profile peak.18 We validated this process with Bland–Altman plots comparing software performance to best human measurement (with a caliper on images that had been enlarged) and found no evidence of systematic bias and a mean difference between human and software measurements for 50 randomly chosen vessels of 0.006 pixels (95% confidence interval [CI] −3.3 to 3.3 pixels).
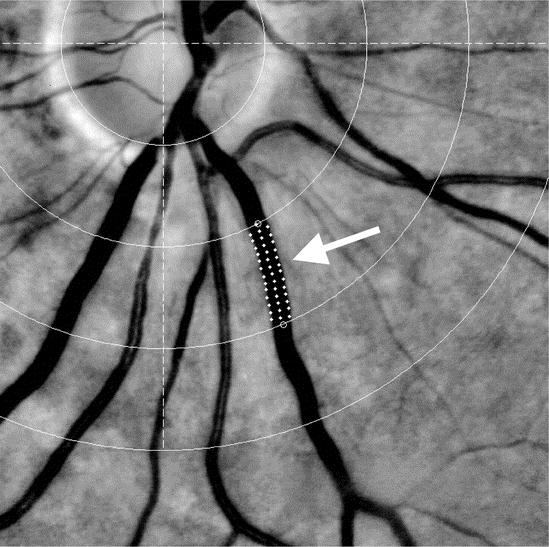
Figure Grayscale retinal photograph showing lower half of optic disk and surrounding arterioles and venules showing vessel tracking and width measurement technique (white arrow)
We summarized the widths of the six largest arterioles by producing a CRAE using a previously described formula which accounts for asymmetry of branching vessels.19 We also summarized the widths of the six largest venules to produce a CRVE and calculated the AVR as CRAE divided by CRVE.
To convert the pixel measurements obtained from the image to absolute measurements (microns), we assumed that the average disk diameter was 1,850 μm.20 We used our cohort mean disk diameter of 381 pixels and multiplied all of our pixel measurements by 4.855 (1,850/381) to convert pixels to microns. In a randomly chosen sample of 20 retinal images, the intraclass correlation coefficients for intrarater reliability (assessments 1 month apart) were excellent (0.94 for CRAE, 0.98 for CRVE, and 0.91 for AVR). Using a second grader (T.J.M.), the intraclass correlation coefficients for interrater reliability were excellent (0.95 for CRAE, 0.89 for CRVE, and 0.90 for AVR).
A physician (F.N.D.) specifically trained in retinal vessel assessment coded the presence (no, questionable, yes) and severity (mild, moderate, severe) of AVN and presence (no, questionable, yes) and severity (mild, severe) of FAN. We defined AVN as a reduced width of a venule on either side of an arteriole where the arteriole crossed the venule and FAN as a focal length of narrowing to at least two-thirds of the width of the proximal and distal arteriole.20 All questionable lesions were graded (present, not present) by an ophthalmologist with a specialist interest in retinal disease. This method of assessment has intrarater κ scores of 0.87 for AVN and 0.80 for FAN.21
Statistical analysis.
We compared baseline characteristics between the lacunar and cortical stroke groups with the Student t test, χ2 test for association, and Fisher exact test. CRAE, CRVE, and AVR were normally distributed, and we compared unadjusted CRAE, CRVE, and AVR between groups with the Student t test. We performed multiple linear regression analysis with stroke subtype, age, and vascular risk factors as explanatory variables and CRAE, CRVE, and AVR as the dependent variable. CRAE and CRVE were covariates and were therefore modeled together. We dichotomized FAN and AVN data into present vs absent and used binary logistic regression to assess differences between stroke subtypes, correcting for vascular risk factors. We used odds ratios (ORs) with 95% CIs to examine associations between retinal and other features. All analysis was performed with Minitab (version 14; Minitab Inc., State College, PA). Sample size calculation based on existing literature on retinal findings in stroke4 suggested that 197 patients would be needed to detect a difference of prevalence of FAN or AVN of 10% with 80% power at the 0.05 significance level between stroke subtypes.
RESULTS
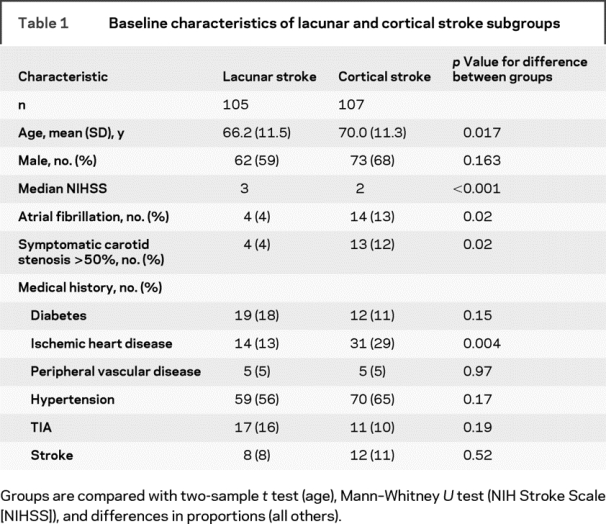
We recruited 220 patients. Eight were excluded (6 had poor-quality photographs; 2 had missing photographs), leaving 212 for analysis of FAN and AVN and 206 with photographic quality permitting analysis of vessel widths. Of the 212 patients, there were 105 lacunar strokes and 107 cortical strokes. The mean age was 68.1 years (SD 11.5 years), and the median NIHSS score was 2 (interquartile range 2–3) (table 1). The lacunar stroke patients were younger, with marginally higher stroke severity and lower rates of atrial fibrillation, symptomatic carotid stenosis >50% NASCET, and ischemic heart disease than cortical strokes.
Table 1 Baseline characteristics of lacunar and cortical stroke subgroups
Vessel widths.
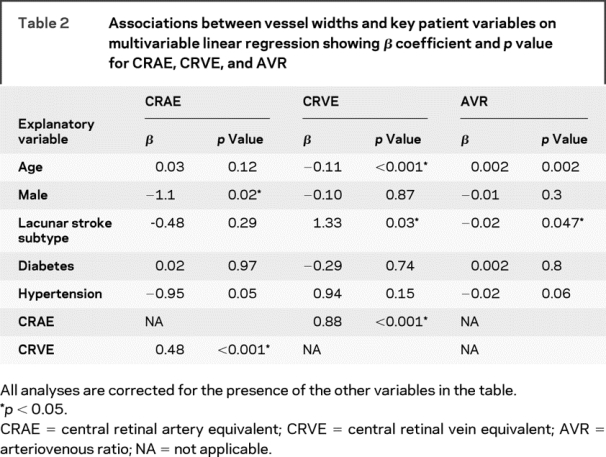
Two hundred six patients contributed to this analysis. For the total study population, the mean CRAE was 33.47 pixels (162 μm), the mean CRVE was 43.87 pixels (212 μm), and the mean AVR was 0.77. CRAE and CRVE were correlated with a Pearson correlation coefficient of 0.66. Patients with lacunar stroke had increased CRVE (44.9 [SD 5.5] vs 42.9 [SD 6.4] pixels, p = 0.01), decreased AVR (0.76 [SD 0.1] vs 0.78 [SD 0.1] pixels, p = 0.03), and similar CRAE compared with patients with cortical stroke (33.2 [SD 4.4] vs 33.7 [SD 4.3] pixels, p = 0.4). Table 2 shows the adjusted associations with arteriolar and venular widths. Increased CRVE was significantly and independently associated with lacunar stroke subtype, younger age, and increased CRAE (after correcting for the presence of diabetes, hypertension, and sex). Increased CRAE was significantly and independently associated with female sex and increased CRVE (correcting for age, diabetes, hypertension, and stroke subtype). Decreased AVR was significantly and independently associated with lacunar stroke subtype and younger age (correcting for diabetes, hypertension, and sex).
Table 2 Associations between vessel widths and key patient variables on multivariable linear regression showing β coefficient and p value for CRAE, CRVE, and AVR
We performed a sensitivity analysis excluding patients with a history of stroke and found that in 186 patients the results were similar to those presented in table 2, although the association between increased venular width and lacunar stroke subtype was strengthened (data not shown).
Arteriovenous nicking and focal arteriolar narrowing.
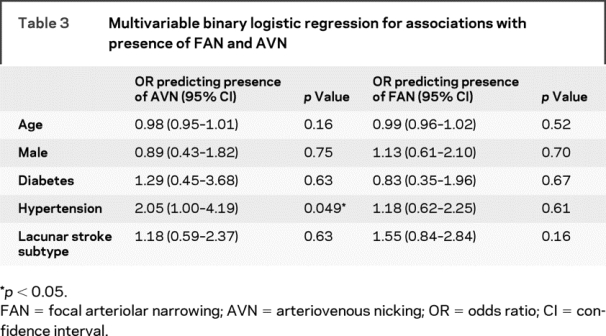
Two hundred twelve patients contributed to this analysis. AVN was present in 83 of 107 patients (78%) with cortical stroke and 85 of 105 patients (81%) with lacunar stroke (difference = 3%, 95% CI −7% to 14%, p = 0.53). FAN was present in 28 of 107 patients (26%) with cortical stroke and 37 of 105 patients (35%) with lacunar stroke (difference = 9%, 95% CI −3% to 21%, p = 0.15). The distributions of FAN and AVN by stroke subtype are given in table e-1 on the Neurology® Web site at www.neurology.org. We performed binary logistic regression to adjust for the baseline risk factor and age imbalances between the cortical and lacunar stroke groups with FAN or AVN as the dependent variable (dichotomized to present or absent). Only hypertension independently predicted the presence of AVN; no risk factors were independently associated with FAN (table 3). Excluding patients with a history of stroke did not alter these results.
Table 3 Multivariable binary logistic regression for associations with presence of FAN and AVN
DISCUSSION
Retinal microvessel morphology differs between lacunar and cortical ischemic stroke subtypes. Increased retinal venular diameters and decreased AVR are both independently associated with lacunar rather than cortical stroke subtype. These results suggest that there may be a distinct small vessel vasculopathy in cerebral small vessel disease. We have not demonstrated a strong association between retinal arteriolar diameter, FAN, or AVN and lacunar stroke subtype.
The cross-sectional design means that we can only report on associations, so we do not know whether these retinal vessel differences are long-standing and predispose to the lacunar phenotype, or are acquired in later life as part of the lacunar disease. Nor can we tell whether they are causative or associative. The parity of arteriolar measurements (width, AVN, and FAN) between stroke subtypes coupled with associations between hypertension and AVN and decreased arteriolar width suggest that arteriolar features reflect exposure to vascular risk factors. In response to these risk factors, some patients may develop lacunar and others a large artery (cortical) stroke phenotype.
No previous studies have compared quantitative vessel width measurement, AVN, or FAN between ischemic stroke subtypes. Previous studies did not subtype stroke and compared retinal arteriolar parameters between subjects with stroke and those without.4 Those that combined arteriolar and venular diameters into the dimensionless AVR produced conflicting results about associations with both future stroke and history of stroke.6–8,22 Changes in AVR were previously thought to reflect only arteriolar narrowing,20 known to change in response to systemic disease, but it is now known that venular diameter also varies.23 Those studies that analyzed arteriolar and venular widths separately found that the increase in AVR predicting the presence of any stroke (of any subtype) compared with absence of stroke was due to venular widening rather than arteriolar narrowing.6,11,24
Reports vary regarding the associations between AVN and FAN and any stroke,8–10,22,25 which may reflect methodologic difficulties in identifying stroke. Studies that showed an association with stroke tended to use MRI-based definitions of cerebral infarct rather than clinical definitions. It is uncertain what “holes” in the brain represent,12 and stroke diagnosis based on case-record assessment is of limited accuracy.26 We did not find differences in retinopathy between lacunar and cortical stroke27 despite many previous reports of associations between retinopathy and stroke.4 The lack of differences in arteriolar changes between stroke subtypes in our study could be because the retinal arteriolar circulation does not reflect cerebrovascular disease or because the arteriolar differences were too small to identify in this study. Alternatively retinal features may simply reflect exposure to systemic risk factors and are not specific for small vs large artery disease.
Two population-based studies showed that increased venular diameter predicted future risk of any clinical stroke.6,24 One study found larger CRVE in subjects with lacunes seen on MRI.11 Increased venular width is associated with plasma markers of inflammation28–30 and decreased arteriolar oxygen saturation levels.31 Thus, increased venular diameter could reflect an inflammatory component in patients with lacunar stroke.
The strengths of the present study are the subtyping of ischemic stroke, the use of cortical stroke patients to control for potential confounding risk factors, and that stroke was diagnosed by a stroke expert with MR at presentation. We maintained careful blinding of brain and retinal images to each other and to clinical features throughout. We used detailed retinal photos, careful training of retinal graders, and validated assessment methods. CRAE and CRVE are correlated; therefore, models assessing multivariable associations with either need to correct for the other, as we did.32 To minimize confounding of stroke subtyping, we performed a sensitivity analysis excluding patients with a history of previous stroke, which did not change the results for the total study population. A larger sample size might be needed to show differences in arteriolar diameters, FAN, and AVN. We have also used a concrete definition of small vessel disease, i.e., clinically evident lacunar stroke with best available imaging backup rather than a purely imaging- or clinical-based definition or one with uncertain relevance even to stroke.
In summary, we have shown that retinal venules are wider in patients with lacunar stroke compared with patients with cortical stroke. This suggests that there may be a distinct vasculopathy causing lacunar stroke.
AUTHOR CONTRIBUTIONS
F.N. Doubal conducted all statistical analysis.
ACKNOWLEDGMENT
Brain imaging took place in the SFC Brain Imaging Research Centre (www.sbirc.ac.uk). Retinal photographs were taken in the Wellcome Trust Clinical Research Facility, Western General Hospital, Edinburgh.
Supplementary Material
Address correspondence and reprint requests to Fergus N. Doubal, Bramwell Dott Building, Western General Hospital, Crewe Road, Edinburgh EH4 2XU, UK fergus.doubal@ed.ac.uk
Supplemental data at www.neurology.org
F.N.D. is funded by the Wellcome Trust (075611). The Chief Scientists Office (Scotland) funded the brain imaging (CZB-4-281).
Disclosure: The authors report no disclosures.
Medical Devices: 1.5T Signa Horizon MR/I 1.5T HDX scanner (operating under a research collaboration with GE Medical Systems, Milwaukee, WI, operating as IGE in the UK); digital retinal camera (CR-DGi; Canon USA Inc., Lake Success, NY).
Received September 11, 2008. Accepted in final form February 18, 2009.
REFERENCES
- 1.Sudlow CLM, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Stroke 1997;28:491–499. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry 2005;76:617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005;206:319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doubal FN, Hokke P, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry 2009;80:158–165. [DOI] [PubMed] [Google Scholar]
- 5.Baker ML, Hand PJ, Wang JJ, Wong TY. Retinal signs and stroke: revisiting the link between the eye and brain. Stroke 2008;39:1371–1379. [DOI] [PubMed] [Google Scholar]
- 6.Ikram MK, De Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology 2006;66:1339–1343. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet 2001;358:1134–1140. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology 2005;65:1005–1009. [DOI] [PubMed] [Google Scholar]
- 9.Longstreth W Jr, Larsen EK, Klein R, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol 2007;165:78–84. [DOI] [PubMed] [Google Scholar]
- 10.Cooper LS, Wong TY, Klein R, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke 2006;37:82–86. [DOI] [PubMed] [Google Scholar]
- 11.Ikram MK, De Jong FJ, Van Dijk EJ, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain 2006;129:182–188. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM. What is a lacune? Stroke 2008;39:2921–2922. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 14.Bamford J, Sandercock P, Dennis M, Warlow C, Burn J. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521–1526. [DOI] [PubMed] [Google Scholar]
- 15.Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. How well does the Oxfordshire Community Stroke Project classification predict the site and size of the infarct on brain imaging? J Neurol Neurosurg Psychiatry 2000;68:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–453. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004;111:1183–1190. [DOI] [PubMed] [Google Scholar]
- 18.Newsom RSB, Sullivan PM, Rassam SMB, Jagoe R, Kohner EM. Retinal vessel measurement: comparison between observer and computer driven methods. Graefes Archiv Clin Exp Ophthalmol 1992;230:221–225. [DOI] [PubMed] [Google Scholar]
- 19.Patton N, Aslam T, MacGillivray T, Dhillon B, Constable I. Asymmetry of retinal arteriolar branch widths at junctions affects ability of formulae to predict trunk arteriolar widths. Invest Ophthalmol Vis Sci 2006;47:1329–1333. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999;106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 21.Sherry LM, Wang JJ, Rochtchina E, et al. Reliability of computer-assisted retinal vessel measurement in a population. Clin Exp Ophthalmol 2002;30:179–182. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Sharrett AR, Klein BEK, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol 2000;20:1644–1650. [DOI] [PubMed] [Google Scholar]
- 23.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004;45:2129–2134. [DOI] [PubMed] [Google Scholar]
- 24.Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med 2006;166:2388–2394. [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the Cardiovascular Health Study. Ophthalmology 2003;110:658–666. [DOI] [PubMed] [Google Scholar]
- 26.Piriyawat P, Smajsova M, Smith MA, et al. Comparison of active and passive surveillance for cerebrovascular disease: the Brain Attack Surveillance in Corpus Christi (BASIC) project. Am J Epidemiol 2002;156:1062–1069. [DOI] [PubMed] [Google Scholar]
- 27.Doubal FN, Dhillon B, Dennis MS, Wardlaw JM. Retinopathy in ischemic stroke subtypes. Stroke 2009;40:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong TY, Islam FMA, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006;47:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R, Klein BEK, Knudtson MD, Wong TY, Tsai MY. are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 2006;124:87–94. [DOI] [PubMed] [Google Scholar]
- 30.de Jong FJ, Ikram MK, Witteman JC, Hofman A, de Jong PT, Breteler MM. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol 2007;61:491–495. [DOI] [PubMed] [Google Scholar]
- 31.de Jong FJ, Vernooij MW, Ikram MK, et al. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters: the Rotterdam Study. Ophthalmology 2008;115:887–892. [DOI] [PubMed] [Google Scholar]
- 32.Liew G, Sharrett AR, Kronmal R, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci 2007;48:52–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


