Abstract
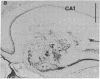
Fetal rat brain cell suspensions prepared from either the locus coeruleus region or hippocampus were implanted bilaterally into the subcortically denervated seizure-prone hippocampus of adult rats. Animals with locus coeruleus grafts were protected against picrotoxin-induced behavioral seizures and had significantly fewer interictal spikes. In contrast, in rats with fetal hippocampal grafts the incidence of interictal spikes was significantly higher than in lesion-only controls, and spontaneous behavioral seizures occurred in almost half of the animals. We suggest that neuronal grafting offers an alternative method for studying the mechanisms and control of epileptic brain activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985 Oct 1;240(1):37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Arnold P. S., Racine R. J., Wise R. A. Effects of atropine, reserpine, 6-hydroxydopamine, and handling on seizure development in the rat. Exp Neurol. 1973 Aug;40(2):457–470. doi: 10.1016/0014-4886(73)90087-3. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C., Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978 Jun 1;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Babb T. L., Carr E., Crandall P. H. Analysis of extracellular firing patterns of deep temporal lobe structures in man. Electroencephalogr Clin Neurophysiol. 1973 Mar;34(3):247–257. doi: 10.1016/0013-4694(73)90252-6. [DOI] [PubMed] [Google Scholar]
- Barry D. I., Kikvadze I., Brundin P., Bolwig T. G., Björklund A., Lindvall O. Grafted noradrenergic neurons suppress seizure development in kindling-induced epilepsy. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8712–8715. doi: 10.1073/pnas.84.23.8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Nornes H., Gage F. H. Cell suspension grafts of noradrenergic locus coeruleus neurons in rat hippocampus and spinal cord: reinnervation and transmitter turnover. Neuroscience. 1986 Jul;18(3):685–698. doi: 10.1016/0306-4522(86)90063-1. [DOI] [PubMed] [Google Scholar]
- Björklund A., Segal M., Stenevi U. Functional reinnervation of rat hippocampus by locus coeruleus implants. Brain Res. 1979 Jul 20;170(3):409–426. doi: 10.1016/0006-8993(79)90961-2. [DOI] [PubMed] [Google Scholar]
- Buzsàki G., Czopf J., Kondàkor I., Björklund A., Gage F. H. Cellular activity of intracerebrally transplanted fetal hippocampus during behavior. Neuroscience. 1987 Sep;22(3):871–883. doi: 10.1016/0306-4522(87)92966-6. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Gage F. H., Kellényi L., Björklund A. Behavioral dependence of the electrical activity of intracerebrally transplanted fetal hippocampus. Brain Res. 1987 Jan 6;400(2):321–333. doi: 10.1016/0006-8993(87)90631-7. [DOI] [PubMed] [Google Scholar]
- CRANDALL P. H., WALTER R. D., RAND R. W. CLINICAL APPLICATIONS OF STUDIES ON STEREOTACTICALLY IMPLANTED ELECTRODES IN TEMPORAL-LOBE EPILEPSY. J Neurosurg. 1963 Oct;20:827–840. doi: 10.3171/jns.1963.20.10.0827. [DOI] [PubMed] [Google Scholar]
- Corcoran M. E., Fibiger H. C., McCaughran J. A., Jr, Wada J. A. Potentiation of amygdaloid kindling and metrazol-induced seizures by 6-hydroxydopamine in rats. Exp Neurol. 1974 Oct;45(1):118–133. doi: 10.1016/0014-4886(74)90105-8. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Ayala G. F. Cellular mechanisms of epilepsy: a status report. Science. 1987 Jul 10;237(4811):157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 1980 Aug;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M., Zimmer J. GABAergic nonpyramidal neurons in intracerebral transplants of the rat hippocampus and fascia dentata: a combined light and electron microscopic immunocytochemical study. J Comp Neurol. 1987 May 8;259(2):266–276. doi: 10.1002/cne.902590207. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. 1983 Mar 31-Apr 6Nature. 302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Hoch D. B., Dingledine R. GABAergic neurons in rat hippocampal culture. Brain Res. 1986 Feb;390(1):53–64. doi: 10.1016/0165-3806(86)90151-3. [DOI] [PubMed] [Google Scholar]
- Hodgson A. J., Penke B., Erdei A., Chubb I. W., Somogyi P. Antisera to gamma-aminobutyric acid. I. Production and characterization using a new model system. J Histochem Cytochem. 1985 Mar;33(3):229–239. doi: 10.1177/33.3.3973378. [DOI] [PubMed] [Google Scholar]
- Hoffer B., Seiger A., Freedman R., Olson L., Taylor D. Electrophysiology and cytology of hippocampal formation transplants in the anterior chamber of the eye. II. Cholinergic mechanisms. Brain Res. 1977 Jan 1;119(1):107–132. doi: 10.1016/0006-8993(77)90094-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Kosaka T. Axon initial segments of the granule cell in the rat dentate gyrus: synaptic contacts on bundles of axon initial segments. Brain Res. 1983 Sep 5;274(1):129–134. doi: 10.1016/0006-8993(83)90527-9. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986 Mar;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre D. C., Racine R. J. Kindling mechanisms: current progress on an experimental epilepsy model. Prog Neurobiol. 1986;27(1):1–12. doi: 10.1016/0301-0082(86)90010-9. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENFIELD W., BALDWIN M. Temporal lobe seizures and the technic of subtotal temporal lobectomy. Ann Surg. 1952 Oct;136(4):625–634. [PMC free article] [PubMed] [Google Scholar]
- Peterson S. L., Albertson T. E. Neurotransmitter and neuromodulator function in the kindled seizure and state. Prog Neurobiol. 1982;19(4):237–270. doi: 10.1016/0301-0082(82)90008-9. [DOI] [PubMed] [Google Scholar]
- Prince D. A. Neurophysiology of epilepsy. Annu Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- Ribak C. E. Axon terminals of GABAergic chandelier cells are lost at epileptic foci. Brain Res. 1985 Feb 11;326(2):251–260. doi: 10.1016/0006-8993(85)90034-4. [DOI] [PubMed] [Google Scholar]
- Schmidt R. H., Ingvar M., Lindvall O., Stenevi U., Björklund A. Functional activity of substantia nigra grafts reinnervating the striatum: neurotransmitter metabolism and [14C]2-deoxy-D-glucose autoradiography. J Neurochem. 1982 Mar;38(3):737–748. doi: 10.1111/j.1471-4159.1982.tb08693.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978 May 19;147(1):117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. II. Activation of the input pathway. Brain Res. 1974 May 31;72(1):99–114. doi: 10.1016/0006-8993(74)90653-2. [DOI] [PubMed] [Google Scholar]
- Seress L., Ribak C. E. A substantial number of asymmetric axosomatic synapses is a characteristic of the granule cells of the hippocampal dentate gyrus. Neurosci Lett. 1985 May 1;56(1):21–26. doi: 10.1016/0304-3940(85)90434-3. [DOI] [PubMed] [Google Scholar]
- Spencer S. S. Depth electroencephalography in selection of refractory epilepsy for surgery. Ann Neurol. 1981 Mar;9(3):207–214. doi: 10.1002/ana.410090302. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. The connections of the septal region in the rat. J Comp Neurol. 1979 Aug 15;186(4):621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982 May 14;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Traub R. D. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2-CA3 region. J Neurophysiol. 1983 Feb;49(2):442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]
- Wyss J. M., Swanson L. W., Cowan W. M. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979;4(4):463–476. doi: 10.1016/0306-4522(79)90124-6. [DOI] [PubMed] [Google Scholar]