Abstract
The current study examined the impact of comorbidity on cognitive and behavioral therapies for generalized anxiety disorder (GAD) as well as the impact of these therapies on diagnoses comorbid to GAD. Seventy-six treatment-seeking adults with principal diagnoses of GAD received 14 sessions of therapy. Most (n = 46; 60.5%) of the sample had at least one comorbid diagnosis. Although the presence of comorbid diagnoses was associated with greater severity of GAD symptoms at pretreatment, greater severity of comorbid major depression, simple phobia, and social phobia was associated with greater change in symptoms of GAD in response to treatment, with no effect on maintenance of gains during a 2-year follow-up. Further, psychotherapy for principal GAD led to a reduction in number of comorbid diagnoses and in severity of social phobia, simple phobia, and major depression at posttreatment. At 2-year follow-up severity of social and simple phobia remained below pretreatment levels, whereas severity of depression was no longer significantly below pretreatment levels. These results suggest that although people with comorbid disorders enter treatment with more severe GAD symptomatology, they demonstrate greater change, and therefore such comorbidity does not diminish the efficacy of cognitive and behavioral therapies for GAD. In addition, the impact of these treatments for GAD may generalize to reduced severity of simple phobia, social phobia, and major depression; however, gains in severity of major depression are not maintained.
Most clients with a principal anxiety disorder meet criteria for at least one additional current anxiety or mood disorder (Brown & Barlow, 1992; Brown, Campbell, Lehman, Grisham, & Mancill, 2001). Such comorbidity is particularly prevalent in generalized anxiety disorder (GAD), with rates ranging from 45% to 98% (Goisman, Goldenberg, Vasile, & Keller, 1995). High comorbidity rates in people with GAD are particularly problematic because of the association between current comorbidity and a number of indices of severity and cost. For example, persons with comorbidity in addition to GAD report more disability days (Hunt, Issakidis, & Andrews, 2002), more interference from symptoms of GAD (Wittchen, Zhao, Kessler, & Eaton, 1994), and increased likelihood of interpersonal problems (Judd et al., 1998). In addition, they are more likely to seek professional help for GAD (Bland, Newman, & Orn, 1997; Mojtabai, Olfson, & Mechanic, 2002; Wittchen et al., 1994) and pose a greater risk of suicide (Boden, Fergusson, & Horwood, 2007; Rudd, Dahm, & Rajab, 1993). Moreover, compared to those with pure GAD, those with current comorbid disorders report more hospitalizations, visits to the emergency room, consultations with medical specialists, laboratory tests, medication use, and work absenteeism (Souetre et al., 1994; Wittchen et al.). Thus, these individuals have more direct and indirect costs than those with pure GAD (Marciniak et al., 2005; Souetre et al., 1994).
Despite the consistent finding of a link between current comorbidity and severity, studies of the influence of comorbidity on the course of principal GAD have been equivocal. Whereas some findings suggest that comorbidity with GAD is unrelated to persistence of the disorder (Kessler et al., 1994; Kessler et al., 2007; Rogers et al., 1999; Yonkers, Dyck, Warshaw, & Keller, 2000), others have found such a relationship with some types of comorbidity. For instance, Kessler et al. (2002) found that simple phobia predicted persistence of GAD, and data from the Harvard/Brown Anxiety Disorders Research Program showed that comorbid major depressive disorder (MDD) or panic disorder predicted decreased likelihood of remission from GAD (Bruce, Machan, Dyck, & Keller, 2001; Bruce et al., 2005).
Studies on the influence of comorbidity on the outcomes of psychotherapy for principal GAD are equivocal. On the one hand, current Axis I comorbidity predicted poorer initial outcome as well as greater relapse from cognitive therapy, analytic therapy, or anxiety management training (Durham, Allan, & Hackett, 1997). Similarly, comorbid MDD predicted reduced responsiveness to brief psychodynamic or supportive nondirective therapy (Crits-Christoph et al., 2004). On the other hand, comorbid panic disorder did not predict outcome at posttreatment or 6-month follow-up for anxiety management training (Butler & Anastasiades, 1988), and the presence of current comorbidity predicted improved outcome from group cognitive behavioral therapy (CBT) for older adults (Wetherell et al., 2005). Thus, it is difficult to draw firm conclusions about the impact of comorbidity on psychotherapy outcome.
In addition to the equivocal findings regarding the impact of comorbidity on psychotherapy outcome for GAD, only one study has examined the impact of psychotherapy for GAD on comorbid disorders. In a sample of clients who received either supportive listening, applied relaxation, or CBT, psychotherapy led to a significant reduction in the number of comorbid diagnoses, especially among clients who no longer met diagnostic criteria for GAD at posttherapy and follow-up assessments (Borkovec, Abel, & Newman, 1995).
For each of these studies, the conclusions that can be drawn are limited. The first study (Durham et al., 1997) included no participants with comorbid MDD, and 66% of the sample was taking psychotropic medication with no requirement that medications be stabilized during the treatment period. Butler and Anastasiades (1988) excluded people who had chronic levels of GAD, comorbid OCD, or comorbid MDD, and provided no information or controls for concurrent medication. In addition, these authors did not examine the predictive value of comorbid diagnoses other than panic disorder. Crits-Christoph and colleagues (2004) examined the prediction of outcome from a brief psychodynamic treatment and findings from this study may not generalize to CBT. Similarly, given that Wetherell and colleagues (2005) examined the impact of group CBT with older adults, their results may not be applicable to younger adults receiving individual CBT. Moreover, only one study has examined the impact of treatment for GAD on the presence of comorbid diagnoses (Borkovec et al., 1995), and no studies have included a follow-up period longer than 1 year. A longer follow-up period allows for a longer-term examination of maintenance of gains.
The goals of the present study were to examine (a) the impact of comorbidity on outcome of individual cognitive and behavioral therapies for adults between the ages of 18 and 65 with GAD and (b) the impact of the therapies on comorbid diagnoses to determine whether results from Borkovec et al. (1995) could be replicated. This is a secondary analysis of data from a published outcome study that compared CBT to purely cognitive and purely behavioral therapies (Borkovec, Newman, Pincus, & Lytle, 2002).
There are several notable differences between the current study and prior psychotherapy studies of comorbidity and primary GAD. In the current study, only two participants were on psychotropic medications, and these medications were stabilized prior to study entry and during the course of therapy. Also, in contrast to Butler and Anastasiades (1988), we did not exclude people with chronic levels of GAD. In addition, whereas participants with MDD were not included in most prior studies (Borkovec et al., 1995; Butler & Anastasiades; Durham et al., 1997), the current study excluded only those with severe depression. Moreover, the present study included a 2-year follow-up. Thus, the current study tested the impact of comorbidity over a longer posttreatment follow-up period than has been previously tested in a sample of clients with principal GAD as well as the impact of psychotherapy for GAD on comorbid MDD.
Given that the two studies using CBT for the whole sample (Butler & Anastasiades, 1988; Wetherell et al., 2005) found no detrimental effect of comorbidity, we hypothesized that although comorbidity would be associated with a number of indices of severity, there would be no detrimental effect of pretreatment comorbidity on outcome for GAD. Based on the findings of Borkovec et al. (1995), we also predicted that psychotherapy for GAD would significantly reduce the presence, number, and clinical severity of comorbid anxiety and mood disorders.
Method
Participants
Four hundred fifty-nine people responded to advertisements in local newspapers or referrals from mental health practitioners. Of these, 320 were ruled out by phone screens for not meeting study inclusion criteria, 54 clients were ruled out via an initial structured interview, and 9 clients were ruled out during a second structured interview, leaving 76 participants with principal GAD who entered treatment. Average age was 36.62 years (SD = 11.56), and average duration of GAD was 12.28 years (SD = 11.87). Clients were mostly Caucasian (89.5%) and women (68.4%). The study included two African American (2.6%), three Hispanic (3.9%), and three Middle Eastern clients (3.9%). Only two clients were taking psychotropic medications for anxiety; they agreed to maintain dosage and frequency during therapy with their physician’s approval. All of these characteristics were nearly equally distributed among treatment conditions and were not significantly different across conditions. All participants consented to the study, and IRB approval was attained.
Procedure
Selection and Assessor Outcome Ratings
Admission criteria included consensus between the two diagnostic interviewers on a principal diagnosis of GAD based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R; American Psychiatric Association, 1987), no diagnosable panic disorder (as recommended by the funding agency’s review committee),1 a Clinician’s Severity Rating (CSR) for GAD of 4 (moderate) or more, absence of concurrent psychosocial therapy, no history of having received prior CBT, no medical contributions to the anxiety, no antidepressant medication, and absence of severe MDD, substance abuse, psychosis, and organic brain syndrome. All but two clients (97.1%) met both DSM-III-R and DSM-IV (American Psychiatric Association, 1994) criteria for GAD.2
Clinical assessors (advanced clinical graduate students trained to reliability in diagnostic interviewing) administered a modified version of the Anxiety Disorders Interview Schedule-III-R (ADIS-R; Di Nardo & Barlow, 1988), which included the Hamilton Anxiety Rating Scale (HARS; Hamilton, 1959), Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), CSRs for GAD and comorbid disorders, and additional questions in the section for GAD corresponding to two criteria being proposed at the time of study initiation by the DSM-IV subcommittee for GAD (i.e., uncontrollable worrying and three of six associated symptoms).
Data suggest that inconsistent reporting on the part of the client is the most common contributing factor to low reliability in the diagnosis of GAD (Brown, Di Nardo, Lehman, & Campbell, 2001). Therefore, a second ADIS-R was administered within 2 weeks by the therapist who would see the client in therapy to reduce the likelihood of false positive cases. The second assessor only interviewed participants who had received a principal diagnosis of GAD from the first assessor. The second assessor was asked to independently judge the presence of GAD and comorbid diagnoses prior to meeting with the first assessor to develop consensus. Of the 54 people ruled out at first ADIS-R, 15 (27.8%) were ruled out because they failed to meet diagnostic criteria for GAD, and 28 (51.9%) were ruled out because they met criteria for another principal diagnosis. Nine individuals (16.7%) were ruled out because they met criteria for panic disorder. In addition, 1 person was ruled out because of an unwillingness to complete any paperwork, and another was ruled out for receiving concurrent psychotherapy. Of the 9 people ruled out following the second ADIS-R, 6 (66.7%) did not meet diagnostic criteria for GAD, and 3 (33.3%) were ruled out because GAD was not the principal diagnosis.
A briefer version of the ADIS-R (assessing only those diagnoses identified at pretherapy) and the rating scales were readministered 10 to 14 days after the last therapy session and at 6- and 12-month follow-up assessments; the complete ADIS-R and rating scales were given at 24-month follow-up. The same assessor administering a pretreatment assessment to a client also administered the posttreatment assessment to that client; this was also the case at follow-up whenever possible. Assessors were kept uninformed of therapy condition and study hypotheses.
Pretreatment diagnoses, both principal and comorbid, were based on consensus between independent structured interviewers. For all other time points, diagnostic information was based on the ratings of the primary assessor. A random subsample of 20% of pretreatment audiotapes of the ADIS-R interviews conducted by the primary assessor (prior to developing consensus) was reviewed for reliability purposes. There was good to excellent agreement on the presence of comorbid diagnoses, with kappa coefficients ranging from .68 to 1.0, and very good agreement on CSRs of comorbid disorders, with intraclass correlations ranging from .77 to 1.0. For the presence of GAD, kappa was 1.0, and Finn’s r (Whitehurst, 1984), which corrects for a restricted range of CSRs for GAD, was .74.
Outcome Measures
Clinician’s Severity Rating (CSR)
This is one of the most commonly used outcome measures in psychotherapy trials for GAD (Fisher & Durham, 1999). For each diagnosis, interviewers assigned a 0-to-8 rating indicating their judgment of the degree of distress and interference in functioning associated with the disorder (0 = none to 8 = very severely disturbing/disabling). Clients who met criteria for any diagnosis were assigned a CSR of 4 (definitely disturbing/disabling) or higher (clinical diagnoses). When key features of a disorder were present but were not judged to be extensive or severe enough to warrant a formal diagnosis (or for disorders in partial remission), a CSR of 1 to 3 was assigned. When no features of a disorder were present, severity ratings of 0 were given. Brown and colleagues (Brown, Di Nardo, et al., 2001) demonstrated good to excellent interrater reliability for CSRs for anxiety and mood disorders except dysthymia (r = .36), with correlations ranging from .65 to .84.
State Trait Anxiety Inventory-Trait version (STAI-T; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983)
This 20-item scale is used to measure trait anxiety. Items are rated from 1 (almost never) to 4 (almost always) to indicate how subjects “generally feel.” Internal consistency reliability was shown to be high (in the .80s and .90s), and retest reliability was much higher for the trait form (high .70s) than the state form (from .27 to .54). Good convergent and discriminant validity has also been demonstrated (Spielberger et al., 1983).
Hamilton Anxiety Rating Scale (HARS; Hamilton, 1959)
This 14-item clinician-administered scale provides a rating of severity of each overarching anxiety symptom cluster on a scale from 0 (not present) to 4 (very severe/incapacitating). Estimates of internal consistency of the HARS range from adequate to good in one study (α = .77 to .81) (Moras, Di Nardo, & Barlow, 1992) to excellent (α = .92) in another (Kobak, Reynolds, & Greist, 1993). Retest reliability was ICC = .86 across 2 days, and interrater reliability ranged from an ICC of .74 to .96 (Bruss, Gruenberg, Goldstein, & Barber, 1994). HARS scores show strong convergent (Beck & Steer, 1991; Maier, Buller, Philipp, & Heuser, 1988) and discriminant validity (Kobak et al., 1993). A version with less overlap between anxiety and depressive symptomatology (Riskind, Beck, Brown, & Steer, 1987) was used in conjunction with the ADIS-R.
Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960)
The HRSD is a 17-item clinician-administered scale that assesses the severity of depressive symptomatology. Severity of each overarching symptom cluster is rated on a scale from 0 (not present) to 4 (very severe/incapacitating). Estimates for the internal consistency of the HRSD range from adequate to good (α = .73 to .81) (Hamilton, 1960; Moras et al., 1992; Riskind et al., 1987). The interrater reliabilities of the total score have been good, ranging from .78 to .82 (Moras et al.; Riskind et al.). The HRSD also shows strong convergent (Steer, McElroy, & Beck, 1983) and discriminant validity (Moras et al.).
Therapy Conditions
Fourteen weekly sessions were administered, with one fading/termination session after posttreatment assessment. Participants were randomly assigned to receive manualized self-control desensitization with applied relaxation (SCD), cognitive therapy (CT), or combined cognitive behavioral therapy (CBT). In all conditions, the first four sessions were 2 hours in duration; remaining sessions were 1.5 hours. The first 30 minutes of each SCD and CT session involved only supportive listening. The methodological purpose of supportive listening was to hold constant the total amount of treatment time while also holding constant the total amount of time devoted to cognitive or behavioral techniques. Therapists delivering CBT used the Borkovec and Costello (1993) manual. The SCD and CT manuals were adapted for this study based on the relevant components of the Borkovec and Costello CBT and nondirective (supportive listening) therapy manuals.
Several aspects were common to the three conditions, although their content differed according to assignment: presentation of a model of anxiety and rationale for therapy, self-monitoring and early identification of anxiety cues, homework assignments, and review of homework, including the results of daily self-monitoring and technique practice and applications. CT entailed cognitive therapy plus supportive listening. In addition to supportive listening, SCD entailed progressive, cue-controlled, and differential relaxation training as described in Bernstein and Borkovec (1973), slowed diaphragmatic breathing, relaxing imagery, meditational relaxation, applied relaxation training, and SCD as described by Goldfried (1971). CBT contained all of the treatment techniques in CT and SCD, except that no supportive listening element was included.
Results
Statistical Analyses
For categorical analyses, clients with at least one comorbid diagnosis of clinical severity were categorized as comorbid, whereas those with CSRs of 0 to 3 for comorbid diagnoses were categorized as noncomorbid.
To reduce the probability of Type I error, we created a single continuous outcome variable for symptoms of GAD, using a composite score of the STAI-T, the CSR for GAD, and the HARS. These are the most frequently used measures in the assessment of outcome of treatment for GAD (Fisher & Durham, 1999). Raw scores for each measure were converted into standardized z-scores and averaged for each participant.
To create a measure of clinically significant change, endstate functioning was calculated by summing the number of the above three outcome measures on which the client either fell within one standard deviation of the mean of nonanxious normative samples and more than a standard deviation from the mean of the anxious sample or a score that exceeded a face-valid level of meaningful change when norms were not available [a score of 2 (mild) or less on the 9-point CSR]. Norms from nonanxious participants for the HARS and STAI-T were based on Butler, Fennell, Robson, and Gelder (1991) and Spielberger et al. (1983), respectively. High endstate functioning was defined as meeting endstate criteria on at least two of the three measures. Responder status was defined as at least 20% change from pretherapy levels on at least two of the three measures.
Unless otherwise noted, linear mixed-effect models, sometimes labeled hierarchical linear modeling (HLM), were used to examine group differences (Raudenbush & Bryk, 2002). Mixed-effect models are ideal for analysis of longitudinal data because they take into account nesting inherent in the repeated measurement of the same individual over time. Random effects, estimates of within-individual variation, are incorporated into the model to allow for a more accurate estimation of fixed effects, systematic sources of variation in the dependent variable.
Various approaches were used in the construction of the mixed-effect models to follow. Therefore, specific details pertaining to the construction of each model are provided along with the accompanying analyses and results. In general, both random and fixed effects were determined with full maximum likelihood estimation. In all cases, the unit of measurement at Level 1 was time, and the unit of measurement at Level 2 was the individual. Random effects for variation at the intercept were entered first into each model, followed by examinations of possible random individual variation in slope, and finally fixed effects.
Mixed-effect models were constructed using a piecewise analysis of time, as opposed to a single time coefficient. Piecewise analyses allow for the representation of discrete multiple time periods by modeling separate variables (and therefore separate coefficients and slopes) for these periods. In the current study, the treatment and follow-up periods can be conceptualized as discrete and yet represented within the same model. Piece 1 was therefore conceptualized as the treatment period, from pretreatment to end-of-treatment. Piece 2 was conceptualized as the posttreatment period including 6, 12, and 24-month follow-ups. All analyses of time were conducted with months as the unit of measurement. Piece 1 was coded to allow for the retention of all observation points while holding posttreatment effects constant during the follow-up period. Piece 2 was coded to remove treatment period effects from the analysis of follow-up effects. Clarke, O’Campo, and Wheaton (2006) determined that HLM sample sizes of 5 or more avoid biased estimations of fixed effects and their accompanying standard errors within multilevel models. Therefore, when examining individual diagnoses, we excluded disorders for which we had fewer than 5 people of clinical severity (i.e., PTSD and agoraphobia).
Data were examined for skew and kurtosis. Averaged z-scores for symptoms of GAD as well as CSRs for most disorders were normally distributed at all time points. CSRs for MDD and dysthymia, as well as count data for number of comorbid disorders were found to be right skewed due to a proliferation of zeros. Appropriate analyses were used to model the distributions (as detailed in conjunction with the description of results).
There were no differences between the therapy conditions on any pretherapy assessment. In addition, the conditions did not differ in number of participants with comorbidity or the distribution of any specific comorbid diagnosis. Treatment credibility and expectancy also did not vary by treatment condition or by presence or absence of comorbidity. Also, there were no significant differences between conditions in treatment efficacy, and all three treatments led to significant improvements that were maintained over the 2-year follow-up period. Furthermore, the separate treatment conditions did not interact with the presence or severity of comorbidity. Therefore, analyses were conducted by collapsing across treatment conditions.
Comorbidity Patterns Before Treatment
Most of the sample (60.5%; n = 46) had at least one additional diagnosis of clinical severity at pretreatment. As shown in Table 1, the most frequent comorbid diagnosis was social phobia, followed by simple phobia, MDD, dysthymia, PTSD, and agoraphobia. Of those who had a comorbid diagnosis, most (78.3%; n = 36) participants had only one, 15.2% (n = 7) had two, and 6.5% (n = 3) had three or more.
Table 1. Breakdown of diagnostic status in percentages of the total sample at pretreatment.
| Diagnosis | % Diagnoses (#) |
|---|---|
| Panic disorder | 0 |
| Agoraphobia | 2.6% (2) |
| PTSD | 2.6% (2) |
| Social phobia | 43.4% (33) |
| Simple phobia | 11.8% (9) |
| Dysthymia | 7.9% (6) |
| MDD | 10.5% (8) |
| Any comorbid disorder | 60.5% (46) |
| No comorbid disorders | 39.5% (30) |
Note. PTSD = Posttraumatic stress disorder; MDD = major depressive disorder.
Pretreatment Differences Between Those With and Without Comorbidity
At pretreatment, participants with comorbidity had higher CSRs for GAD, t(74) = -3.18, p < .005, ηp2 = 0.12, CI.95 = 0.23, 1.00, and higher HARS scores, t(74) = -2.39, p = .02, ηp2 = 0.07, CI.95 = 0.58, 6.49, than did those without comorbidity (see Table 2). There were no differences between those with and without comorbidity on other dimensional measures of GAD.
Table 2. Means and standard deviations for outcome measures at all time points for comorbid and noncomorbid groups.
| Measure | Pre N = 76 |
Post N = 69 |
FU6 N = 67 |
FU12 N = 65 |
FU24 N = 63 |
|
|---|---|---|---|---|---|---|
| Comorbid | CSR | 5.79 (0.89) |
2.19 (1.23) |
2.05 (1.54) |
2.29 (1.77) |
2.39 (1.63) |
| Non-Comorbid | 5.16 (0.74) |
1.90 (0.97) |
1.99 (1.36) |
2.57 (1.29) |
2.30 (1.32) |
|
| Comorbid | HARS | 26.11 (6.76) |
9.63 (6.66) |
10.57 (7.24) |
12.33 (8.19) |
12.55 (7.77) |
| Non-Comorbid | 22.38 (5.67) |
8.84 (5.45) |
9.69 (6.08) |
11.87 (5.71) |
12.37 (6.31) |
|
| Comorbid | STAI-T | 58.82 (7.25) |
43.04 (9.42) |
42.46 (10.12) |
42.01 (10.29) |
42.83 (10.27) |
| Non-Comorbid | 56.11 (7.40) |
42.14 (11.21) |
43.21 (10.16) |
43.46 (8.65) |
43.37 (9.62) |
Note. Pre = pretreatment; Post = posttreatment; FU6 = 6-month follow-up; FU12 = 12-month follow-up; FU24 = 24-month follow-up; CSR = Clinician’s Severity Rating; HARS = Hamilton Anxiety Rating Scale; STAI-T = Trait Version of the State Trait Anxiety Inventory.
Attrition
Seven people dropped out early in the course of treatment. There were no significant differences between dropouts and completers in percentage taking medication (p = .84), ethnicity (p = .92), age (p = .22), or marital status (p = .81). Likewise, the difference between completers and dropouts in number of people with pretreatment comorbidity failed to reach significance, χ2(1, N = 76) = 3.30, p = .07; although there was a trend for those with comorbid disorders to be less likely to drop out. Whereas 63.8% (n = 44) of completers had comorbid diagnoses, only 28.6% (n = 2) of dropouts received comorbid diagnoses at pretreatment.
Impact of Pretreatment Comorbidity on GAD Treatment Outcome
A model was constructed contrasting participants with and without pretreatment comorbidity. Random effects for individual variation at the intercept and across slopes for Piece 1 and Piece 2 were entered into the model and found to be significant. Fixed effects consisted of those for Piece 1, Piece 2, comorbid status, and the interaction between comorbid status and Piece 1. Results demonstrated a significant difference in the rate of change in the composite measure of GAD symptoms over the course of treatment, such that those with comorbid diagnoses at pretreatment demonstrated a greater rate of change from pretreatment to posttreatment, β (Piece 1 × Comorbid Status) = -.089, SE = .038, t = -2.32, p < .05, compared to those without comorbid diagnoses. A t-test showed that there was no significant difference between those with and without comorbidity at posttreatment. There was a small but significant main effect for Piece 2, indicating an increase in severity of symptoms of GAD over the follow-up period, β = .008, SE = .004, t = 2.01, p < .05. However, the severity of symptoms of GAD at 24-month follow-up remained significantly below pretreatment levels, t(62) = -16.72, p < .001. We then examined whether pretreatment comorbidity predicted a differential course over the follow-up period. This effect was found to be nonsignificant, β (Piece 2 × Comorbid Status) = -.006, SE = .008, t = -.77, p = .47, indicating no significant difference between those with and without pretreatment comorbidity on the maintenance of gains over the follow-up period. Table 2 shows the means and standard deviations for the three outcome measures for those with and without comorbidity, across treatment and follow-up periods.
Given the dimensional nature of these disorders and the significant loss of information inherent in examining data at a categorical level, we used CSRs for each comorbid diagnosis to predict changes in severity of symptoms of GAD. A model was constructed with random effects for individual variation at the intercept as well as individual variation in slope for Piece 1 and Piece 2. Fixed effects for Piece 1 and Piece 2, CSRs for MDD, social phobia, simple phobia, and dysthymia, as well as the interactions between Piece 1 and each of the four comorbid diagnoses, were all entered into the model simultaneously. All fixed and random effects were significant with the exception of dysthymia, β (Piece 1 × Dysthymia Severity) = .022, SE = .013, t = 1.67, p = .16. Effects for other individual diagnoses during the treatment period were as follows: MDD, β (Piece 1 × MDD Severity) = -.021, SE = .009, t = -2.31, p < .05; simple phobia, β (Piece 1 × Simple Phobia Severity) = -.026, SE = .010, t = -2.63, p < .01; social phobia, β (Piece 1 × Social Phobia Severity) = -.014, SE = .007, t = -1.97, p < .05. For all three diagnoses, a greater pretreatment CSR for the comorbid disorder predicted a greater rate of change in the decrease of symptomatology of GAD over the treatment period. Once again, a small but significant main effect was found for Piece 2, β = .008, SE = .004, t = 2.15, p < .05, reflecting the overall increase in symptoms during the follow-up period. However, as noted above, level of symptoms of GAD at 24-month follow-up remained 1.43 standard deviations below that found at pretreatment.
Fixed effects for interactions between Piece 2 and CSRs for each specific diagnosis were added to the model indicated above, but all were nonsignificant, indicating that the severity of pretreatment comorbidity did not significantly predict the maintenance of treatment gains during the follow-up period.3
Effect of Treatment on Comorbid Diagnoses
A piecewise analysis using a quasi-Poisson distribution was conducted to examine the effect of treatment for GAD on number of comorbid diagnoses, first across the treatment period and then during follow-up. The quasi-Poisson distribution estimates a dispersion parameter, Φ, to model the variance greater than the mean, while allowing for the estimation of random variation at the individual level. The quasi-Poisson distribution has been shown to be an accurate extension of the generalized linear model for dependent variables containing rare outcomes above zero (Elston et al., 2001). This analysis demonstrated a significant decline in the number of comorbid diagnoses from pretreatment to posttreatment, β = -.58, SE = .07, t = -8.32, p < .001, and a small but significant increase from posttreatment across the follow-up period, β = .048, SE = .015, t = 3.13, p < .001. However, the number of comorbid disorders at 24-month follow-up (M = 0.25) remained significantly smaller than at pretreatment (M = 0.81), t(62) = 5.99, p < .001.
We also examined the impact of treatment for GAD on CSRs of individual comorbid diagnoses. As noted above, there was skewness in CSRs for MDD and dysthymia due to a proliferation of zeros in the data. To account for such a distribution we used the Tobit regression model (Tobin, 1958), which involves the designation of a threshold value, here zero, at or below which the data are considered to be censored. Within the Tobit model, the subthreshold values represent an unobservable extension of a continuous distribution.
Tobit regression models have been shown to provide superior estimates of coefficients and standard errors compared to ordinary least squares regression (Austin, Escobar, & Kopec, 2000). Although CSRs for social phobia and simple phobia were normally distributed, Tobit regressions were carried out for these variables, as well as MDD and dysthymia to be consistent in the conceptualization of zero values as unobservable extensions of the continuous distribution.
Analyses of the piecewise effects of treatment of GAD on CSRs of individual diagnoses revealed a significant decrease over the treatment period for MDD, β = -.29, SE = .11, z = -2.60, p < .01; social phobia β = -.44, SE = .06, z = -7.02, p < .001; and simple phobia β = -.21, SE =. 06, z = -3.50, p < .001. In addition, there was a small but significant increase in CSRs over the follow-up period for MDD, β = .07, SE = .02, z = 2.95, p < .01, and social phobia β = -.44, SE = .06, z = -7.02, p < .001, with a trend in the same direction for simple phobia, β = .03, SE = .02, z = 1.83, p = .07. Post hoc t-tests revealed that social and simple phobia remained below baseline, ts = 7.16 and 4.09, respectively, ps < .001. However, CSRs for MDD were no longer significantly different from baseline, t = 1.19, p = .24. There were no significant effects for dysthymia during the treatment period or over the course of the follow-up period.
As described above, only the ADIS-R modules for disorders identified at baseline were assessed at posttreatment, 6-, and 12-month follow-ups. Given that CSRs for MDD at the 2-year assessment were no longer different from baseline, it might be reasonably inferred that the significant decrease in CSRs for MDD during the treatment period and subsequent increase over the follow-up period was an artifact of the ADIS-R interviewing protocol. However, the HRSD was administered to all participants at all time points, allowing for an additional analysis of depressive symptomatology. Piecewise analysis also showed a significant decrease in the severity of depressive symptomatology during the treatment period, β = -1.95, SE = .21, t = -9.40, p < .001, and an increase over the follow-up period, β = .10, SE = .04, t = 2.30, p < .01.
Within-Individual Analyses
We also examined the longitudinal course of comorbidity with GAD at the level of the individual client in order to determine whether (a) the number of people with comorbid disorders changed over time, (b) types of comorbid diagnoses changed over time for clients with comorbid diagnoses at pretreatment, and (c) whether any clients who had no comorbid diagnoses at pretreatment developed comorbidity at the 2-year assessment. In addition, we examined whether success in the treatment of principal GAD was significantly associated with a reduction in the number of people with comorbid diagnoses.
Change Over Time in Number of People With Comorbid Diagnoses
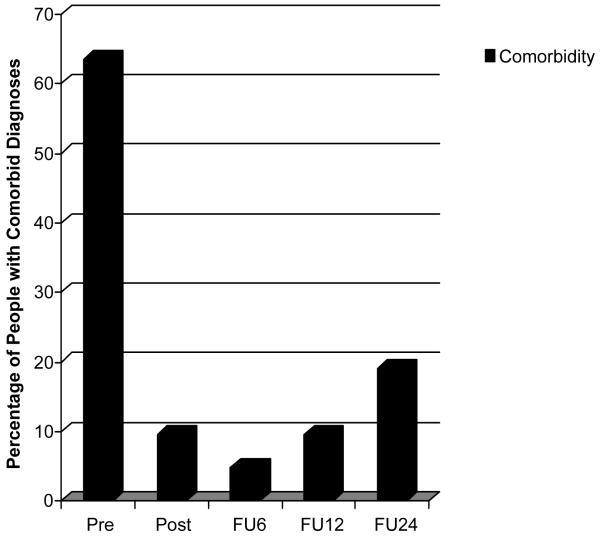
Within the 76 original participants, 7 people dropped out of treatment, and an additional 6 people were not available for the 24-month follow-up as well as other time points. Thus, there were 63 participants who completed treatment, were assessed at all time points, and were assessed with the full ADIS-R at the 2-year follow-up. The frequencies of people with at least one comorbid disorder are shown in Figure 1. As illustrated, 40 (63.5%) of the 63 participants had comorbidity at pretreatment, 6 (9.5%) at posttreatment, 3 (4.8%) at 6-month follow-up, 6 (9.5%) at 12-month follow-up, and 12 (19%) at 2-year follow-up. McNemar repeated measures chi-square tests demonstrated significant changes in the percentage of people with comorbid diagnoses from pretreatment to posttreatment, χ2 (1, N = 63) = 32.03, p < .001, 6-month, χ2 (1, N = 63) = 35.03, p < .001, 12-month, χ2 (1, N = 63) = 30.25, p < .001, and 2-year follow-up, χ2 (1, N = 63) = 22.78, p < .001.
Figure 1.
Percentage of clients with comorbid diagnoses at each assessment (Pre = pretreatment, Post = posttreatment, FU6 = 6-month follow-up, FU12 = 12-month follow-up, and FU24 = 24-month follow-up.)
Change Over Time in Types of Comorbidity
Similar to data reported for panic disorder by Brown and colleagues (1995), 6 of the 10 (60%) clients who had comorbidity at pretreatment and who continued to carry one or more comorbid diagnosis at the 24-month follow-up were assigned a different diagnosis from any of the comorbid diagnoses they had received at pretreatment [the new diagnoses were major depressive disorder (n = 2), simple phobia (n = 2), OCD (n = 1) and panic disorder (n = 1)]. Interestingly, however, 3 of these clients also continued to carry at least one diagnosis that had been made at pretreatment in addition to developing a new diagnosis, whereas the other 3 no longer carried any of the comorbid diagnoses assigned at pretreatment. Of the 23 treatment completers who were available for the 2-year assessment and who had no comorbid diagnoses at pretreatment, 2 (8.7%) were given a diagnosis other than GAD at 2-year follow-up. The diagnoses they received were simple phobia (n = 1) and major depression (n = 1).
Relationship Between Successful Therapy for GAD and Percentages of People With Comorbidity
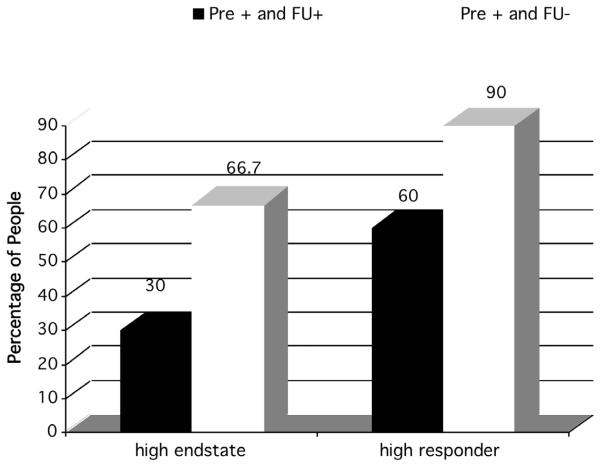
We classified clients into those who did and did not meet high endstate functioning criteria at 2-year follow-up. Of the 40 clients with complete data who had been diagnosed with comorbid disorders at pretreatment, 25% (n = 10) continued to have comorbid diagnoses at 2-year follow-up, and 42.5% (n = 17) had low endstate functioning at 2-year follow-up. A chi-square analysis demonstrated that successful therapy for GAD (defined as high endstate functioning) was associated with a reduction in the percentage of people with comorbid diagnoses at 2 year-follow-up, χ2(1, N = 40) = 4.13, p < .05. Similar results were found when examining high responder status, χ2(1, N = 40) = 4.68, p < .05. As demonstrated in Figure 2, at the 2-year follow-up assessment, successful treatment for GAD was more likely to be associated with reduced percentages of people with comorbidity than was unsuccessful treatment, replicating the effect found in Borkovec et al. (1995).
Figure 2.
Percentage of endstate and responder status of individuals with and without comorbidity after treatment among those with pretreatment comorbidity (Pre + FU+ = those with comorbid diagnoses at pretreatment who continued to have comorbidity at specific timepoint after treatment. Pre + FU- = Those who had comorbid diagnoses at pretreatment but no comorbidity at 2-year follow-up.)
For the 2 people who had no comorbidity at pretreatment but who received additional diagnoses at 2-year follow-up, neither met criteria for high endstate functioning at the 2-year assessment. However, the number of people for whom additional diagnoses later emerged was obviously too small for any meaningful statistical tests.
Impact of Pretreatment Comorbid Diagnoses on Additional Treatment Seeking
Twenty-three people sought additional treatment at some point during the 2-year follow-up period. Ten of these individuals (43.5%) sought psychotherapy, and 19 (82.6%) sought psychotropic medication. Analyses revealed a significant difference in the number of people seeking additional treatment when we compared those who had comorbid disorders at pretreatment to those who did not, χ2(1, N = 66) = 4.67, p < .05. Whereas 52.2% (12/23) of those without comorbid diagnoses sought additional treatment, only 25.6% (11/43) of those with comorbid diagnoses sought additional treatment.
Discussion
In the present sample, 60.5% had at least one comorbid disorder. The most common comorbid disorders were social phobia, mood disorders, and simple phobia, similar to the findings of other studies (Brown, Campbell, et al., 2001; Garyfallos et al., 1999; Wittchen et al., 1994; Yonkers, Warshaw, Massion, & Keller, 1996). Also comparable to other studies (e.g., Kessler et al., 2005) and consistent with our prediction, the presence of pretreatment comorbidity was associated with pretreatment severity of GAD.
Despite having greater severity of symptoms of GAD at pretreatment, clients with comorbidity evidenced a greater amount of change in these symptoms from pretreatment to posttreatment than clients without comorbidity. In fact, following treatment, the level of symptoms of GAD in clients with pretreatment comorbidity was comparable to symptoms of GAD in individuals without comorbidity. These patterns were replicated in analyses of the impact of baseline CSRs for simple phobia, social phobia, and MDD on treatment outcome. Thus, those with a higher CSR for these disorders demonstrated more change in their symptoms of GAD and thus ended up at a comparable level at posttreatment and across the follow-up period than those without comorbidity. Moreover, whereas the whole sample exhibited a slight deterioration in gains from posttreatment to 2-year follow-up, participants continued to have significantly lower levels of symptoms of GAD at 2-year follow-up compared to pretreatment rates. Those with comorbidity at pretreatment were also significantly less likely to seek additional treatment during the 2-year follow-up period than those without comorbidity. In addition, fewer participants with comorbidity at pretreatment dropped out of treatment compared to those with no comorbid diagnoses, although this finding failed to reach significance. These results are consistent with our initial prediction that there would be no negative effect of the presence of pretreatment comorbidity on treatment outcome.
A possible explanation for the finding that presence of comorbid disorders predicted more change in symptoms of GAD from pretreatment to posttreatment might be regression to the mean. People with GAD and comorbid disorders begin treatment with more severe symptoms of GAD compared to those without comorbidity and thus may be more likely to exhibit regression in their symptomatology. However, GAD is a highly chronic disorder with a low probability of naturalistic remission (Yonkers et al., 1996). Further, no naturalistic study has found that the presence of comorbidity increases the odds of remission from GAD. In fact, a number of these studies have found that comorbidity to GAD predicts greater chronicity and reduced likelihood of remission from symptoms of GAD when compared to pure GAD (Bruce et al., 2001; Bruce et al., 2005; Kessler et al., 2002). Also, because we assessed every participant twice at pretreatment for symptoms of GAD (using the ADIS-R), if regression to the mean were to occur, it would likely occur at the second assessment before we began treatment and the participant would not have been included in our study. In fact, dual pretreatment assessments are recommended as a way to avoid treatment results that might be due to regression to the mean (cf. Kazdin, 2003). Furthermore, although this study did not include a placebo condition, Borkovec and Costello (1993) found that the version of CBT for GAD we used was significantly superior to placebo. Thus, it is unlikely that the changes we found in the symptomatology of GAD for those with comorbid disorders were due to regression effects.
Another possible explanation for our results is that they are due to systematic diagnostic measurement error (e.g., overdiagnosis at pretreatment due to higher levels of general distress, underdiagnosis at posttreatment due to demand characteristics such as social desirability). However, as noted earlier, the treatment used in this study was superior to a placebo control condition (Borkovec & Costello, 1993). In addition, assessors were uninformed of study hypotheses and therapy conditions, reducing the likelihood of a systematic bias on their part.
Our findings replicate the findings of Wetherell and colleagues (2005) that the presence of comorbidity predicted more reliable change in response to group CBT with a geriatric sample. In addition, results of our study are consistent with data suggesting that people with comorbidity to GAD are more likely to seek treatment than are people with pure GAD (Bland et al., 1997; Mojtabai et al., 2002; Wittchen et al., 1994). Given that the presence of comorbidity likely provides further motivation for individuals with GAD to pursue psychotherapy, perhaps it also motivates them to work harder at getting better. Also, because comorbidity is associated consistently with greater severity of GAD (Kessler et al., 2005), these individuals have more room to change than do those without comorbidity and for whom a floor effect may exist.
Although our findings replicate Wetherell et al. (2005), they do not replicate the findings of Durham et al. (1997). This may be due to differences between the treatments used in the two studies. Whereas their participants received cognitive therapy, anxiety management training, or dynamic psychotherapy, our participants received CT, SCD, or combined CBT. Also, whereas we found no differences in the efficacy of our compared treatments and all treatments led to clinically and statistically significant change (Borkovec et al., 2002), Durham et al. (1997) found that cognitive therapy was a predictor of positive outcome and not receiving cognitive therapy was a predictor of relapse. On the other hand, our treatments were more similar to the treatment employed by Wetherell et al., who also examined a form of CBT for GAD that led to clinically and statistically significant reductions in anxiety symptoms.
The present study also found that cognitive and behavioral therapies were associated with a reduction in the number of comorbid disorders per person, in the number of people with comorbid disorders, and in CSRs for comorbid depression, social phobia, and simple phobia between pretreatment and posttreatment. Similar to the pattern for symptomatology of GAD, the number of comorbid disorders and CSRs for simple phobia, social phobia, and MDD increased from posttreatment to the 2-year follow-up, demonstrating some return of psychopathology over the long-term follow-up period. However, participants had significantly fewer comorbid disorders at the 2-year follow-up than they had at pretreatment, and CSRs for simple and social phobia remained below baseline. On the other hand, CSRs for MDD were no longer significantly different from baseline, demonstrating a failure to maintain treatment gains. These results must be qualified, however, by our failure to administer the full ADIS-R at posttreatment, 6-month, and 1-year assessment.
Similar to findings of Brown et al. (1995) for panic disorder, we found that six people diagnosed with disorders comorbid to GAD at pretreatment developed a comorbid disorder different from any of their pretreatment diagnoses at the 2-year assessment. Similarly, we diagnosed two people with comorbid disorders at the 2-year follow-up who had no comorbid diagnoses at pretreatment. Given that at posttreatment assessment, 6-month, and 1-year follow-up, we only administered the sections of the ADIS-R that corresponded to participants’ pretreatment diagnoses, we may have failed to detect the presence of comorbid diagnoses that were different from participants’ pretreatment diagnoses at these assessment points. Therefore, it is possible that the numbers, presence, and severity ratings were not decreased during these time points as much as our data suggest. However, in most treatment studies (including the current study), there is a tendency for the strongest impact of therapy to occur closest in time to the ending of the treatment as well as a greater tendency for deterioration to occur as assessments move further away in time from the final treatment session. At the 2-year assessment, when we administered the full ADIS-R, the number and presence of comorbid disorders were significantly reduced compared to pretreatment, and CSRs for simple and social phobia were also significantly smaller. Therefore, it is likely that if we had administered the full ADIS-R, we would have detected a pattern in which participants were also significantly lower in presence, and numbers of comorbid disorders and in CSRs for simple and social phobia compared to pretreatment.
Because CSRs for MDD were not significantly below baseline levels at 2-year assessment, there was more of a possibility that for this disorder, the significantly reduced CSRs at posttreatment were an artifact of our failing to administer the full ADIS-R at posttreatment. Nonetheless, we conducted confirmatory analyses using the HRSD (which was administered to all participants at all time points) and replicated the pattern we found for CSRs for MDD (i.e., symptoms of depression decreased significantly from pretreatment to posttreatment and increased significantly during the follow-up period). This suggests reduced likelihood that our findings related to CSRs for MDD were an artifact of our protocol.
The reduction in presence and number of comorbid disorders from pretreatment at the 2-year assessment is consistent with our initial prediction. In addition, similar to Borkovec and colleagues (1995), we found that reducing the number of people with comorbid diagnoses appears to be more likely when the therapy target is successfully eliminated (as indicated by high endstate functioning and high responder status) than when the therapy is not successful. The current study also extends the findings of Borkovec and colleagues from 1-year to 2-year follow-up and to a sample that included clients with mild and moderate levels of MDD. Moreover, the administration of the full ADIS-R at the 2-year assessment is an improvement over Borkovec and colleagues, who administered only the sections of the ADIS-R corresponding to pretreatment diagnoses at all follow-up assessments.
Although explanations for these findings are speculative, it is possible that individuals with comorbid depression, simple phobia, and social phobia are likely to remain in and benefit from cognitive and behavioral therapies for GAD because of overlapping symptoms between these disorders and GAD. People with behavioral avoidance and specific fears often worry about feared situations; therefore, cognitive restructuring and behavioral experiments to test such worries are likely to be beneficial. Similarly, given the close association between worry and rumination (Watkins, Moulds, & Mackintosh, 2005), it is likely that treatments targeting worries would generalize to the rumination associated with depressed mood.
Our GAD protocol encompasses CBT techniques that are used successfully for most anxiety and mood disorders. This treatment includes cognitive restructuring, stimulus control, applied relaxation training, and self-control coping desensitization. Once clients learn these techniques and the principles behind them, they can easily use them to address any anxiety or mood symptoms. In fact, Barlow and associates (Barlow, Allen, & Choate, 2004) have proposed the idea of developing a unified treatment for all emotional disorders, based on the concept that many of the protocols contain a common set of techniques based on common principles. On the other hand, our findings suggest that although severity of MDD is decreased by CBT, such a decrease is not maintained across the 2-year follow-up period. This may be due to the recurrent nature of MDD compared to the other disorders assessed. Perhaps CBT for GAD decreased the immediate symptoms of MDD but not clients’ vulnerability to future episodes. Depressed clients may benefit from a series of booster sessions.
These results, as well as the results of Borkovec and associates (1995), suggest that cognitive and behavioral therapies for GAD may generalize beyond the targeted disorder to social phobia, simple phobia, and mild to moderate MDD and thus may possess a potential cost-effectiveness (Newman, 2000). This is particularly important because the presence of a comorbid disorder tends to predict future cost associated with medical utilization, quality of life, work absenteeism, unemployment, disability, and limitations in physical functioning (Hunt et al., 2002; Maier et al., 2000; Souetre et al., 1994; Wittchen et al., 1994).
Several additional caveats should be mentioned regarding limitations of this study. As noted in the methods section, clients were ruled out if they were diagnosed with clinical levels of panic disorder (based on the request of the funding agency) or with very severe MDD. Therefore, our findings do not generalize to these groups. However, this study is an improvement over most of the other examinations of the impact of comorbidity to GAD on psychotherapy because most of these studies excluded MDD altogether. In addition, our study population was mostly Caucasian, and the findings may not generalize to more diverse groups.
Future studies should continue to examine the impact of comorbidity on psychotherapy efficacy and the impact of psychotherapy on comorbidity with GAD. If the current results are replicated, additional studies should also examine longitudinally whether or not the reduction in number of people with comorbidity or severity of comorbidity serves as a mediator of reduced cost. In addition, future studies could examine whether there are specific pretreatment predictors of unaddressed comorbidity at posttreatment.
Table 3. Means and standard deviations for Clinician’s Severity Ratings for each comorbid diagnosis, for the entire sample, across the treatment and follow-up periods.
| Pretreatment (n = 76) |
Post-Treatment (n = 69) |
6-Month Follow-up (n = 68) |
12-Month Follow-up (n = 65) |
24-Month Follow-up (n = 63) |
|
|---|---|---|---|---|---|
| Social phobia | 2.62 (2.27) | 1.16 (1.49) | 1.06 (1.46) | 1.03 (1.41) | 0.92 (1.62) |
| Simple phobia | 1.35 (1.59) | 0.50 (1.09) | 0.49 (1.00) | 0.59 (1.09) | 0.54 (1.26) |
| MDD | 0.78 (1.73) | 0.12 (0.56) | 0.15 (0.63) | 0.36 (1.25) | 0.45 (1.46) |
| Dysthymia | 0.37 (1.21) | 0.02 (0.18) | 0.10 (0.74) | 0.12 (0.70) | 0.10 (0.70) |
Note. MDD = major depressive disorder; n = number of observations.
Acknowledgments
This research was supported in part by National Institute of Mental Health Research Grant MH-39172.
Footnotes
Psychiatric research and history were the basis for the request from the NIMH review committee to exclude panic disorder. In DSM-III (American Psychiatric Association, 1980), GAD and panic disorder were considered to be the two “anxiety states” distinguished from the other anxiety disorders, and pharmacological research was at the time arguing for their separate pathogenesis. The committee wanted us in this context to keep the research separated for the two disorders.
Because the last author was on the DSM-IV task force for GAD, he knew the likely changes in diagnostic criteria that were coming, so our ADIS included interview questions that covered both sets of criteria, although admission was to be based on the existing (DSM-III-R) criteria.
We also examined whether nonnormality of CSRs for MDD and dysthymia at baseline would significantly degrade the fit of the linear mixed-effect models for the composite outcome measure when used as a predictor for outcome of GAD treatment. Nested model chi-square difference tests were performed between the null model, containing only fixed and random effects for Piece 1 and Piece 2 and a random effect for the intercept, and models with additional fixed effects for MDD and dysthymia. Chi-square tests revealed that rather than lead to degraded fit, the addition of the CSR for MDD and dysthymia led to significant increases in the goodness of fit, χ2(1, N = 76) = 9.71, p < .01, and χ2(1, N = 76) = 6.07, p < .05, respectively. There was therefore no degradation in model fit due to the nonnormality of the independent variables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed. American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed., rev. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Austin PC, Escobar M, Kopec JA. The use of the Tobit model for analyzing measures of health status. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation. 2000;9:901–910. doi: 10.1023/a:1008938326604. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behavior Therapy. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Relationship between the Beck Anxiety Inventory and the Hamilton Anxiety Rating Scale with anxious outpatients. Journal of Anxiety Disorders. 1991;5:213–223. [Google Scholar]
- Bernstein DA, Borkovec TD. Progressive relaxation training: A manual for the helping professions. Research Press; Champaign, IL: 1973. [Google Scholar]
- Bland RC, Newman SC, Orn H. Help-seeking for psychiatric disorders. Canadian Journal of Psychiatry. 1997;42:935–942. doi: 10.1177/070674379704200904. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ. Anxiety disorders and suicidal behaviours in adolescence and young adulthood: Findings from a longitudinal study. Psychological Medicine. 2007;37:431–440. doi: 10.1017/S0033291706009147. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Costello E. Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 1993;61:611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Abel JL, Newman H. Effects of psychotherapy on comorbid conditions in generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 1995;63:479–483. doi: 10.1037//0022-006x.63.3.479. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Newman MG, Pincus AL, Lytle R. A component analysis of cognitive-behavioral therapy for generalized anxiety disorder and the role of interpersonal problems. Journal of Consulting and Clinical Psychology. 2002;70:288–298. [PubMed] [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Diagnostic comorbidity in panic disorder: Effect on treatment outcome and course of comorbid diagnoses following treatment. Journal of Consulting and Clinical Psychology. 1995;63:408–418. doi: 10.1037//0022-006x.63.3.408. [DOI] [PubMed] [Google Scholar]
- Brown TA, Barlow DH. Comorbidity among anxiety disorders: Implications for treatment and DSM-IV. Journal of Consulting and Clinical Psychology. 1992;60:835–844. doi: 10.1037//0022-006x.60.6.835. [DOI] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110:49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Machan JT, Dyck I, Keller MB. Infrequency of “pure” GAD: Impact of psychiatric comorbidity on clinical course. Depression and Anxiety. 2001;14:219–225. doi: 10.1002/da.1070. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. American Journal of Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss GS, Gruenberg AM, Goldstein RD, Barber JP. Hamilton Anxiety Rating Scale Interview Guide: Joint interview and test-retest methods for interrater reliability. Psychiatry Research. 1994;53:191–202. doi: 10.1016/0165-1781(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Butler G, Anastasiades P. Predicting response to anxiety management in patients with generalised anxiety disorders. Behaviour Research and Therapy. 1988;26:531–534. doi: 10.1016/0005-7967(88)90150-7. [DOI] [PubMed] [Google Scholar]
- Butler G, Fennell M, Robson P, Gelder M. Comparison of behavior therapy and cognitive behavior therapy in the treatment of generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 1991;59:167–175. doi: 10.1037//0022-006x.59.1.167. [DOI] [PubMed] [Google Scholar]
- Clarke P, O’Campo P, Wheaton B. Data sparseness in contextual population health research: Effects of small group size and cluster analysis on linear and nonlinear multilevel models; Proceedings of Statistics Canada Symposium: Methodological Issues in Measuring Population Health; Ottawa: Canada. 2006, March; Retrieved July 21, 2008, from http://www.statcan.ca/english/freepub/11-522-XIE/11-522-XIE2006001.htm. [Google Scholar]
- Crits-Christoph P, Gibbons MBC, Losardo D, Narducci J, Schamberger M, Gallop R. Who benefits from brief psychodynamic therapy for generalized anxiety disorder? Canadian Journal of Psychoanalysis. 2004;12:301–324. [Google Scholar]
- Di Nardo PA, Barlow DH. Anxiety Disorders Interview Schedule-Revised. Center for Stress and Anxiety Disorders; Albany: 1988. [DOI] [PubMed] [Google Scholar]
- Durham RC, Allan T, Hackett CA. On predicting improvement and relapse in generalized anxiety disorder following psychotherapy. British Journal of Clinical Psychology. 1997;36:101–119. doi: 10.1111/j.2044-8260.1997.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Elston DA, Moss R, Boulinier T, Arrowsmith C, Lambin X. Analysis of aggregation, a worked example: Numbers of ticks on red grouse chicks. Parasitology. 2001;122:563–569. doi: 10.1017/s0031182001007740. [DOI] [PubMed] [Google Scholar]
- Fisher PL, Durham RC. Recovery rates in generalized anxiety disorder following psychological therapy: An analysis of clinically significant change in the STAI-T across outcome studies since 1990. Psychological Medicine. 1999;29:1425–1434. doi: 10.1017/s0033291799001336. [DOI] [PubMed] [Google Scholar]
- Garyfallos G, Adamopoulou A, Karastergiou A, Voikli M, Milis V, Donias S, Giouzepas J, Parashos A. Psychiatric comorbidity in Greek patients with generalized anxiety disorder. Psychopathology. 1999;32:308–318. doi: 10.1159/000029104. [DOI] [PubMed] [Google Scholar]
- Goisman RM, Goldenberg I, Vasile RG, Keller MB. Comorbidity of anxiety disorders in a multicenter anxiety study. Comprehensive Psychiatry. 1995;36:303–311. doi: 10.1016/s0010-440x(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Goldfried MR. Systematic desensitization as training in self-control. Journal of Consulting and Clinical Psychology. 1971;37:228–234. doi: 10.1037/h0031974. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Issakidis C, Andrews G. DSM-IV generalized anxiety disorder in the Australian National Survey of Mental Health and Well-Being. Psychological Medicine. 2002;32:649–659. doi: 10.1017/s0033291702005512. [DOI] [PubMed] [Google Scholar]
- Judd LL, Kessler RC, Paulus MP, Zeller PV, Wittchen HU, Kunovac JL. Comorbidity as a fundamental feature of generalized anxiety disorders: Results from the National Comorbidity Study (NCS) Acta Psychiatrica Scandanavica. 1998;98(Suppl 393):6–11. doi: 10.1111/j.1600-0447.1998.tb05960.x. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Research design in clinical psychology. 4th ed. Allyn & Bacon; Needham Heights, MA: 2003. [Google Scholar]
- Kessler RC, Andrade LH, Bijl RV, Offord DR, Demler OV, Stein DJ. The effects of co-morbidity on the onset and persistence of generalized anxiety disorder in the ICPE surveys. Psychological Medicine. 2002;32:1213–1225. doi: 10.1017/s0033291702006104. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Major depression and generalized anxiety disorders in the National Comorbidity Survey Follow-up Survey; Paper presented at the American Psychological Association DSM-IV Workshop on Depression and GAD; London, England. 2007, June; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Study. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Reynolds WM, Greist JH. Development and validation of a computer-administered version of the Hamilton Rating Scale. Psychological Assessment. 1993;5:487–492. [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders. 1988;14:61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Maier W, Gansicke M, Freyberger HJ, Linz M, Heun R, Lecrubier Y. Generalized anxiety disorder (ICD-10) in primary care from a cross-cultural perspective: A valid diagnostic entity? Acta Psychiatrica Scandinavica. 2000;101:29–36. doi: 10.1034/j.1600-0447.2000.101001029.x. [DOI] [PubMed] [Google Scholar]
- Marciniak MD, Lage MJ, Dunayevich E, Russell JM, Bowman L, Landbloom RP, Levine LR. The cost of treating anxiety: The medical and demographic correlates that impact total medical costs. Depression and Anxiety. 2005;21:178–184. doi: 10.1002/da.20074. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Mechanic D. Perceived need and help-seeking in adults with mood, anxiety, or substance use disorders. Archives of General Psychiatry. 2002;59:77–84. doi: 10.1001/archpsyc.59.1.77. [DOI] [PubMed] [Google Scholar]
- Moras K, Di Nardo PA, Barlow DH. Distinguishing anxiety and depression: Reexamination of the reconstructed Hamilton scales. Psychological Assessment. 1992;4:224–227. [Google Scholar]
- Newman MG. Recommendations for a cost-offset model of psychotherapy allocation using generalized anxiety disorder as an example. Journal of Consulting and Clinical Psychology. 2000;68:549–555. [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; Newbury Park, CA: 2002. [Google Scholar]
- Riskind JH, Beck AT, Brown G, Steer RA. Taking the measure of anxiety and depression: Validity of the reconstructed Hamilton scales. Journal of Nervous and Mental Disease. 1987;175:474–479. doi: 10.1097/00005053-198708000-00005. [DOI] [PubMed] [Google Scholar]
- Rogers MP, Warshaw MG, Goisman RM, Goldenberg I, Rodriguez-Villa F, Mallya G, Freeman SA, Keller MB. Comparing primary and secondary generalized anxiety disorder in a long-term naturalistic study of anxiety disorders. Depression and Anxiety. 1999;10:1–7. [PubMed] [Google Scholar]
- Rudd MD, Dahm PF, Rajab MH. Diagnostic comorbidity in persons with suicidal ideation and behavior. American Journal of Psychiatry. 1993;150:928–934. doi: 10.1176/ajp.150.6.928. [DOI] [PubMed] [Google Scholar]
- Souetre E, Lozet H, Cimarosti I, Martin P, Chignon JM, Ades J, J T, Darcourt G. Cost of anxiety disorders: Impact of comorbidity. Journal of Psychosomatic Research. 1994;38(Suppl 1):151–160. doi: 10.1016/0022-3999(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory STAI (Form Y) Mind Garden; Palo Alto, CA: 1983. [Google Scholar]
- Steer RA, McElroy MG, Beck AT. Correlates of self-reported and clinically assessed depression in outpatient alcoholics. Journal of Clinical Psychology. 1983;39:144–149. doi: 10.1002/1097-4679(198301)39:1<144::aid-jclp2270390128>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- Watkins E, Moulds M, Mackintosh B. Comparisons between rumination and worry in a non-clinical population. Behaviour Research and Therapy. 2005;43:1577–1585. doi: 10.1016/j.brat.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Hopko DR, Diefenbach GJ, Averill PM, Beck JG, Craske MG, Gatz M, Novy DM, Stanley MA. Cognitive-behavioral therapy for late-life generalized anxiety disorder: Who gets better? Behavior Therapy. 2005;36:147–156. [Google Scholar]
- Whitehurst GJ. Interrater agreement for journal manuscript reviews. American Psychologist. 1984;39:22–28. [Google Scholar]
- Wittchen HU, Zhao S, Kessler RC, Eaton WW. DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:355–364. doi: 10.1001/archpsyc.1994.03950050015002. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Warshaw MG, Massion AO, Keller MB. Phenomenology and course of generalised anxiety disorder. British Journal of Psychiatry. 1996;168:308–313. doi: 10.1192/bjp.168.3.308. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Dyck IR, Warshaw M, Keller MB. Factors predicting the clinical course of generalised anxiety disorder. British Journal of Psychiatry. 2000;176:544–549. doi: 10.1192/bjp.176.6.544. [DOI] [PubMed] [Google Scholar]