Abstract
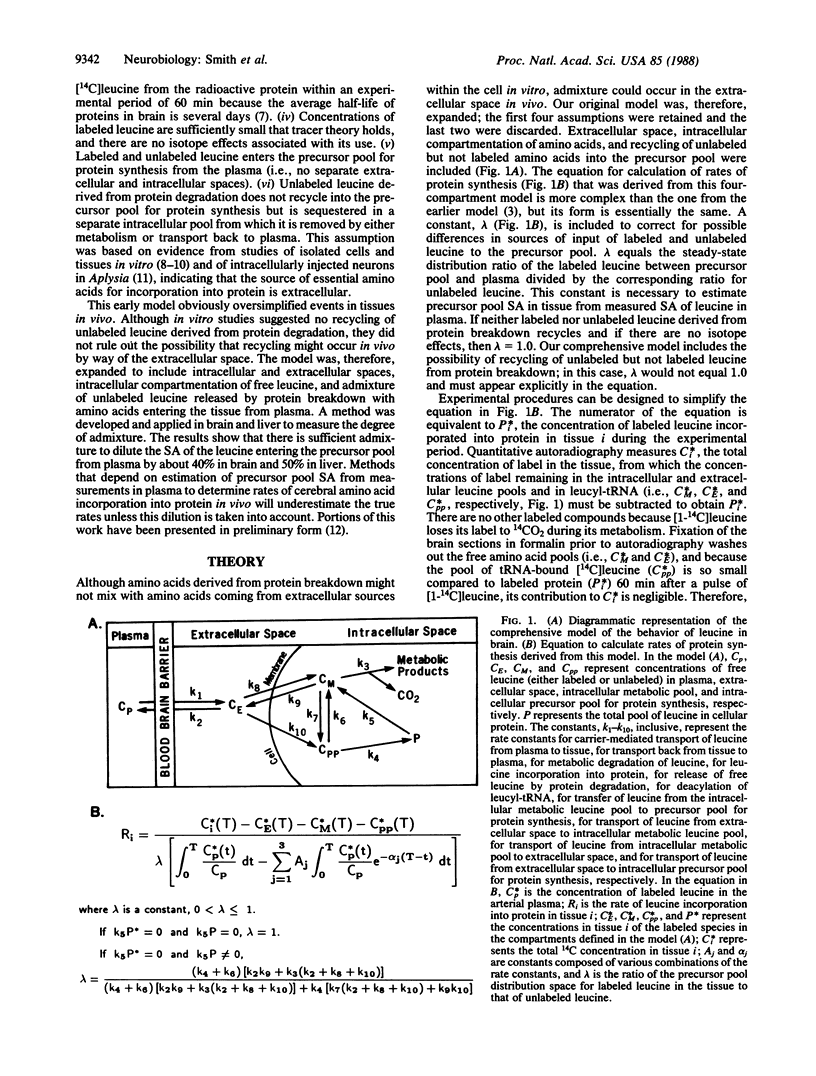
A quantitative autoradiographic method for the determination of local rates of protein synthesis in brain in vivo is being developed. The method employs L-[1-14C]leucine as the radiolabeled tracer. A comprehensive model has been designed that takes into account intracellular and extracellular spaces, intracellular compartmentation of leucine, and the possibility of recycling of unlabeled leucine derived from steady-state degradation of protein into the precursor pool for protein synthesis. We have evaluated the degree of recycling by measuring the ratio of the steady-state precursor pool distribution space for labeled leucine to that of unlabeled leucine. The values obtained were 0.58 in whole brain and 0.47 in liver. These results indicate that there is significant recycling of unlabeled amino acids derived from steady-state protein degradation in both tissues. Any method for the determination of rates of cerebral protein synthesis in vivo with labeled tracers that depends on estimation of precursor pool specific activity in tissue from measurements in plasma must take this recycling into account.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G., Cotman C. W. Characteristics of different amino acids as protein precursors in mouse brain: advantages of certain carboxyl-labeled amino acids. Arch Biochem Biophys. 1971 Feb;142(2):565–573. doi: 10.1016/0003-9861(71)90520-0. [DOI] [PubMed] [Google Scholar]
- Dunlop D. S., van Elden W., Lajtha A. A method for measuring brain protein synthesis rates in young and adult rats. J Neurochem. 1975 Feb;24(2):337–344. doi: 10.1111/j.1471-4159.1975.tb11885.x. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J. 1981 Jan 15;194(1):365–368. doi: 10.1042/bj1940365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H., Barker J. L., Wollberg Z. Preferential incorporation of extracellular amino acids into neuronal proteins. J Neurochem. 1975 Aug;25(2):177–179. doi: 10.1111/j.1471-4159.1975.tb12246.x. [DOI] [PubMed] [Google Scholar]
- Gan J. C., Jeffay H. Origins and metabolism of the intracellular amino acid pools in rat liver and muscle. Biochim Biophys Acta. 1967 Nov 28;148(2):448–459. doi: 10.1016/0304-4165(67)90141-9. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Preferential channeling of exogenously supplied methionine into protein by sea urchin embryos. J Biol Chem. 1981 Mar 25;256(6):2830–2834. [PubMed] [Google Scholar]
- Ingvar M. C., Maeder P., Sokoloff L., Smith C. B. Effects of ageing on local rates of cerebral protein synthesis in Sprague-Dawley rats. Brain. 1985 Mar;108(Pt 1):155–170. doi: 10.1093/brain/108.1.155. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., REISS E., HELMREICH E. Functional heterogeneity of the intracellular amino acid pool in mammalian cells. Biochim Biophys Acta. 1961 Aug 19;51:519–524. doi: 10.1016/0006-3002(61)90608-4. [DOI] [PubMed] [Google Scholar]
- LOFTFIELD R. B., HARRIS A. Participation of free amino acids in protein synthesis. J Biol Chem. 1956 Mar;219(1):151–159. [PubMed] [Google Scholar]
- Lajtha A., Latzkovits L., Toth J. Comparison of turnover rates of proteins of the brain, liver and kidney in mouse in vivo following long term labeling. Biochim Biophys Acta. 1976 Apr 2;425(4):511–520. doi: 10.1016/0005-2787(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Pettigrew K. D. A method to obtain infusion schedules for prescribed blood concentration time courses. J Appl Physiol. 1976 Mar;40(3):458–463. doi: 10.1152/jappl.1976.40.3.458. [DOI] [PubMed] [Google Scholar]
- Robertson J. H., Wheatley D. N. Pools and protein synthesis in mammalian cells. Biochem J. 1979 Mar 15;178(3):699–709. doi: 10.1042/bj1780699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Crane A. M., Kadekaro M., Agranoff B. W., Sokoloff L. Stimulation of protein synthesis and glucose utilization in the hypoglossal nucleus induced by axotomy. J Neurosci. 1984 Oct;4(10):2489–2496. doi: 10.1523/JNEUROSCI.04-10-02489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]



