Abstract
Purpose
To identify significant covariates in addition to spirometry that predict mortality in elderly subjects with obstructive airway disease (OAD).
Methods
268 participants with OAD from the `Health, Aging and Body Composition' study, a community-based observational cohort of well-functioning elderly aged 70–79 years, were followed on average for 6.1 years. Covariates related to pulmonary and physical function, comorbidity, demographics, and three inflammatory markers (IL-6, TNF-α, C-reactive protein) were evaluated for their association with all-cause mortality (31%) using Kaplan Meier analysis and Cox proportional modeling.
Results
Percent predicted forced expiratory volume in one second (PPFEV1; hazard ratio (HR)=2.03, p<0.0001), knee extensor strength (HR=1.36, P=0.0002), interleukin-6 (HR=1.37, P=0.0002) and 400m corridor walk time (HR=1.24, P=0.008) significantly predicted mortality. A multidimensional index, the PILE score, was constructed from PPFEV1, IL-6 and knee extensor strength. Each one point increase in PILE score (range 1–10) was associated with a 30% increase in mortality (95% confidence interval: 16–47%) after adjusting for age, race, gender, smoking and comorbidity, resulting in a 10.4-fold higher risk of death between the highest and lowest risk category.
Conclusion
Subjects with OAD have a wide gradient of risk for mortality that can potentially be incorporated in clinical decision making.
Keywords: Obstructive airway disease, COPD, Mortality, Elderly, Interleukin-6, Cox proportional hazard modeling
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the fourth most common cause of death in the United States (1). Percent predicted forced expiratory volume in one second (PPFEV1) is the major prognostic variable used to grade disease severity and predict risk of death in COPD patients, but it fails to capture important systemic manifestations of the disease (2, 3). Thus, it has only limited predictive power for mortality in COPD patients. Celli et al.(4) introduced a multidimensional index, the BODE index, that includes not only PPFEV1 (as a measure of airway obstruction, O), but also three other markers of disease severity: body mass index (B), dyspnea (D) and exercise capacity (E). The BODE index was shown to be better than PPFEV1 alone at predicting all-cause and respiratory mortality among patients with COPD. The BODE index highlights that the prognostic assessment of COPD patients should include factors other than lung function (4, 5).
Since then, several composite indices have been reported to predict mortality in COPD patients. These include the CPI (COPD Prognostic Index) (6), the DOSE index (Dyspnea, airflow Obstruction, Smoking status, Exacerbation frequency) (7), and the ADO index (Age, Dyspnea, airflow Obstruction) (8). The CPI index was developed from a dataset of more than 8000 patients and was shown to predict COPD exacerbations and hospital episodes in addition to mortality. This index, however, is difficult to construct in routine clinical settings as it comprises seven variables. Moreover, the CPI index does not include variables which capture the systemic manifestations of COPD such as loss in endurance and strength. The DOSE index has the advantage of being simple and not requiring any specialized test or equipment other than a spirometer to capture its variables. The DOSE index, however, does also not fully capture the systemic manifestations of COPD and was not compared to the already established BODE index. The ADO index has only three variables and thus is easy to construct. It was compared with the BODE index and was found to be superior in predicting mortality in COPD patients in two different European cohorts, but the authors caution that different protocols were used for assessing the respective variables. In summary, it is important to note that (a) all the above indices were developed in COPD patients and (b) none of them included markers of systemic inflammation.
The Health, Aging and Body Composition Study (Health ABC) is a prospective community-based observational cohort study in well-functioning elderly (9). In this article we explore the validity of the BODE index in Health ABC participants with obstructive airway disease (OAD), examine the role of other covariates associated with airway obstruction in predicting mortality, and develop a multivariate model (the PILE score) to predict all-cause mortality in this population.
Materials and Methods
Study Population
The Health ABC study is a longitudinal study of 3075 well-functioning individuals (50% men; 40% Black) aged 70–79 residing in Pittsburgh, PA and Memphis, TN (10). Participants were included if they reported no difficulty walking a quarter mile, climbing ten steps without resting or performing basic activities of daily living. Exclusion criteria included any life-threatening condition, participation in any research study involving medications or modification of eating or exercise habits, plans to move out from the geographic area within 3 years, difficulty in communicating with the study personnel or cognitive impairment. The protocol was approved by the Institutional Review Boards of the clinical sites. All participants gave written informed consent.
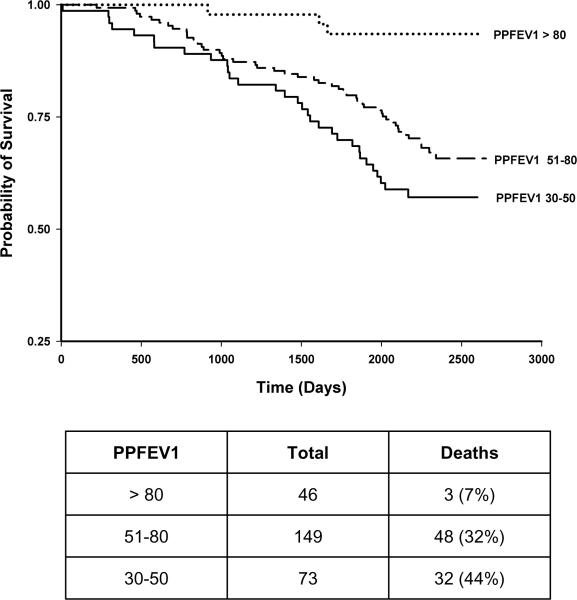
The present analysis was performed on data collected at enrollment into Health ABC and is limited to 268 participants with OAD as identified by spirometry based on ATS criteria (Figure 1) (11). OAD was defined as reduced FEV1/FVC as determined by age, gender and race-normalized values from the third National Health and Nutrition Examination Survey (NHANESIII) (12). These values were used because they were obtained from a random sample in the United States representing the age range, gender and racial groups present in the Health ABC study. Subjects with both airway obstruction and low FVC (mixed pattern) usually due to hyperinflation or obesity were also included in the OAD population.
FIGURE 1.
268 participants with obstructive airways disease (OAD) were selected from the Health ABC cohort based on ATS criteria.
Survival Time
Surveillance for survival was conducted by in-person visit alternating with telephone interview every 6 months. The date of death was determined based on hospital records, death certificates or informant interviews. During an average follow-up period of 6.1 years (range: 4.0 – 7.2 years), 83 (31.0%) of the 268 participants with OAD died from all causes. 11 (4.1%) were lost during follow-up and their survival-time was censored based on their date of last contact.
Covariates
Demographics (age, gender, race) and comorbidity (smoking status, pack years of smoking, history of congestive heart failure, cardiovascular disease, and other prevalent health conditions) were assessed based on self-report and medication inventory. Prevalence of diabetes was assessed based upon blood glucose and oral glucose tolerance test. Body mass index (BMI) was calculated from measured weight and height and was evaluated as continuous variable as well as categorical variable based on the previously described cutoff of BMI<21 (4, 13).
PPFEV1 was determined by spirometry as previously reported (14), following ATS criteria of reproducibility and acceptability (15). In subjects taking bronchodilators, spirometry was only performed after their use, and bronchodilator challenge was not part of the protocol (14). Once subjects were classified with OAD, they were grouped under different stages of severity according to ATS criteria (16). OAD severity was classified as `mild' (PPFEV1>80), `moderate' (50–80) or `severe' (<50).
Dyspnea was assessed by self-report and classified as `none', `mild' or `moderate' based on the following questions. The individuals were asked whether they stopped for breath when hurrying on a level surface or walking up a slight hill. If answering yes, they were questioned whether or not they have to stop for breath when walking at their own pace on a level surface. Subjects who answered yes to both questions were classified as having moderate dyspnea, while those answering no to both questions were classified as having no dyspnea. Individuals answering yes only to the first question were classified as having mild dyspnea.
Exercise capacity was quantified as the performance time of a 400-meter long distance corridor walk (LDCW) as previously described (10, 17), for which participants were instructed to complete the distance as quickly as they could at a pace they could maintain throughout the test.
Upper extremity strength was measured as the average of the maximum grip strength of the left and right hand using an adjustable, Jamar hydraulic hand dynamometer.
Lower extremity strength was assessed by dynamometer measurement of the mean concentric isokinetic knee extensor strength at 60° per second using a Kin-Com 125-AP Dynamometer (Chattanooga Group, Chattanooga, TN) (18). The measure was obtained in the right leg only, unless it had been injured or affected by a condition, such as osteoarthritis, that would impair joint motion. Best overlapping torque curves were generated using at least three but not more than 6 trials. Subjects were asked to exert maximum force while extending the knee from 90° to 30° at 60°/second. Strength was measured as mean maximal torque (Nm) average of three best trials. There were no mean differences in tests for inter-examiner reliability while the intra-examination coefficient variation was 11% (19). For 32 participants with missing strength measurements, values were imputed using age, race, gender, grip strength and leg lean mass as variables.
Plasma concentrations of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) were determined by immunoassays in peripheral blood specimens collected by venipuncture after an overnight fast (11, 20). Cytokines (IL-6 and TNF-α) were measured in duplicate by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). The detectable limits for IL-6 (HS600 Quantikine kit) and TNF-α (HSTA50 kit) were 0.10 pg/mL and 0.18 pg/mL, respectively. Serum CRP was also measured in duplicate by ELISA on the basis of purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA) with a detection limit of 0.007 mg/L. Blind duplicate analyses (n=150) for IL-6, CRP, and TNF-α showed coefficients of variation of 10.3, 8.0, and 15.8%, respectively (20). For missing values of IL-6 (7%), quintiles corresponding to CRP were assigned as their concentrations were highly correlated.
PILE score and BODE index
For constructing the PILE score, each subject received points ranging from 0 to 4 according to their inclusion in quintiles for PPFEV1 (P), plasma levels of IL-6 (IL) and knee extensor strength (E) (Table 3a). The final score for each subject was obtained by summing up the points corresponding to each variable. PILE scores ranged from 1 to 10 with higher scores implying greater risk of death.
TABLE 3a.
Variables and point values used for construction of the PILE score
| Points on PILE Score |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| PPFEV1 [P] | >80 (mild) | 51–80 (moderate) | 30–50 (severe) | - | - | |
| IL-6 (pg/mL) [IL] | 0.45–1.39 | 1.40–1.85 | 1.86–2.41 | 2.42–3.77 | 3.78–13.25 | |
| Knee Extensor Strength (Nm) [E] | Men | 131.0–221.1 | 115.7–130.9 | 99.3–113.5 | 83.4–98.9 | 19.5–81.6 |
| Women | 86.4–137.7 | 72.5–84.7 | 62.4–72.3 | 51.3–61.4 | 12.9–49.9 | |
A modified BODE index was also constructed in a similar manner as described by Celli et al. (4) and compared with the PILE score in its predictive capability for all-cause mortality (Table 3b). It was modified from the original BODE index in two aspects: a) exercise capacity was measured using LDCW instead of a 6 min walk test; and b) the gradual differences in the dyspnea assessment in Health ABC were less detailed.
TABLE 3b.
Variables and point values used for construction of the modified BODE Index.
| Points on BODE index |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Body-mass index (kg/m2) [B] | >21 | <21 | - | - | - | |
| PPFEV1 [O] | >80 (mild) | 51–80 (moderate) | 30–50 (severe) | - | - | |
| Dyspnea [D] | None | Mild | Moderate | - | - | |
| Performance time for LDCW (sec) [E] | Men | 241–292 | 294–320 | 323–348 | 351–354 | 377–494* |
| Women | 242–305 | 307–331 | 333–360 | 363–388 | 392–512* | |
Participants who did not complete or were excluded from LDCW were also assigned 4 points.
Statistical Analysis
Covariates between survivors and non-survivors were compared with the student t-test and χ2-test and correlation analyses were performed to explore potential associations.
Initially, univariate Kaplan-Meier analysis and Cox proportional hazards modeling were performed to identify covariates of prognostic importance and to obtain hazard ratios (HR) (21). Continuous covariates were coded into quintiles, with upper and lower extremity strength and LDCW coded separately for men and women to account for gender effects. Survival functions among subgroups were compared by log rank test. Breslow's approximation was used to handle ties in survival times. Subsequently, multivariate Cox regression analysis was performed using forward, backward and stepwise regression with likelihood ratio, Wald and Score statistics to identify significant covariates at a 95% confidence level. The proportionality assumption for Cox regression modeling was satisfied. A multidimensional index was formulated from final selected covariates and independently evaluated by Cox modeling adjusting for age, race, gender, smoking status and comorbid conditions. The model was evaluated internally using bootstrap generated confidence intervals for hazard ratios (22, 23).
Akaike information criteria (AIC) and Bayesian information criteria (BIC) were computed as diagnostics for model fits. C-statistic was computed to compare the new index to BODE or PPFEV1 alone in their predictive performance for risk of death (24).
Results
Survivors and non-survivors differed significantly in PPFEV1, IL-6 concentration, knee extensor strength and LDCW (Table.1). There was also a significant gender difference with 41% deaths in men compared to only 18% deaths in women.
TABLE 1.
Comparison between survivors and non-survivors among 268 Health ABC participants with obstructive airway disease for demographics, physical function, pulmonary function and inflammatory markers.
| Variable | Category | Total | Dead | Alive | P value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | N | 268 | 83 (31%) | 185 (69%) | ||
| Mean (SD) | 74.0 (2.8) | 72.8 (2.8) | 0.002 | |||
| Gender | Men | 154 (57%) | 63 (41%) | 91 (59%) | < 0.0001 | |
| Women | 114 (43%) | 20 (18%) | 94 (82%) | |||
| Race | White | 149 (56%) | 41 (28%) | 108 (72%) | 0.17 | |
| Black | 119 (44%) | 42 (35%) | 77 (65%) | |||
| Smoking Status | Never | 46 (17%) | 10 (22%) | 36 (78%) | 0.09 | |
| Former | 147 (55%) | 43 (29%) | 104 (71%) | |||
| Current | 75 (28%) | 30 (40%) | 45 (60%) | |||
| Pack years of smoking | N | 263 | 82 (31%) | 181 (69%) | 0.09 | |
| Mean (SD) | 45 (32) | 38 (34) | ||||
| Body mass index (BMI) | N | 268 | 83 (31%) | 185 (69%) | 0.31 | |
| Mean (SD) | 25.0 (4.8) | 25.6 (4.7) | ||||
| BMI >21 | N | 218 (81%) | 66 (30%) | 152 (70%) | 0.53 | |
| BMI <21 | N | 50 (19%) | 17 (35%) | 33 (65%) | ||
| Weight (kg) | N | 268 | 83 (31%) | 185 (69%) | 0.94 | |
| Mean (SD) | 72.0 (14.2) | 72.0 (15.5) | ||||
| Physical Function | ||||||
| Time for Long Distance Corridor | Men | N | 116 (63%) | 45 (39%) | 71 (61%) | 0.02 |
| Mean (SD) | 350 (50) | 328 (50) | ||||
| Walk (LDCW) (sec) | Women | N | 68 (37%) | 9 (13%) | 59 (87%) | 0.08 |
| Mean (SD) | 384 (59) | 349 (55) | ||||
| Knee extensor strength (Nm) | Men | N | 152 (57%) | 62 (41%) | 90 (59%) | 0.0004 |
| Mean (SD) | 96.4 (27.5) | 114 (30.3) | ||||
| Women | N | 114 (43%) | 20 (18%) | 94 (82%) | 0.05 | |
| Mean (SD) | 58 (19.8) | 69.4 (22.4) | ||||
| Grip Strength (kg) | Men | N | 153 (58%) | 62 (41%) | 91 (59%) | 0.18 |
| Mean (SD) | 37.7 (9.0) | 39.5 (8.2) | ||||
| Women | N | 111 (42%) | 20 (18%) | 91 (82%) | 0.22 | |
| Mean (SD) | 21.2 (4.0) | 22.7 (5.3) | ||||
| Pulmonary Function | ||||||
| PPFEV1 | N | 268 | 83 (31%) | 185 (69%) | 0.0006 | |
| Mean (SD) | 57.2 (15.9) | 65.4 (18.4) | ||||
| Mild (>80) | N | 46 (17%) | 3 (7%) | 43 (93%) | < 0.0001 | |
| Moderate (51–80) | N | 149 (56%) | 48 (32%) | 101 (68%) | ||
| Severe (30–50) | N | 73 (27%) | 32 (44%) | 41 (56%) | ||
| Dyspnea | None | 135 (50%) | 37 (27 %) | 98 (73%) | 0.10 | |
| Mild | 106 (40%) | 33 (31%) | 73 (69%) | |||
| Moderate | 27 (10%) | 13 (48%) | 14 (52%) | |||
| Inflammatory Markers | ||||||
| IL-6 (pg/mL) | N | 249 | 76 (31%) | 173 (69%) | 0.0003 | |
| Mean (SD) | 3.6 (2.5) | 2.5 (1.9) | ||||
| CRP (mg/L) | N | 262 | 82 (31%) | 180 (69%) | 0.23 | |
| Mean (SD) | 4.5 (5.5) | 3.6 (6.3) | ||||
| TNF-α (pg/mL) | N | 244 | 77 (46%) | 167 (54%) | 0.53 | |
| Mean (SD) | 3.5 (1.8) | 3.4 (1.6) | ||||
N Number of participants
Kaplan Meier and univariate Cox proportional hazards analysis both indicate that all-cause mortality in the study participants was significantly predicted by PPFEV1 (HR=2.03, P<0.0001), knee extensor strength (HR=1.36, P=0.0002), IL-6 concentration (HR=1.37, P=0.0002), and time for LDCW (HR=1.24, P=0.008) (Table.2). Thus, participants having more severe airway obstruction, less knee extensor strength, elevated plasma IL-6 levels, and a slower walking pace were at greater risk of death. Smoking status (HR=1.45, P=0.03), gender (HR=2.6, P=0.0002), and comorbid conditions (HR=1.32, P=0.01) also significantly predicted mortality. The risk of death increased from never smokers to former to current smokers, from women to men, and with increasing number of comorbid conditions. Dyspnea (HR=1.36, P=0.053) and plasma concentrations of CRP (HR=1.18, P=0.04) showed only marginal significance in predicting mortality (Table.2).
TABLE 2.
Investigated covariates in 268 Health ABC participants with obstructive airway disease as predictors of survival using Kaplan Meier and Cox proportional hazards regression analysis.
| Kaplan Meier | Cox Proportional Hazards | |||||
|---|---|---|---|---|---|---|
| Strata | P value | Unadjusted HRΔ | P value | Adjusted HR* | P value | |
| Age | 0–4 | 0.008 | 1.26 (1.1, 1.5) | 0.002 | - | - |
| Gender | Men, Women | 0.0001 | 2.60 (1.6, 4.3) | 0.0002 | - | - |
| Race | White, Black | 0.14 | 1.37 (0.9, 2.1) | 0.15 | - | - |
| Smoking status | 0, 1, 2η | 0.083 | 1.45 (1.0, 2.0) | 0.03 | - | - |
| Comorbid conditions† | 0, 1, 2 | 0.0002 | 1.32 (1.1, 1.7) | 0.01 | - | - |
| Pack years of smoking | 0–4 | 0.04 | 1.15 (1.0–1.4) | 0.06 | 0.95 (0.8, 1.1) | 0.6 |
| BMI | 0, 1Θ | 0.34 | 1.30 (0.7, 2.1) | 0.34 | 1.50 (0.9, 2.7) | 0.12 |
| 0–4 | 0.45 | 1.12 (1.0, 1.3) | 0.15 | 1.12 (0.9, 1.3) | 0.2 | |
| Weight | 0–4 | 0.7 | 0.98 (0.8, 1.1) | 0.8 | 1.10 (0.9, 1.3) | 0.3 |
| Time for LDCW | 0–4 | 0.055 | 1.24 (1.1, 1.5) | 0.008 | 1.22 (1.0, 1.4) | 0.02 |
| Knee extensor strength | 0–4 | 0.003 | 1.36 (1.2, 1.6) | 0.0002 | 1.34 (1.1, 1.6) | 0.0008 |
| Grip strength | 0–4 | 0.47 | 1.13 (0.98, 1.4) | 0.11 | 1.1 (0.9, 1.5) | 0.22 |
| PPFEV1Ω | 0, 1, 2 | 0.0002 | 2.03 (1.4, 2.9) | < 0.0001 | 1.8 (1.3, 2.6) | 0.0004 |
| Dyspneaσ | 0, 1, 2 | 0.06 | 1.36 (0.99, 1.9) | 0.053 | 1.36 (0.98, 1.9) | 0.07 |
| IL6 | 0–4 | 0.001 | 1.37 (1.2, 1.6) | 0.0002 | 1.24 (1.1, 1.5) | 0.01 |
| TNF-α | 0–4 | 0.23 | 1.01 (0.9, 1.2) | 0.86 | 0.95 (0.8, 1.9) | 0.56 |
| CRP | 0–4 | 0.37 | 1.18 (1.0, 1.4) | 0.04 | 1.12 (0.9, 1.3) | 0.16 |
Hazard Ratio with 95% confidence intervals
Hazard Ratio adjusted for age, race, gender, smoking status and comorbid conditions with 95% confidence intervals
0= Never Smoker, 1=Former Smoker, 2=Current Smoker
Diabetes, cardiovascular disease, congestive heart failure
0=BMI >21, 1=BMI <21
0=Mild, 1=Moderate, 2=Severe
0=None, 1=Mild, 2=Severe
Multivariate analysis by Cox regression resulted in the selection of PPFEV1 (P), IL-6 (IL) and knee extensor strength (E) as the most significant covariates after adjusting for age, race, gender, smoking status and comorbid conditions. The mean PILE score for non-survivors (mean {SD}: 6.3 {2.0}) was significantly higher (p<0.0001) than survivors (4.6 {2.1}). There was no significant difference between mean PILE scores of men and women (5.4 {2.3} vs. 4.9 {2.2}, P=0.08), Whites and Blacks (5.1 {2.2} vs. 5.3 {2.3}, P=0.83), and participants with or without cardiovascular disease or congestive heart failure. The mean hazard ratio per PILE score point increase was 1.30 (95% confidence interval: 1.16–1.47). Thus, each point increase in PILE score was associated with a 30% increase in all-cause mortality after adjusting for age, gender, race, smoking status and comorbid conditions. Participants with a PILE score of 10 had a 10.4-fold higher risk of death compared to individuals with a PILE score of 1 (Table.4). There were no deaths in the group of individuals with a PILE score of 1 in contrast to 80% deaths in the group having a PILE score of 10.
TABLE 4.
Relationship between PILE score, hazard ratio and percentage of deaths during an average follow-up period of 6.1 years in 268 Health ABC participants with obstructive airway disease.
| PILE score | Hazard ratio | Number of participants | % Deaths |
|---|---|---|---|
| 1 | 1 | 17 | 0 |
| 2 | 1.3 | 23 | 13 |
| 3 | 1.7 | 24 | 25 |
| 4 | 2.2 | 32 | 22 |
| 5 | 2.8 | 51 | 22 |
| 6 | 3.7 | 38 | 37 |
| 7 | 4.8 | 45 | 50 |
| 8 | 6.2 | 16 | 63 |
| 9 | 8.0 | 17 | 58 |
| 10 | 10.4 | 5 | 80 |
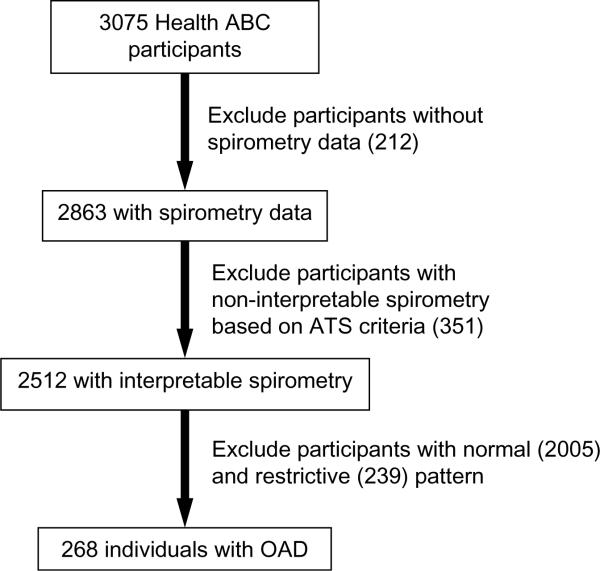
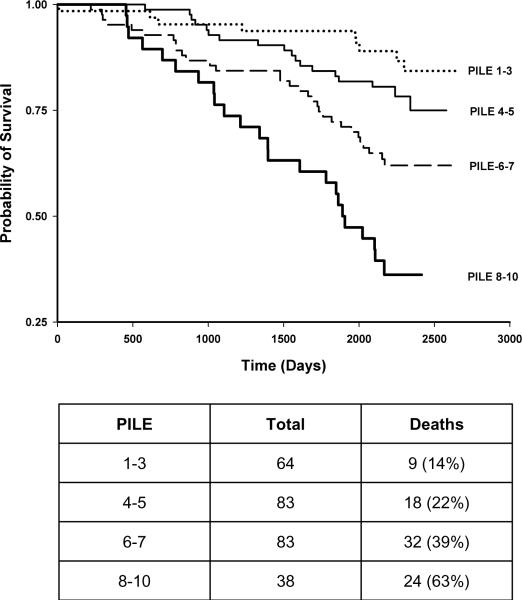
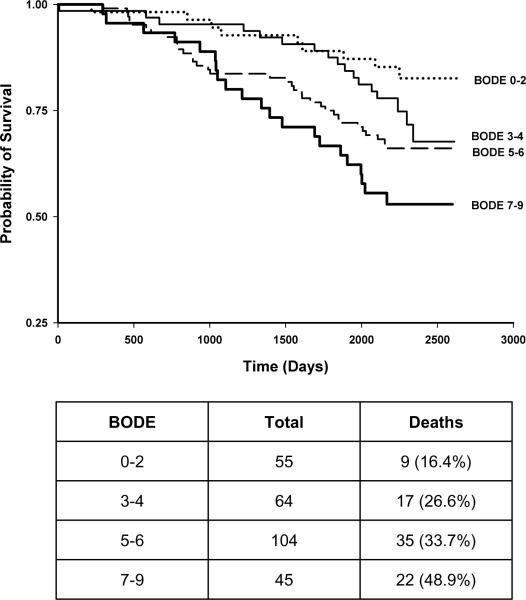
The modified BODE index for the studied Health ABC cohort was constructed similar to the PILE score (Table 3b). Kaplan Meier plots were constructed with quartiles for PILE score or modified BODE index as different strata (Figures 2 and 3), as well as PPFEV1 (Figure 4). Each quartile increase in PILE score caused a significant increase in mortality. The highest quartile was associated with 64% mortality in a 6-year period. The survivorship functions for the modified BODE index and PPFEV1 for the same data were also significantly different among quartiles (P<0.0001). Both AIC (844) and BIC (858) of the survival model with the PILE score were lower when compared to the BODE index (AIC=856, BIC=870) indicating better model fit of the data with the PILE score. Moreover, the C-statistic for the ability of the PILE score to predict risk of death was 0.71 as compared to 0.64 for the modified BODE index and 0.63 for PPFEV1 alone, indicating a superior predictive capability of the PILE score for mortality in the studied Health ABC population.
FIGURE 2.
Kaplan Meier plot using quartiles of PILE score as stratum (P < 0.0001). Higher PILE scores indicate greater risk of death. The table indicates the number of individuals in each PILE score quartile.
FIGURE 3.
Kaplan Meier plot using quartiles of the modified BODE index as stratum (P = 0.003). The table indicates the number of individuals in each BODE index quartile.
FIGURE 4.
Kaplan Meier plot using PPFEV1 as stratum (>80%, 51–80%, <50%) (P = 0.0002). The table indicates the number of individuals in each PPFEV1 category.
Discussion
PPFEV1 is an important health indicator that has been associated with disease progression and mortality in pulmonary and extrapulmonary conditions (25–28). It has been recognized, however, that a single measurement of PPFEV1 incompletely reflects the complex clinical consequences of COPD and its systemic manifestations (4, 16). In the present analysis, we used mortality over a 6-year period among Health ABC participants with OAD to investigate nonspirometric predictors of mortality including BMI, self-reported dyspnea, plasma concentrations of inflammatory markers, and various measures of strength and endurance. PPFEV1, plasma IL-6 concentration, and knee extensor strength were identified as independent predictors for mortality in participants with OAD. As our study involved well-functioning elderly from a community-based cohort study in whom airway obstruction has been suggested to be under-recognized (14) rather than participants diagnosed with COPD, it may be more suited to understand the relationship between the investigated covariates, OAD and mortality in a general population of elderly. Only 44% (118) participants of our study cohort with OAD reported any previous diagnosis of airway disease (24.3% asthma, 20.5% chronic bronchitis, 19% emphysema, 3.7% COPD).
Low BMI is a common clinical observation in COPD, and BMI has been shown to be inversely related to mortality in COPD, with a threshold value of 21 (1, 13, 16). In the present analysis, BMI did neither predict mortality as a categorical covariate with a threshold of 21 nor as a continuous covariate, most likely because the mean BMI of the present cohort was 25, with only a minor proportion of individuals having a BMI<21 (19%).
Similarly, dyspnea showed only marginal significance (P=0.07) in predicting mortality in the present analysis although several studies reported the degree of dyspnea to be stronger correlated with mortality in COPD than PPFEV1 (29, 30). Dyspnea, however, is a multifactorial condition whose etiology is often not clear and most elderly people are reluctant complaining about dyspnea, associating their symptoms to age rather than disease (31, 32). In Health ABC, 50% of participants did not report of dyspnea despite suffering from obstructive airway disease (14). In addition, study enrollment was limited to well-functioning elderly, so that only 10% of the studied OAD population had moderate dyspnea.
While OAD is often discussed in spirometric terms, much of the disability caused by it is attributed to its systemic manifestations characterized by decreased lower extremity lean body mass, strength and endurance (33–35). In Health ABC, LDCW, knee extensor strength, and grip strength were used to assess exercise tolerance, and lower and upper extremity strength, respectively (10). Both time for LDCW and knee extensor strength were found to predict mortality after adjusting for confounding factors, but both were significantly correlated (r=−0.28, P<0.0001). This is in agreement with previous reports that peripheral muscle weakness is significantly associated to low exercise tolerance (36, 37). Only knee extensor strength as the better predictor entered the multivariate regression model. Although grip strength has been widely used as a general indicator of frailty, it was not a significant predictor of mortality in the present cohort, probably due to a preferential distribution of muscle weakness in lower as compared to upper extremities in COPD (36, 38, 39). This is further supported by the observation that quadriceps strength is a better predictor than grip strength to differentiate normal from OAD participants in Health ABC (11).
Systemic inflammation correlates well with disease severity in COPD (33) and has been suggested as the `missing link' between airway dysfunction and extrapulmonary manifestations of COPD (40). Increased circulating levels of IL-6 and TNF-α have been reported in COPD (41–44), and IL-6 was inversely correlated with PPFEV1 in both obstructive and normal individuals (11). In our analysis, only IL-6, but not TNF-α was a significant predictor of mortality in OAD. A potential explanation might be the very short half-life of TNF-α (6–7 minutes) as compared to IL-6 (3–4 hours) (11), resulting in less stable, more fluctuating TNF-α concentrations as compared to IL-6, which might have resulted in its reduced predictive power for mortality in OAD. Surprisingly, CRP did not predict survival in our cohort, despite having been described as elevated in COPD (45–48).
We combined the identified three independent predictors for mortality in a multidimensional index, the PILE score. While the previously described BODE index is limited to measures of pulmonary function, dyspnea, endurance and BMI, addition of a measure of systemic inflammation seems to increase the predictive capability of the PILE score in the present study. The differences between PILE score and BODE index may further be explained by the fact that we used a modified BODE index with different measures for exercise capacity and dyspnea, and that Celli et al. (4) used a population of COPD patients rather than well-functioning elderly to develop the BODE index, including substantially more individuals with very severe obstruction (PPFEV1<35), 38% vs. 1%, respectively.
Recently, a new approach has been suggested for determining lower limits of normal lung function parameters across all ages (49). This approach takes into account that the between subject variability in FEV1 and FVC is not constant, but increases with age in the elderly. As the normal range for these parameters is defined by the median ± 2 times the coefficient of variation, the range of normal increases with increasing age. Failure to account for this increased variability in the elderly could misclassify some normal subjects as `below normal' (49). So far, however, the equations presented for this approach are limited to non-hispanic White subjects, and are thus not applicable to the Health ABC population with its substantial representation of Black subjects. In order to evaluate whether our analysis was affected by this potential misclassification, however, we performed a sensitivity analysis by removing 10% our study subjects (N=27) with the highest FEV1/FVC ratio and repeated our analysis. The PILE score with the reduced population (N=241) was 1.30 (95% CI 1.16–1.47, P <0.0001) which is identical to that obtained with all 268 subjects thus indicating the robustness of the PILE score and a lack of effect of this potential misclassification.
Several of the variables tested in the present analysis (IL-6, knee extensor strength, long distance corridor walk) have been independently linked to increased mortality in elderly subjects (10, 19, 50). This suggests that the PILE score may be applicable to general elderly populations, including subjects with obstructive as well as restrictive lung function patterns. However, the scope of the present manuscript was to test the influence of these variables (including the composite PILE score) on mortality in subjects having OAD (N=268), as these variables have been consistently shown to be associated with COPD.
Our study has several other limitations that need to be considered: First, the findings from this analysis are only applicable to community dwelling elderly people due to the strict inclusion criteria employed, according to which only individuals free of disability and mobility impairment were enrolled in the Health ABC study. Moreover, the PILE score, in contrast to the BODE index, has not yet been independently evaluated in a different population. Thus, its predictive capability for mortality may be limited to populations with similar age and functional status as the Health ABC cohort. Second, our analysis is limited to all-cause mortality rather than mortality from respiratory causes, predominantly because our cohort comprised well-functioning elderly with mild-to-moderate OAD rather than diagnosed COPD patients with higher disease severity. Thus, only a small fraction of deaths was documented to be caused solely by respiratory conditions. Third, as bronchodilator challenge was not part of the protocol, participants were tested for spirometry only after their use, and some of the OAD participants may have a mixture of OAD and asthma (51). As only a relatively small proportion of study participants used bronchodilators (1.5%), this is unlikely to affect the interpretation of our results. Finally, we did not have LDCW performance times for 83 participants as they could not complete the test. These participants were combined with the worst performers for LDCW which might have created a potential bias in our analysis.
There are several studies that have utilized multivariate approaches to assess various domains affected by COPD (4, 6, 8) in clinically diagnosed COPD patients. As our analysis involved well-functioning elderly from a community-based cohort study in whom airway obstruction has been suggested to be under-recognized (14) rather than subjects diagnosed with COPD, it may be more suited to understand the relationship between the investigated covariates, OAD and mortality in a general population of elderly. Therefore, the results should not be extrapolated to predict survival in COPD patients. In summary, our investigation suggests that there is a wide gradient of mortality risk in elderly individuals with OAD, and multidimensional indexes such as the PILE score should be further explored in clinical practice to determine their utility for a more differentiated prognosis of survival in this population.
Acknowledgements
This work was supported by research grants and contracts R01-HL-074104, N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106 by the National Institutes of Health. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
List of Abbreviations
- AIC
Akaike information criterion
- ATS
American Thoracic Society
- BIC
Bayesian information criterion
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- ELISA
Enzyme linked immunosorbent assay
- Health ABC
Health, Aging and Body Composition Study
- HR
Hazard ratio
- IL-6
Interleukin-6
- LDCW
Long-distance corridor walk
- OAD
Obstructive airway disease
- PPFEV1
Percent predicted forced expiratory volume in one second
- TNF-α
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes. Thorax. 1990;45:579–585. doi: 10.1136/thx.45.8.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tockman MS, Comstock GW. Respiratory risk factors and mortality: longitudinal studies in Washington County, Maryland. Am Rev Respir Dis. 1989;140:S56–63. doi: 10.1164/ajrccm/140.3_Pt_2.S56. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 5.Steyerberg EW, Borsboom GJ, van Houwelingen HC, Eijkemans MJ, Habbema JD. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med. 2004;23:2567–2586. doi: 10.1002/sim.1844. [DOI] [PubMed] [Google Scholar]
- 6.Briggs A, Spencer M, Wang H, Mannino D, Sin DD. Development and validation of a prognostic index for health outcomes in chronic obstructive pulmonary disease. Arch Intern Med. 2008;168:71–79. doi: 10.1001/archinternmed.2007.37. [DOI] [PubMed] [Google Scholar]
- 7.Jones RC, Donaldson GC, Chavannes NH, et al. Derivation and Validation of a Composite Index of Severity in Chronic Obstructive Pulmonary Disease - The DOSE Index. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200902-0271OC. [DOI] [PubMed] [Google Scholar]
- 8.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Aging Health ABC Description. http://www.nia.nih.gov/ResearchInformation/ScientificResources/HealthABCDescription.htm. Accessed May 15, 2009.
- 10.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 11.Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61:10–16. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 14.Waterer GW, Wan JY, Kritchevsky SB, et al. Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc. 2001;49:1032–1038. doi: 10.1046/j.1532-5415.2001.49205.x. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society Standards for the Diagnosis and Management of COPD. http://www.thoracic.org/sections/copd/resources/copddoc.pdf. Accessed May 15, 2009.
- 17.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 19.Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 20.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 21.Lee ET, Wang JW. Statistical Methods for survival data analysis. 3rd ed. John Wiley & Sons Inc; Hoboken, NJ: 2003. [Google Scholar]
- 22.Henderson AR. The bootstrap: a technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta. 2005;359:1–26. doi: 10.1016/j.cccn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294:1071–1075. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 26.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. Bmj. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knuiman MW, James AL, Divitini ML, Ryan G, Bartholomew HC, Musk AW. Lung function, respiratory symptoms, and mortality: results from the Busselton Health Study. Ann Epidemiol. 1999;9:297–306. doi: 10.1016/s1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 28.Weiss ST, Segal MR, Sparrow D, Wager C. Relation of FEV1 and peripheral blood leukocyte count to total mortality. The Normative Aging Study. Am J Epidemiol. 1995;142:493–498. doi: 10.1093/oxfordjournals.aje.a117665. [DOI] [PubMed] [Google Scholar]
- 29.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 31.Isoaho R, Puolijoki H, Huhti E, Kivela SL, Laippala P, Tala E. Prevalence of chronic obstructive pulmonary disease in elderly Finns. Respir Med. 1994;88:571–580. doi: 10.1016/s0954-6111(05)80004-6. [DOI] [PubMed] [Google Scholar]
- 32.Littlejohns P, Ebrahim S, Anderson R. Prevalence and diagnosis of chronic respiratory symptoms in adults. Bmj. 1989;298:1556–1560. doi: 10.1136/bmj.298.6687.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jette DU, Manago D, Medved E, Nickerson A, Warzycha T, Bourgeois MC. The disablement process in patients with pulmonary disease. Phys Ther. 1997;77:385–394. doi: 10.1093/ptj/77.4.385. [DOI] [PubMed] [Google Scholar]
- 35.Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest. 2002;121:127S–130S. doi: 10.1378/chest.121.5_suppl.127s. [DOI] [PubMed] [Google Scholar]
- 36.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–980. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton AL, Killian KJ, Summers E, Jones NL. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med. 1995;152:2021–2031. doi: 10.1164/ajrccm.152.6.8520771. [DOI] [PubMed] [Google Scholar]
- 38.Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 39.Heijdra YF, Pinto-Plata V, Frants R, Rassulo J, Kenney L, Celli BR. Muscle strength and exercise kinetics in COPD patients with a normal fat-free mass index are comparable to control subjects. Chest. 2003;124:75–82. doi: 10.1378/chest.124.1.75. [DOI] [PubMed] [Google Scholar]
- 40.Sin DD, Man SF. Skeletal muscle weakness, reduced exercise tolerance, and COPD: is systemic inflammation the missing link? Thorax. 2006;61:1–3. doi: 10.1136/thx.2005.044941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Godoy I, Donahoe M, Calhoun WJ, Mancino J, Rogers RM. Elevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patients. Am J Respir Crit Care Med. 1996;153:633–637. doi: 10.1164/ajrccm.153.2.8564110. [DOI] [PubMed] [Google Scholar]
- 42.Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- 43.Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 44.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 45.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 46.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131:1058–1067. doi: 10.1378/chest.06-2336. [DOI] [PubMed] [Google Scholar]
- 47.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 51.Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest. 2004;126:117S–124S. doi: 10.1378/chest.126.2_suppl_1.117S. discussion 159S–161S. [DOI] [PubMed] [Google Scholar]