Abstract
The purpose of this study was to determine the effect (if any) of significant sensory loss on the long-range correlations normally observed in the stride intervals of human gait. Fourteen patients with severe peripheral neuropathy, and 12 gender-, age-, height-, and weight-matched non-diabetic cotrols participated. Subjects walked around an approximately 200m open level walkway for 10 minutes at their comfortable pace. Continuous knee joint kinematics were recorded and used to calculate a stride interval time series for each subject. Power spectral density and detrended fluctuation analyses were used to determine if these stride intervals exhibited long-range correlations. Two alternative hypotheses were proposed. First, if the loss of long-range correlations stems from deteriorated central control of gait, then changes in peripheral sensation should have no effect. Second, if these changes evolve from detrimental changes in control of locomotor timing in general, then similar changes should also occur for patients with peripheral locomotor disorders. Both power spectral density analyses and detrended fluctuation analyses showed that temporal correlations in the stride times of neuropathic and control subjects were statistically identical (p = 0.954 and p = 0.974, respectively), despite slower gait speeds (p = 0.008) and increased stride time variability (p = 0.036) among the neuropathy patients. All subjects in both groups exhibited long-range correlations. These findings demonstrate that the normal long-range correlation structure of stride intervals is unaltered by significant peripheral sensory loss. This further supports the hypothesis that the central nervous system is involved in the regulation of long-range correlations.
Keywords: Walking, Stride Times, Detrended Fluctuation Analysis, Diabetes, Neuropathy
1.INTRODUCTION
Peripheral neuropathy is a significant long-term complication of Diabetes Mellitus that results in a “dying back” of nerve function from the periphery to more proximal regions (24). Neuropathic patients are fifteen times more likely to experience an injury while walking than appropriately matched subjects with intact sensation (3). Neuropathic patients also have a greater incidence of repetitive falls (21) and their increased risk of falling is independent of other comorbidities (20, 22). One predictive factor that has been linked to increased fall risk is increased stride-to-stride variability (15)). However, while neuropathic patients do exhibit increased stride-to-stride variability in both gait cycle timing and walking kinematics, these increases are mainly due to their slower walking speeds and are not directly related to their sensory loss (5). These findings raise the question of whether or not, and to what extent, the loss of peripheral sensory feedback affects these patients’ ability to appropriately regulate gait cycle timing.
One proposed indicator of a person’s capacity to regulate gait cycle timing that is independent of measures of variability, is the presence of long-range correlations in stride time (10, 11). The time it takes to complete each stride varies slightly in individuals walking over a long period of time. These changes are typically small and are often assumed to represent an uncorrelated random process (9). If this were true, the duration of each stride would be completely independent of the duration of the previous stride or strides. In a complex system like the human body that uses a variety of inputs and feedback to regulate movements, it seems unlikely that the duration of one stride would have no effect on the duration of subsequent strides. Instead, it seems more likely, for example, that if you took one longer than average stride, the following stride would be shorter than average to maintain a relatively constant mean. This then raises the question that if such correlations exist, how is a particular stride related to previous strides? If each stride time only depends on a few previous strides, the series is said to exhibit short-range correlations (11). Alternatively, if each stride time depends in some way on many previous strides, the full sequence of stride times may exhibit long-range correlations that ‘decay in a scale-free (fractal-like) power-law fashion’ (11).
In healthy adults walking at a variety of speeds over level ground, the time series of sequential stride intervals exhibited just such long-range correlations (12). Stride intervals become more uncorrelated (random) in elderly subjects, patients with Huntington’s disease (10), Parkinson’s disease (7), and healthy people walking in time with a metronome (12). Herman et al. examined subjects with higher level gait disorders who had experienced falls compared with those that did not. They found that the stride intervals of fallers were more uncorrelated than those of non-fallers (13). Based on these findings, it has been postulated that long-range correlations in stride interval are regulated by supraspinal control mechanisms. The loss of these correlations would then be due to the deterioration of central processing mechanisms resulting from aging or central nervous system disease (11, 13). Recent efforts to model these phenomena have been based on this assumption that stride interval correlations are governed by central mechanisms (1, 25).
To date, however, the question of how (if at all) changes in the peripheral mechanisms involved in the control and regulation of locomotion affect these long-range correlations in gait cycle timing has not been examined. Peripheral sensory feedback mechanisms related to muscle and load receptor reflexes are also thought to play a significant role in regulating gait cycle timing (26). In patients with peripheral sensory neuropathy, these mechanisms would presumably be significantly disrupted. If the loss of the normal long-range correlation structure of stride interval time series reflects changes in the mechanisms that regulate gait cycle timing in general, then similar changes should also occur in patients with peripheral sensory neuropathy. If on the other hand, this loss of long-range correlation structure indicates deterioration specifically of the central control of gait, as has been suggested (10, 13), then changes in peripheral sensation should not affect these long-range correlations. The purpose of this study was therefore to directly test these two competing hypotheses by determining if significant deterioration of peripheral sensory feedback alters the fractal structure of gait stride timing in the same way that changes in central nervous system structures have been shown to.
2.METHODS
The data analyzed in this paper were originally collected as part of a separate study on the variability and local dynamic stability of walking in patients with diabetic neuropathy (5, 6). Briefly, 14 diabetic patients with significant peripheral neuropathy (NP) and twelve control subjects with no history of diabetes or neuropathic illness (CO) participated. These groups were matched on marginal distributions (i.e. similar mean and variance in each group) according to age, gender, height, weight, and BMI (Table 1). Subjects in both groups were overweight (BMI > 25) or obese (BMI > 30), based on CDC guidelines. All participants signed institutionally approved consent forms and were screened to ensure that no subject had a history of medications, surgeries, injuries, or illnesses (other than diabetes and neuropathy where appropriate) that might have affected their walking. NP patients had a significantly reduced passive range of motion at the first metatarsophalangeal joint and at the knee compared to controls (p<0.05), but not at the ankle (p = 0.298). NP subjects also had slightly reduced (p ≥ 0.063) lower extremity muscle strength compared to controls. Refer to (5) for details.
Table 1.
Subject characteristics (mean ± SD) for the diabetic neuropathy (NP) patients and control (CO) subjects, including gender ratio, age, height, weight, and body mass index (BMI).
| NP | CO | p-value | |
|---|---|---|---|
| Gender Ratio (M/F) | 12 / 2 | 10 / 2 | --- |
| Age (yrs) | 61.0± 6.6 | 57.6±7.7 | 0.234 |
| Height (m) | 1.77±0.07 | 1.76±0.08 | 0.689 |
| Weight (kg) | 95.2±14.1 | 91.1±9.8 | 0.411 |
| BMI (kg/m2) | 30.3±4.4 | 29.4±2.2 | 0.498 |
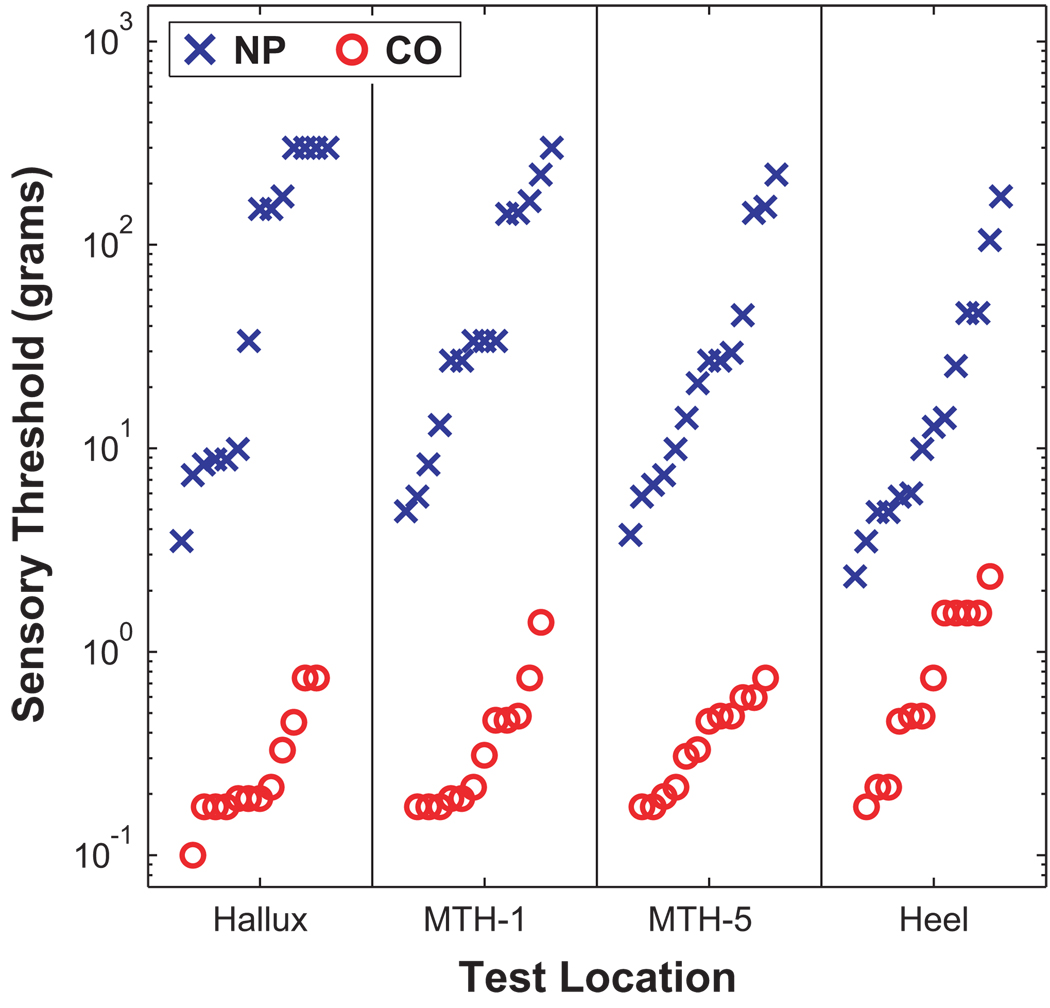
Peripheral sensory status was quantified in all subjects at several locations on the bottom of the foot using touch / pressure sensation (TPS) and vibration perception threshold (VPT) tests. TPS was evaluated with the full set of 20 Semmes-Weinstein monofilaments (North Coast Medical, Inc., San Jose, CA) using a forced choice method. Log transformed minimum detectable buckling forces (grams) were recorded. Subjects unable to feel the largest monofilament (279.4g) were given a score of 300g for “off-scale”. All NP patients had “loss of protective sensation” as determined by the TPS tests (2) and exhibited substantial sensory loss compared to CO subjects (Fig. 1). VPT tests (not shown) yielded similar results (5).
Figure 1.
Minimum sensory detection thresholds (in grams) for each NP and CO subject for 4 locations on the bottoms of the feet: Hallux, 1st metatarsal head (MTH-1), 5th metatarsal head (MTH-5), and Heel. Horizontal displacements of the symbols within each column were made only to distinguish individual subjects. Sensory thresholds in NP subjects were generally 2 to 3 orders of magnitude greater than in CO subjects. Five of the fourteen NP patients exhibited “off-scale” TPS measurements. All differences were highly statistically significant (p = 0.000), demonstrating the severity of sensory loss in these NP patients.
Subjects wore their own comfortable low-rise rubber-soled walking shoes. Three NP patients wore extra-depth shoes, and one NP patient wore custom molded inserts in their shoes. Each subject was fitted with a custom-made self-contained programmable data collection system that received input from a strain gauge electrogoniometer (Penny & Giles, Inc., Santa Monica, CA) placed across the knee joint of the right leg (5). Each subject walked around an approximately rectangular, 7-m wide by 200-m long, open level indoor walking track at his/her self-selected speed and was instructed “to walk in as consistent a manner as possible.” Electrogoniometer data were sampled at 200 Hz continuously for 10 minutes. The total distance walked was measured with a rolling measuring wheel and was used to determine each subject’s average walking speed.
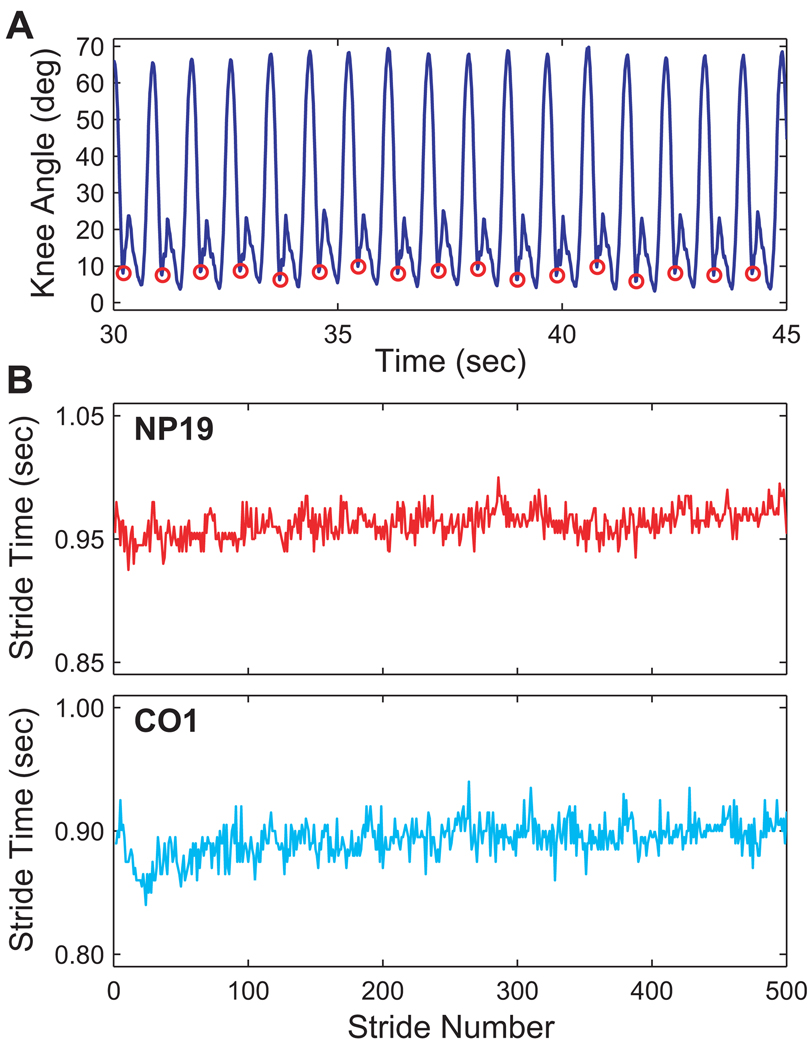
To compute the total number of strides and stride times for each subject, points of maximum knee joint extension just prior to heel strike were extracted for each successive stride from the continuous knee goniometer data (Fig. 2A). While these points did not exactly correspond to the actual times of heel contact, the parameters quantified from these data required only that the duration of each complete stride of gait be known. Thus, the definition of where exactly each stride began or ended was arbitrary, as long as the same point during each gait cycle was chosen consistently. The point of maximum end-swing knee extension was used because it was easy to extract and because it was relatively close to the actual heel strike times. We then analyzed the time series of stride times obtained from each subject (e.g., Fig. 2B). The number of strides recorded ranged from 445 to 620 for NP patients and from 488 to 668 for CO subjects. The measurement of stride time had a resolution of ±0.005 sec.
Figure 2.
A) Example segment of knee angle data with points of maximum end-swing knee extension highlighted (red circles). Stride times were defined as the elapsed time between these successive points. B) Example time series of stride times for two representative subjects; NP19 and CO1. NP patients exhibited greater variability in their stride intervals than CO subjects. However, both time series appear to exhibit random variations.
Average walking speeds were calculated by dividing the total distance each subject walked by 10 min. Average stride times and standard deviations of stride times were calculated for each subject from the individual stride times extracted from the continuous time series data. These values were used as independent measures in a single-factor general linear model (GLM) analysis of variance (ANOVA) for a randomized block design to test for differences between NP and CO groups (5).
To determine if subjects exhibited long-range correlations, stride interval time series were analyzed using two different methods. The first method used was based on calculating the power spectral density (PSD) distribution for each time series. The entire time series of stride times for each subject was used for these analyses. For each time series, the log of the squared Fourier transform amplitude, S(f), was plotted as a function of the log of the frequency, f. In the present analysis, the units on f were inverse stride number. These log-log PSD plots were then fitted with a linear function using a standard least squares regression. The negative slope of this line defined the scaling exponent β. A value of β = 0 indicates that the time series is completely uncorrelated (i.e., white noise). β = 1 for 1/f or “pink” noise and β = 2 for brown noise (i.e., integrated white noise or Brownian motion) (11). A single value of β was calculated for the stride time data for each subject. These values were then used as independent measures in a single-factor GLM ANOVA for a randomized block design to test for differences between NP and CO groups.
The second method applied was detrended fluctuation analysis (DFA). DFA is based on a classic root-mean square analysis of a random walk. This method is advantageous because it reduces noise effects and removes local trends making it less likely to be affected by nonstationarities (11). Complete details of the methodology are published elsewhere (11, 17–19). In brief, the data sequence was first integrated and then divided into equal, non-overlapping segments of length n. In each segment, the series was detrended by subtracting a least squares linear regression line fit to that segment. The squares of the integrated, detrended data points (i.e, residuals) were then averaged over the entire data set and the square root of the mean residual, F(n), was calculated. This process was repeated for different values of segment lengths, n, ranging from 4 to 400 strides. A variety of other ranges were also tested. Statistical results were not sensitive to the range chosen. This particular range was used to eliminate end effects while allowing determination of correlations over several decades.
Typically, F(n) increases with n and a graph of log[F(n)] versus log(n) will exhibit a power-law relationship indicating the presence of scaling, such that F(n) ≈ nα. These log[F(n)] versus log(n) plots were then fitted with a linear function using a standard least squares regression approach. The slope of this line defines the scaling exponent α. A value of α = 0.5 indicates the stride intervals are completely uncorrelated (i.e., white noise). When, α < 0.5, the stride intervals contain short-range correlations. Long-range correlations are present when 0.5 < α ≤ 1.0 (11). For stationary data of infinite length, this scaling exponent is directly related to the exponent extracted from the log-log PSD algorithm through the equation α = (1+β)/2 (11, 19). A single value of α was calculated for the stride time data for each subject. These values were then used as independent measures in a single-factor GLM ANOVA for a randomized block design to test for differences between NP and CO groups.
To determine statistically whether long-range correlations were different from an uncorrelated process, we used surrogate data (11, 23). For each subject, 20 surrogate data sets were generated by shuffling their original time series in random order. Thus, each surrogate represented a time series of temporally independent values with the exact same amplitude distribution as the original time series. DFA analysis was run on each of these surrogates to obtain a value of α. The mean and standard deviation of α for these 20 surrogates was then calculated and compared with the value from the original series. For each subject, if the original α was more than 3 standard deviations away from the mean of the 20 surrogates, the result was considered significant (11, 23).
3.RESULTS
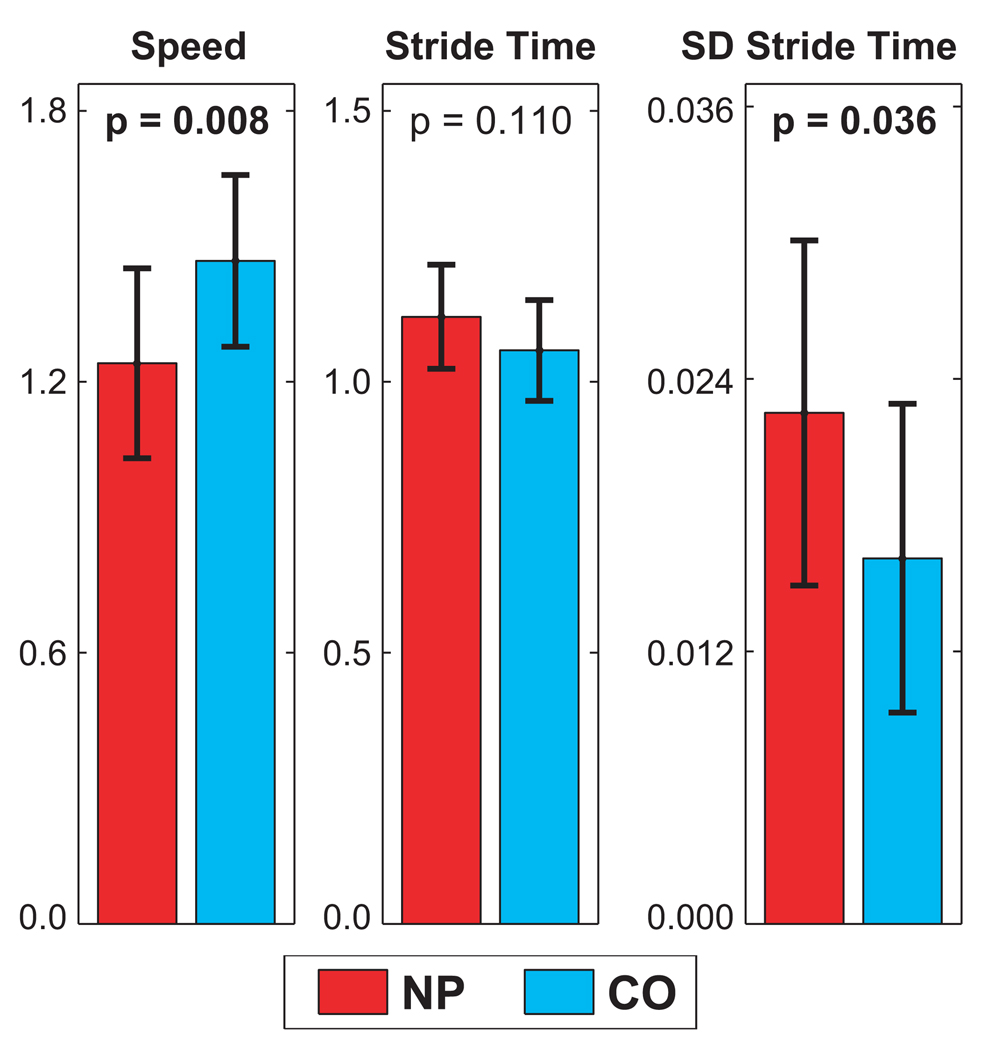
Average self-selected walking speeds were significantly slower (p = 0.008) for NP patients compared to CO subjects (Fig. 3). These findings were similar to those of previous studies in NP patients (4, 14, 16). Average stride times for NP patients were not significantly different from those of CO subjects (p=0.110). However, the difference between NP patients and CO subjects for the standard deviations of stride times was statistically significant (p=0.036; Fig. 3). This increased variability can also be seen qualitatively in Fig. 2B. The NP patients therefore exhibited greater variability in their gait cycle timing.
Figure 3.
Average walking speed (m/s), average stride times (s), and standard deviations of stride times (s) for neuropathic (NP) and control (CO) subjects. Error bars represent the between-subject standard deviations for each group. ANOVA p-values are given for each comparison (adapted from Dingwell, 2001 (5)).
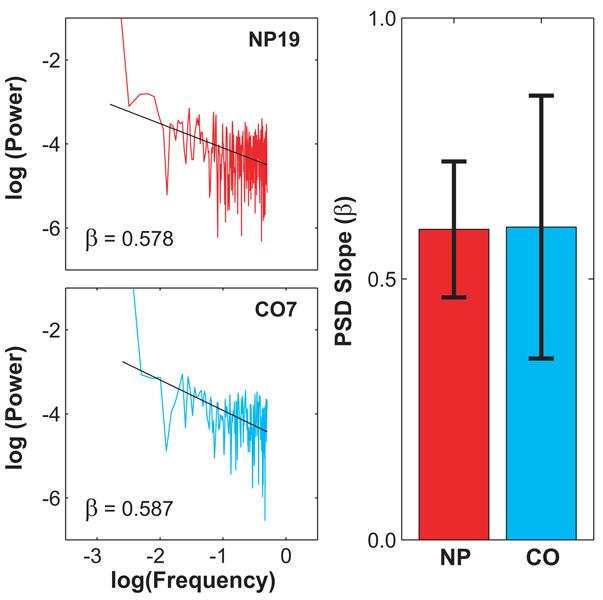
Figure 4A shows representative log-log PSD plots for one representative NP patient and one representative CO subject. Both plots exhibit a smooth and approximately linear decay in spectral power at increasing frequencies. Similar results were obtained from all subjects tested. The scaling exponents, β, defined by the slopes of linear fits to these plots were 0.595 ± 0.130 for the NP patients and 0.599 ± 0.252 for the CO subjects (Fig. 4B). Statistically, these values were nearly identical (p = 0.954). Thus, the structure of the long-range correlations in the NP patients walking patterns, as quantified by β, were completely unaffected by the significant deterioration in peripheral sensory feedback that these patients experienced.
Figure 4.
A) An example of the effect of neuropathy on the results of power spectral analysis of the stride interval series. Plots of two representative subjects illustrate that there are no differences between the control (CO7) and neuropathic patient (NP19). The values of β for these two subjects were 0.587 and 0.578, respectively. B) Average values of β for the NP and CO groups. Error bars represent the between-subject standard deviations for each group. These values were statistically identical (p = 0.954).
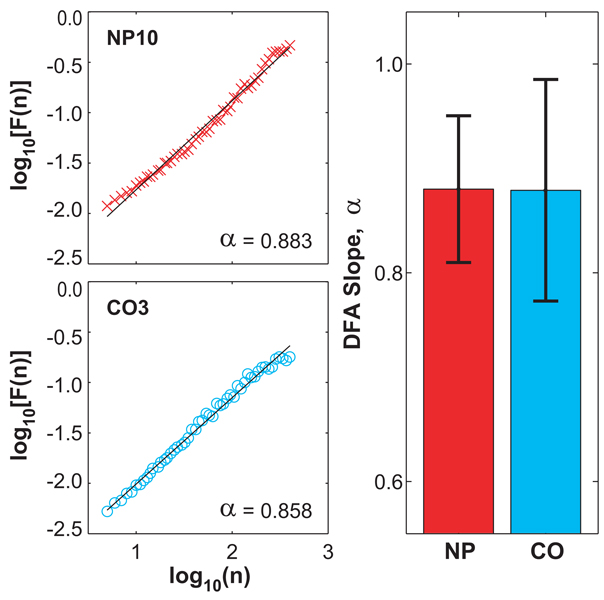
Figure 5A shows the results of the DFA analysis for the same subjects shown in Fig. 4A. Both plots exhibit a smooth and approximately linear increase in log[F(n)] with increasing log(n). All fits in all subjects were highly statistically significant (r2 ≥ 0.97, p < 0.001). All subjects in both groups exhibited long-range correlations in their stride intervals (α > 0.5). The scaling exponents, α, as defined from the slopes of linear fits to these curves, were 0.880 ± 0.070 for the NP patients and 0.879 ± 0.106 for the CO subjects (Fig. 5B). These values of α for the NP and CO groups were also statistically identical (p = 0.974; Fig. 5B). Thus, values of the scaling exponents, α, were also completely unaffected by the significant peripheral neuropathy experienced by these NP patients.
Figure 5.
A) An example of the effect of neuropathy on the results of detrended fluctuation analysis of the stride interval series. Plots of two representative subjects illustrate that there are no differences between the control (CO3, ‘o’) and neuropathic patient (NP10, ‘x’). The values of α for these two subjects were 0.858 and 0.883, respectively. B) Average values of α for the NP and CO groups. Error bars represent the between-subject standard deviations for each group. These values were statistically identical (p = 0.974).
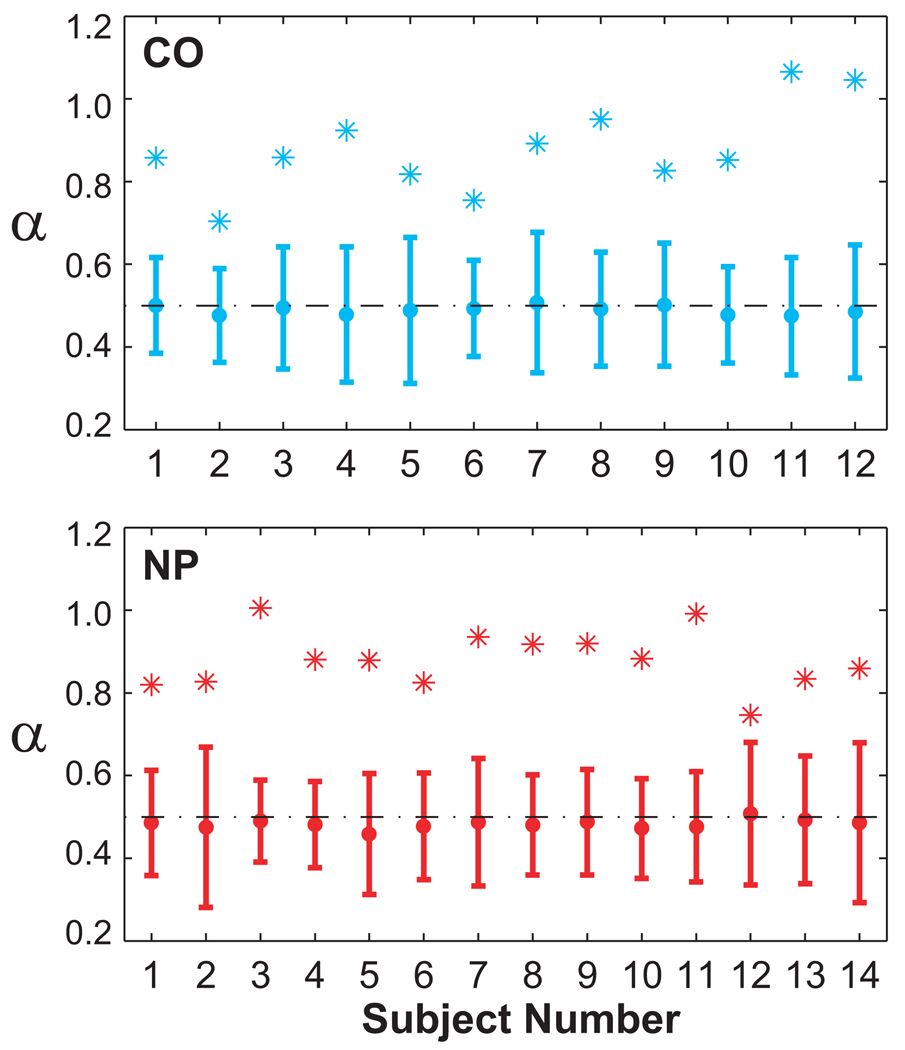
The surrogate analyses further verified the presence of long-range correlations in all subjects. Figure 6 shows the mean and ±3 standard deviations of the surrogate data. For all subjects, the average scaling exponents for the surrogates were very close to 0.5 (i.e., white noise). However, the scaling exponents for the original time series for all subjects were significantly different from 0.5 and from an uncorrelated random process (i.e., white noise).
Figure 6.
Results of surrogate analyses for all subjects (Top: CO, Bottom: NP). Slope values, α, for each original stride time series are shown as (*). Mean α values for each set of 20 surrogate data sets are shown with error bars representing ±3 standard deviations from the mean. All original α values fell outside of this range. Therefore, each subject’s stride time series was significantly different from an uncorrelated, random series.
For subjects in both groups, the average values of α and β agreed with the theoretical prediction that α ≈ (1+β)/2 (11, 19). In the case of both measures, these findings demonstrate that the normal long-range correlation structure of stride intervals was unaltered by significant peripheral neuropathy. Thus, the hypothesis that changes in α and/or β reflect deterioration of the mechanisms in general that regulate gait cycle timing was rejected. Instead, the alternative hypothesis that loss of these long-range correlations results primarily from deterioration of the central mechanisms controlling gait cycle timing was supported.
4. DISCUSSION
Peripheral sensory feedback from muscle and load receptor reflexes is thought to play a significant role in regulating gait cycle timing (26). This sensory feedback is severely disrupted in patients with significant diabetic neuropathy, suggesting that the ability of these patients to appropriately regulate gait cycle timing should also be significantly disrupted. Indeed, neuropathic patients do exhibit increased stride-to-stride variability in gait cycle timing (5). The presence of long-range correlations in stride interval time series has been proposed as an indicator of a healthy nervous system’s capacity to regulate gait cycle timing, independent of variability (11). Statistical measures quantifying these long-range correlations have been shown to be a sensitive indicator of the degeneration of central nervous system mechanisms involved in regulating gait cycle timing (10, 13). The purpose of the present study was to determine if these measures were equally sensitive to the degeneration of the peripheral nervous system.
Despite their significant peripheral sensory loss, patients with even severe diabetic neuropathy maintained normal long-range correlations in their stride intervals. In fact, for both of the dependent measures collected (α and β), the NP and CO groups were statistically identical (p > 0.95). The very large magnitudes of the p-values we obtained strongly suggest that the statistical possibility of Type II error was extremely small.
One reason neuropathic patients retained long-range correlations may be that diabetic neuropathy has little or no effect on the central nervous system, which is thought to be the primary source of these correlations (1, 10, 13). The findings of the present study generally support this view. Of course, central and peripheral mechanisms are closely coupled and changes in one are likely to affect changes in the other. If so, however, this would also be true for Huntington’s disease, the elderly, and the other central deteriorations that have been studied previously. Somehow, people with peripheral deficits (at least diabetic neuropathic patients) can accommodate and adapt to their peripheral sensory loss, whereas people with central deficits do not.
It should also be noted that the sensory loss in these neuropathic patients was not complete: i.e., they still retained proximal somatosensory inputs, as well as visual and vestibular feedback information. The present findings cannot completely rule out the possibility that long-range stride interval correlations might still be disrupted in patients with more complete somatosensory loss and/or significant degradation of multiple sensory systems. However, in either case, the present results clearly demonstrate that distal sensory feedback is not necessary for maintaining the complex structure of stride cycle timing exhibited by healthy humans.
Another possible reason that differences in long-range correlations were not found in these patients could be adaptation effects. Peripheral neuropathy is a slowly advancing disease, and these patients had been living with significant and progressive sensory loss for many years. Their walking patterns reflect not only the direct effects of the neuropathy, but also of any adaptive mechanisms they developed to compensate for their sensory loss (e.g., perhaps by relying more heavily on other sources of sensory information). These adaptations may enable them to maintain normal long-range correlations. However, general aging is also a slow process where reduced proprioception and muscle weakness may also cause people to adapt their gait patterns in different ways. Likewise, although the rate of progression of the disease does vary, Huntington’s disease results from the gradual degeneration of neurons that progresses over many years. Unlike the peripheral neuropathy subjects examined here, however, elderly individuals and patients with Huntington’s disease experience a loss of long-range correlations in their stride intervals (10). It is more likely then, that this loss of correlation structure in this population is due to the deterioration of central nervous system mechanisms that regulate gait cycle timing, rather than a general adaptation effect.
NP and CO groups were matched for age, height, weight, and gender. Ideally, to make completely valid comparisons, all subjects would also have walked barefoot or worn the same shoes. In patients with diabetic neuropathy, however, there is a high risk of injury to the feet when walking barefoot or in unfamiliar shoes. For this reason, all subjects were permitted to wear their own shoes. Only 4 of the 14 NP subjects wore corrective shoes, and these included only relatively minor modifications. There were no differences for our final dependent measures (α and β) between these 4 subjects and the rest of the NP group. Therefore, we are confident that these minor differences in footwear did not affect our final results.
The presence of long-range (fractal) correlations is believed to reveal something about its behavior in normal and diseased conditions. Analysis of the time-series of heartbeats for patients with severe heart disease and elderly showed that these populations could be differentiated from healthy individuals by quantifying the scaling exponent, α (8, 18). The value of α is reduced in the elderly and approaches 0.5 for the diseased population, indicating a loss of long-range correlations. If this scaling exponent could be used as a predictor of heart disease, could it also be used as a predictor of falls? Previous research found that people with higher level gait disorders who had a history of falls had more uncorrelated stride intervals than non-fallers (13). Based on these results, it could be suggested that the loss of long-range correlations might also indicate an increased risk of falls. The results of the present study do not support this belief, however, since there were no differences in either α or β measures for the NP patients, even though they are well known to be at greater risk of falling (20, 22). It should be noted that while the patients tested in the present study were considered to be at high risk, none of them had a history of falls in the previous year. Thus, while the loss of these long-range correlations may suggest a history of falls (13), the present findings clearly demonstrate that these measures may give no indication of a patient’s future risk of falls. Therefore, the use of measures of α and/or β to predict fall risk prospectively is strongly cautioned against.
It is still unclear what specific mechanisms are involved in generating and regulating the fractal dynamics of the stride interval. We now know that the fractal dynamics of the stride interval do not change in patients with significant peripheral neuropathy, whereas they do for patients with Huntington’s disease, the elderly, children, and people walking in time with a metronome. Together, these findings do suggest that these long-range correlations are centrally controlled. Further research needs to be done to determine the clinical relevance (if any) of long-range correlations in predicting disease: e.g., can we identify whether a person has a peripheral or central disorder based purely on the α or β measures? Additional research should also focus on identifying those specific mechanisms within the central nervous system (e.g., the cerebellum, basal ganglia, etc.) that give rise to these long-range correlations.
ACKNOWLEDGEMENTS
Partial funding for this project was provided by grant # RG-02-0354 from the Whitaker Foundation and by grant #EB003425 from the National Institutes of Health. The authors also gratefully thank Dr. Peter R. Cavanagh and Mrs. Mary B. Becker, R.N. for their assistance in collecting the data used in this study.
REFERENCES
- 1.Ashkenazy Y, Hausdorff JM, Ivanov PC, Stanley HE. A stochastic model of human gait dynamics. Physica A. 2002;316:662–670. [Google Scholar]
- 2.Birke JA, Sims DS. Plantar Sensory Threshold in the Ulcerative Foot. Lepr Rev. 1986;57:261–267. doi: 10.5935/0305-7518.19860028. [DOI] [PubMed] [Google Scholar]
- 3.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with Gait and Posture in Neuropathic Patients with Insulin-Dependent Diabetes Mellitus. Diabet Med. 1992;9:469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 4.Courtemanche R, Teasdale N, Boucher P, Fleury M, Lajoie Y, Bard C. Gait Problems in Diabetic Neuropathic Patients. Arch Phys Med Rehabil. 1996;77:849–855. doi: 10.1016/s0003-9993(96)90269-5. [DOI] [PubMed] [Google Scholar]
- 5.Dingwell JB, Cavanagh PR. Increased Variability of Continuous Overground Walking in Neuropathic Patients Is Only Indirectly Related to Sensory Loss. Gait & Posture. 2001;14:1–10. doi: 10.1016/s0966-6362(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 6.Dingwell JB, Cusumano JP, Sternad D, Cavanagh PR. Slower Speeds in Neuropathic Patients Lead to Improved Local Dynamic Stability of Continuous Overground Walking. J Biomech. 2000;33:1269–1277. doi: 10.1016/s0021-9290(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 7.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson's disease. 2005;20:1109–1114. doi: 10.1002/mds.20507. [DOI] [PubMed] [Google Scholar]
- 8.Goldberger AL, Amaral LAN, Hausdorff JM, Ivanov PC, Peng C-K, Stanley HE. Fractal Dynamics in Physiology: Alterations with Disease and Aging. Proc Natl Acad Sci USA. 2002;99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin L, West DJ, West BJ. Random Stride Intervals with Memory. J Biol Phys. 2000;26:185–202. doi: 10.1023/A:1010322406831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered Fractal Dynamics of Gait: Reduced Stride Interval Correlations with Aging and Huntington's Disease. J Appl Physiol. 1997;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL. Is Walking a Random Walk? Evidence for Long-Range Correlations in Stride Interval of Human Gait. J Appl Physiol. 1995;78:349–358. doi: 10.1152/jappl.1995.78.1.349. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff JM, Purdon PL, Peng CK, Ladin Z, Wei JY, Goldberger AL. Fractal Dynamics of Gait: Stability of Long-Range Correlations in Stride Interval Fluctuations. J Appl Physiol. 1996;80:1448–1457. doi: 10.1152/jappl.1996.80.5.1448. [DOI] [PubMed] [Google Scholar]
- 13.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a "cautious" gait: why do certain older adults walk fearfully? Gait Posture. 2005;21:178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Katoulis EC, Ebdon-Parry M, Lanshammar H, Vileikyte L, Kulkarni J, Boulton AJM. Gait Abnormalities in Diabetic Neuropathy. Diabetes Care. 1997;20:1904–1907. doi: 10.2337/diacare.20.12.1904. [DOI] [PubMed] [Google Scholar]
- 15.Maki BE. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? J Am Ger Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 16.Mueller MJ, Sinacore DR, Hoogstrate S, Daly L. Hip and Ankle Walking Strategies: Effect on Peak Plantar Pressures and Implications for neuropathic Ulceration. Arch Phys Med Rehabil. 1994;75:1196–1200. doi: 10.1016/0003-9993(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 17.Peng C-K, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic Organization of DNA Nucleotides. Phys Rev E. 1994;49:1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- 18.Peng C-K, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 19.Peng C-K, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-Range Anticorrelations and Non-Gaussian Behavior of the Heartbeat. Phys Rev Let. 1993;70:1343–1346. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- 20.Richardson JK. Factors Associated With Falls in Older Patients With Diffuse Polyneuropathy. J Am Ger Soc. 2002;50:1760–1766. doi: 10.1046/j.1532-5415.2002.50503.x. [DOI] [PubMed] [Google Scholar]
- 21.Richardson JK, Ching C, Hurvitz EA. The Relationship Between Electromyographically Documented Peripheral Neuropathy and Falls. J Am Geriatr Soc. 1992;40:1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 22.Richardson JK, Hurvitz EA. Peripheral Neuropathy: A True Risk Factor for Falls. J Gerontol A Biol Sci Med Sci. 1995;50:M211–M215. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- 23.Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for Nonlinearity in Time Series: the Method of Surrogate Data. Physica D. 1992;58:77–94. [Google Scholar]
- 24.Vinik AI, Holland MT, LeBeau JM, Liuzzi FJ, Stansberry KB, Colen LB. Diabetic Neuropathies. Diab Care. 1992;15:1926–1975. doi: 10.2337/diacare.15.12.1926. [DOI] [PubMed] [Google Scholar]
- 25.West BJ, Scafetta N. Nonlinear dynamical model of human gait. Phys Rev E. 2003;67:051917. doi: 10.1103/PhysRevE.67.051917. [DOI] [PubMed] [Google Scholar]
- 26.Zehr EP, Stein RB. What Functions do Reflexes Serve During Human Locomotion? Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]