Abstract
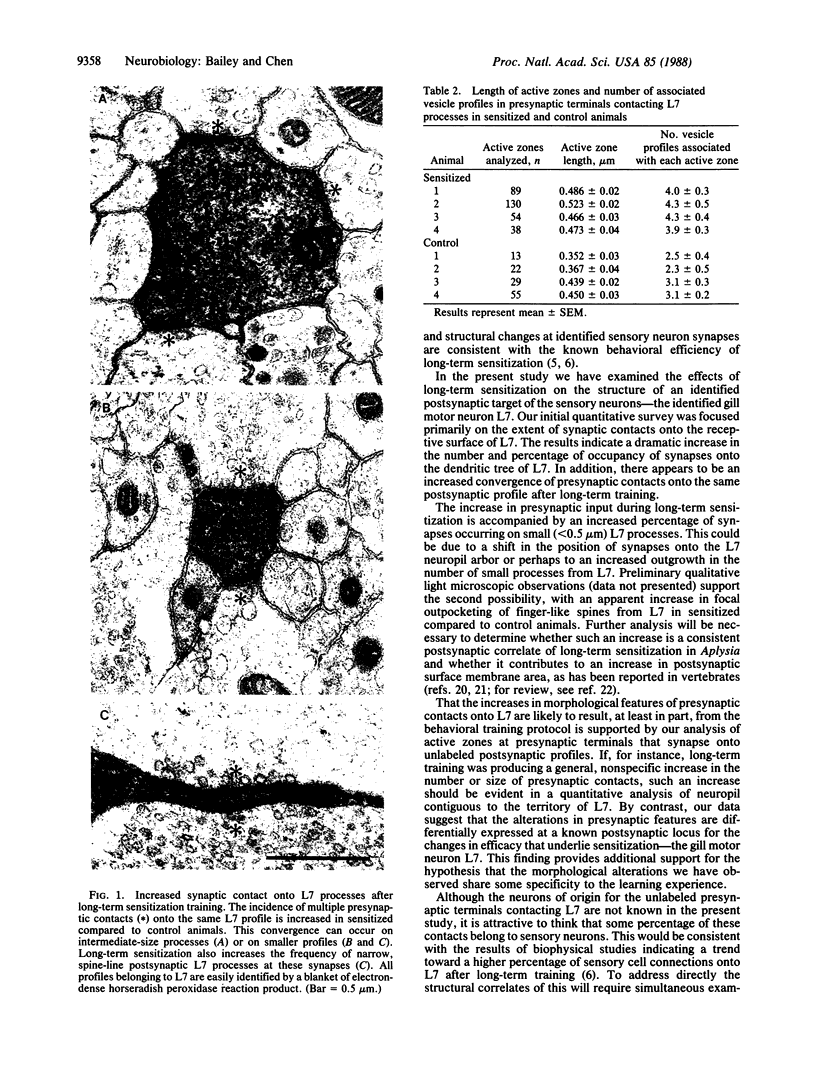
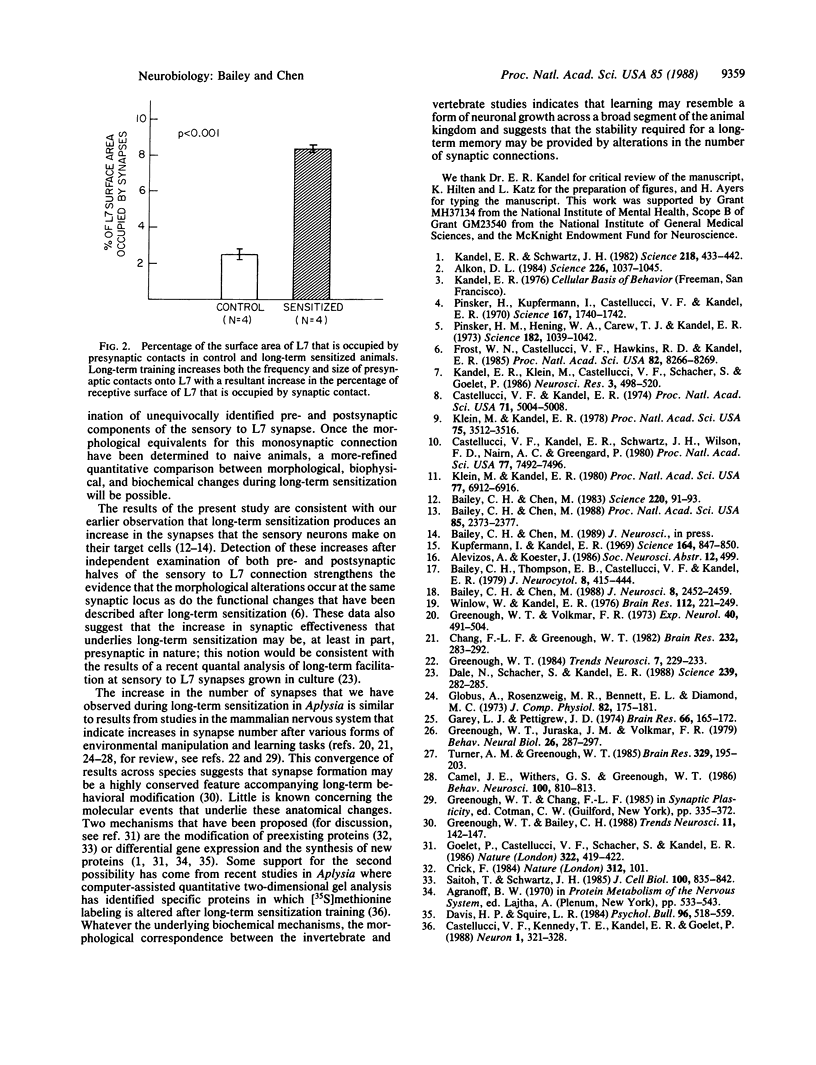
We have used the gill and siphon withdrawal reflex of Aplysia to study the morphological basis of the persistent synaptic plasticity that underlies long-term sensitization. One critical locus for storage of the memory for sensitization is the set of monosynaptic connections between identified siphon sensory neurons and gill and siphon motor neurons. To complement previous morphological studies of the presynaptic terminals of identified sensory neurons, we examined the effects of long-term sensitization on the structure of an identified postsynaptic target--the gill motor neuron L7. We found an increase in the frequency, size, and vesicle complement of presynaptic contacts onto L7 processes in sensitized compared to control animals. Combined, these data indicate a striking increase in the percentage of the surface area of L7 that is occupied by synaptic contacts after long-term training. These results are consistent with our observations that sensitization produces an increase in the synapses that the sensory neurons make on their target cells and provide additional support for the hypothesis that changes in synapse number may represent a mechanism underlying long-term memory.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983 Apr 1;220(4592):91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Morphological basis of short-term habituation in Aplysia. J Neurosci. 1988 Jul;8(7):2452–2459. doi: 10.1523/JNEUROSCI.08-07-02452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Thompson E. B., Castellucci V. F., Kandel E. R. Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. J Neurocytol. 1979 Aug;8(4):415–444. doi: 10.1007/BF01214801. [DOI] [PubMed] [Google Scholar]
- Camel J. E., Withers G. S., Greenough W. T. Persistence of visual cortex dendritic alterations induced by postweaning exposure to a "superenriched" environment in rats. Behav Neurosci. 1986 Dec;100(6):810–813. doi: 10.1037//0735-7044.100.6.810. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kennedy T. E., Kandel E. R., Goelet P. A quantitative analysis of 2-D gels identifies proteins in which labeling is increased following long-term sensitization in Aplysia. Neuron. 1988 Jun;1(4):321–328. doi: 10.1016/0896-6273(88)90080-3. [DOI] [PubMed] [Google Scholar]
- Chang F. L., Greenough W. T. Lateralized effects of monocular training on dendritic branching in adult split-brain rats. Brain Res. 1982 Jan 28;232(2):283–292. doi: 10.1016/0006-8993(82)90274-8. [DOI] [PubMed] [Google Scholar]
- Crick F. Memory and molecular turnover. Nature. 1984 Nov 8;312(5990):101–101. doi: 10.1038/312101a0. [DOI] [PubMed] [Google Scholar]
- Dale N., Schacher S., Kandel E. R. Long-term facilitation in Aplysia involves increase in transmitter release. Science. 1988 Jan 15;239(4837):282–285. doi: 10.1126/science.2892269. [DOI] [PubMed] [Google Scholar]
- Davis H. P., Squire L. R. Protein synthesis and memory: a review. Psychol Bull. 1984 Nov;96(3):518–559. [PubMed] [Google Scholar]
- Frost W. N., Castellucci V. F., Hawkins R. D., Kandel E. R. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus A., Rosenzweig M. R., Bennett E. L., Diamond M. C. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973 Feb;82(2):175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Juraska J. M., Volkmar F. R. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979 Jul;26(3):287–297. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Volkmar F. R. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973 Aug;40(2):491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Klein M., Castellucci V. F., Schacher S., Goelet P. Some principles emerging from the study of short- and long-term memory. Neurosci Res. 1986 Sep;3(6):498–520. doi: 10.1016/0168-0102(86)90050-7. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I., Kandel E. R. Neuronal controls of a behavioral response mediated by the abdominal ganglion of Aplysia. Science. 1969 May 16;164(3881):847–850. doi: 10.1126/science.164.3881.847. [DOI] [PubMed] [Google Scholar]
- Pinsker H. M., Hening W. A., Carew T. J., Kandel E. R. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973 Dec 7;182(4116):1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Pinsker H., Kupfermann I., Castellucci V., Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. M., Greenough W. T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985 Mar 11;329(1-2):195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Winlow W., Kandel E. R. The morphology of identified neurons in the abdominal ganglion of Aplysia californica. Brain Res. 1976 Aug 13;112(2):221–249. doi: 10.1016/0006-8993(76)90282-1. [DOI] [PubMed] [Google Scholar]






