Abstract
Pigmentation of murine cardiac tricuspid valve leaflet is associated with melanocyte concentration, which affects its stiffness. Owing to its biological and viscoelastic nature, estimation of the in situ stiffness measurement becomes a challenging task. Therefore, quasi-static and nanodynamic mechanical analysis of the leaflets of the mouse tricuspid valve is performed in the current work. The mechanical properties along the leaflet vary with the degree of pigmentation. Pigmented regions of the valve leaflet that contain melanocytes displayed higher storage modulus (7–10 GPa) than non-pigmented areas (2.5–4 GPa). These results suggest that the presence of melanocytes affects the viscoelastic properties of the mouse atrioventricular valves and are important for their proper functioning in the organism.
Keywords: melanocytes, storage modulus, pigmentation, nanoindentation, valve, heart
The cardiac valves display complex biomechanical properties that allow them to function in directed blood flow during the cardiac cycle. The understanding of cardiac valve function requires analyses at the organ, tissue and cellular scales since mechanical stimuli occur and have effects at all these levels. The mature atrioventricular (AV) valves (mitral and tricuspid) have leaflets composed of extracellular matrix (ECM), valvular interstitial cells and overlying endothelial cells. The mechanical requirements of the valve for elasticity, compressibility, stiffness and strength, as well as durability throughout the lifespan of an individual are achieved primarily by the highly organized and compartmentalized ECM composition of the leaflets (Rabkin-Aikawa et al. 2005; Schoen 2005). Valvular interstitial and endothelial cells are affected by the local stress environments of the valve leaflet and appear to interact in managing ECM formation and remodelling (Rabkin-Aikawa et al. 2004; Butcher & Nerem 2006; Sacks & Yoganathan 2007). Recently, a few studies have indicated that, apart from these two cell lineages, cells of neural crest origin can also be found in the murine developing AV valves. At least some of these cells differentiate as melanocytes, produce melanin and persist into adulthood. It is still unknown whether these neural crest-derived cells contribute to valve development and whether the mature melanocytes exert a specific function (Mjaatvedt et al. 2005; Nakamura et al. 2006; Brito & Kos 2008; Yajima & Larue 2008).
Mutant mice that lack cutaneous and cardiac melanocytes live well into adulthood and do not present any gross abnormalities in the heart (Brito & Kos 2008). This suggests that the function of cardiac melanocytes may be more subtle and become critical in situations of stress. Skin melanocytes have a protective role against UV damage (Miyamura et al. 2007). Inner ear melanocytes have a structural function and also seem to participate in controlling ionic balance for the maintenance of the endocochlear potential (Tachibana 2001). Except for the structural role, these other functions do not seem to pertain to cardiac melanocytes. Rather, their location and timing of arrival in the heart suggest that they may be involved in AV valve development from the endocardial cushions (Brito & Kos 2008). This process requires the expression of specific ECM proteins and remodelling enzymes such as metalloproteases (Lincoln et al. 2006). Given that melanocyte precursors have been shown to express and secrete metalloproteases (Lei et al. 2002), they may contribute to valve remodelling. At a slower pace, this function could continue during adulthood when melanocytes along with interstitial cells could be involved in the maintenance of tissue homeostasis and influence the mechanical properties of the AV valves. Therefore, we sought to establish how the mechanical properties of the valve leaflets are affected by the presence of melanocytes.
There has been considerable interest in correlating the multi-scale structure of biological systems (such as toucan beak, human hair, cartilage, bacteria) with their mechanical behaviour (Seki et al. 2005; Wei & Bhushan 2006; Yuan & Verma 2006; Franke et al. 2007; Wang et al. 2007). Seki et al. (2005) demonstrated the viscoelastic response of the outer keratin layer of the toucan beak that comprises hexagonal scales glued together and the internal fibrous network of closed cells containing calcium-rich proteins. The keratin layer showed strain rate sensitivity with a transition from slippage of the scales owing to the release of organic glue, whereas the closed cell foam fibres displayed increased stiffness (approx. 12.7 GPa) owing to their higher calcium content. Nanotribological testing on the cuticle cells of human hair depicted differences in the coefficient of friction and nature of Caucasian and Asian hairs (Wei & Bhushan 2006). It was also observed that hair surface (approx. 200 nm) is softer than the hair core. Consequently, scratching against the cuticle resulted in higher hair damage, with the mode of fracture depending upon the hardness of the cuticle (Wei & Bhushan 2006). Wang et al. (2007) applied nanoindentation techniques on bacterial cells and showed that the elastic modulus of nickel-coated Escherichia coli was 17 times larger than that of bare bacterial cells (approx. 71.48 GPa and approx. 4.38 GPa, respectively) (Wang et al. 2007). Apart from the generic and conventional histological evaluation as a measure of tissue repair, nanomechanical analysis has shown differences in the contact stiffness of repair cartilage being 10 times lower than that of hyaline cartilage (approx. 4.0 kN m−1) at 0.625–10 mN load (Franke et al. 2007). Hence, nanomechanical testing also serves as a complementary measure of predicting the health of cartilage.
Most of the research concerned with heart valve mechanics has been conducted using the porcine or ovine models (Sacks & Yoganathan 2007). To our knowledge, there have been no reports documenting the biomechanical properties of the murine valve. Nevertheless, over the last decade, the mouse has emerged as a powerful model for studies of genetic mutations that underlie a variety of pediatric and/or adult cardiovascular disorders (Yutzey & Robbins 2007). The very small size of the murine valve leaflets (between 500 µm and 1 mm) makes them unsuitable for conventional macroscale mechanical testing techniques. As such, in this study, we have used nanoindentation as a means to evaluate the quasi-static and dynamic mechanical properties of the tricuspid valve leaflet of the wild-type mouse. Given the localized homogeneity of the valve leaflet, this technique has also allowed us to probe for differences in the mechanical properties of pigmented versus non-pigmented areas.
The animals used in this study were obtained from our colony housed in the Animal Care Facility at Florida International University (Miami, FL, USA). Wild-type mice (C57BL6/J) were originally obtained from The Jackson Laboratory, Maine. The hearts from adult mice (two months old) were dissected and placed in phosphate-buffered saline (PBS; pH 7.3) on ice. They were immediately opened with fine forceps from their ventral sides to visualize the AV valves. The tricuspid valve was then removed and the leaflets gently separated. Each leaflet was placed on a glass microscope slide and kept cold (approx. 2°C) until measurements could be taken in the next 15–20 min. It took approximately 10 min to dissect the leaflet and approximately 30–45 min to perform the nanoindentation analysis. It was critical to keep the tissue moist at all times with a minimal amount of PBS. A special fluid-cell Berkovich diamond tip (with an extended shaft of 4 mm) was used to allow testing under a moist environment.
Nanomechanical testing involved: (i) tracing the test location to identify the area of interest; (ii) performing a quick surface contact for probe calibration so as to retain the tissue properties; and (iii) subsequent testing of the tissue, which yielded the corresponding nanomechanical properties. The tissues are very soft and, thus, require high sensitivity to soft loading (tens of micronewtons) to evaluate their nanodynamic response. Therefore, the testing required extending the nanoindenter limit, which is usually used for much harder materials. Additionally, the procedure was quite demanding in terms of time constraints owing to the complexity of the dissection protocol and the requirements for tissue integrity and longevity, restricting our sample size (preventing us from obtaining measurements from large numbers of samples). The results reported from the nanodynamic mechanical tests on the tricuspid leaflets of three wild-type mice were consistent for at least three data points for each test region. The average hardness is reported from quasi-static test results obtained from five tests at different loads (30–60 µN) on a single mouse tricuspid leaflet.
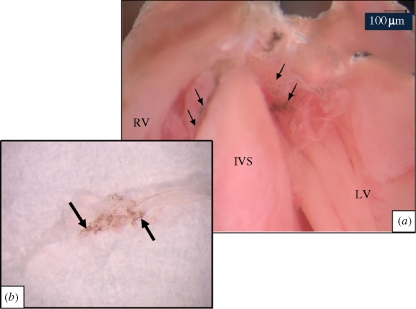
A whole-mount preparation of the wild-type mouse heart is shown in figure 1a and that of a dissected leaflet is shown in figure 1b indicating the presence of melanocytes in the tricuspid valve. Regions containing melanocytes could be detected by the presence of dark spots in the leaflets.
Figure 1.
Melanocyte pigmentation is observed in whole mount preparations of the (a) murine heart and (b) triscupid leaflet. (a) The heart of a two-month-old wild-type mouse was carefully sectioned along the frontal plane with a razor blade to expose the AV valves. The presence of melanocytes on the tricuspid and mitral valve leaflets is clearly seen as dark spots (marked by arrows). IVS, intraventricular septum; RV, right ventricle; LV, left ventricle. (b) The tricuspid valve was dissected from the heart and a leaflet was gently teased apart with the help of fine forceps. Pigmented areas (marked by arrows) are mostly localized to the atrial side of the leaflet.
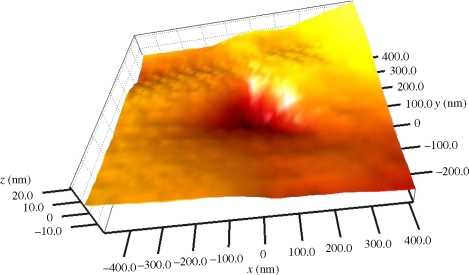
Quasi-static and nanodynamic mechanical analysis (nano-DMA) of the tricuspid valve was evaluated using TriboIndenter (Hysitron, Minneapolis, MN, USA) with a pyramidal fluid-cell Berkovich tip of radius 100 nm. Quasi-static tests were performed at a load of 30 µN to evaluate the hardness and visualize the residual contact depth after indentation. Standard operational calibrations for transducer and Berkovich diamond tip (as recommended/provided by Hysitron) such as air sweep, tip area, tip to optics, microscopic and digital feedback and drift calibrations were performed for quasi-static indentation, followed by frequency sweep calibrations for consequent nanodynamic testing. Dissected leaflet was fixed on a charged glass slide to avoid its lateral movement and sticking to the indenter tip. This technique can be done in any laboratory with suitable equipment and expertise. Often a custom-made fixture is required for the nanomechanical testing of the histological samples depending on their nature and the type of testing. A typical scanning probe microscope (SPM) indent image of the indented tricuspid leaflet at a load of 30 µN is shown in figure 2. Quasi-static nanoindentation of the leaflet at a depth of approximately 67 nm yielded an average hardness of 106.9 ± 19.4 MPa. This test was immediately followed by the scanning of a 0.8 µm × 0.8 µm region at a contact force of 0.5 µN and a probe scan speed of approximately 6.4 µm s−1. The restoration of indentation depth down to 20 nm (from approx. 67 nm after indentation), as seen in the scanning probe microscopy (figure 2), demonstrates the high viscoelasticity of the tricuspid leaflet. Owing to the viscoelastic nature of cardiac leaflets, Young's modulus alters and static stiffness does not remain constant (Merryman et al. 2006). Hence, in situ measurement of leaflet stiffness is performed via more appropriate nano-DMA (Yuan & Verma 2006).
Figure 2.
SPM image of the indented tricuspid leaflet at a load of 30 µN. Quasi-static indentation depth was measured to be 40 nm, which is recovered to 20 nm indicating highly viscoelastic nature of the cardiac leaflet.
Nano-DMA tests were done at 100 Hz with a load sweep in the range of 2–30 µN resulting in different contact depths. All the tests were conducted within 1 h of the heart's dissection keeping the tissue partially moist. From the principal quasi-static and much smaller dynamic load of the probe tip, displacement amplitude in the range of 0.5–1.5 nm was maintained to achieve comparable results. The equation of motion balances as (Balani & Agarwal 2008)
| 1 |
where displacement response at the same frequency oscillation is x = X sin(ωt − ϕ), F0 is the maximum force, m is the mass of centre plate of the nanoindenter transducer, C and k are combined damping and stiffness, respectively, X is the amplitude of displacement oscillation, ω is the angular frequency and ϕ is the phase shift of displacement. Consequently, displacement and phase lag can be calculated from the response of the tissue to loading, from which the stiffness of the tissue can be evaluated by subtracting the stiffness of the instrument.
Correspondingly, storage modulus (E′) can be calculated as (Balani & Agarwal 2008)
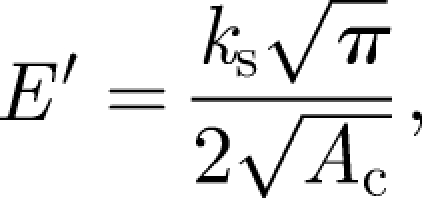 |
2 |
where ks is the stiffness of tissue and Ac is the contact area, which is dependent on the contact depth. Contact depth of the indenter is described through tip area function during instrument calibration. Storage modulus relates to stiffness of the tissue, as it falls in direct phase with the tissue's response to loading. The contact depth response of similar regions of the tissue marginally varied with similar load cycle at varying time; hence, three times repeatable results are reported without statistical error analysis. In the load sweeps, the paired t-test was performed because the storage modulus varies with the indentation depth. Assuming a null hypothesis, we obtained p < 0.005 for nine test points (N = 9), which confirms greater than 99.5 per cent confidence in stating that the differences in the mean of different test conditions are statistically extremely significant.
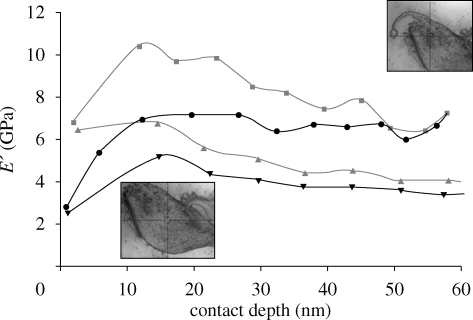
The nanodynamic mechanical testing of various regions in the tricuspid leaflet yielded a wide distribution of storage modulus ranging between 2.5 and 10 GPa (figure 3). Storage modulus is the in-phase response of the leaflet upon loading, i.e. a direct measure of its stiffness. An inverted bell-shaped modulus distribution was observed in the near-surface region. As the leaflet is very soft, the initial increase in the surface depth showed increased modulus. Consequently, the values reach a maximum and revert to an equilibrium value straightening the modulus plateau. The non-pigmented areas of the leaflet showed storage modulus of 2.5–5 GPa, which increased to 7–10 GPa in heavily pigmented regions. Areas with moderate amounts of pigmentation had a modulus of approximately 7 GPa at varying indentation depths. It should be mentioned that the indentation depth for heavily pigmented regions was lower (approx. 60 nm) than that for non-pigmented regions (approx. 75 nm) for similar loads (2–30 µN) at constant frequency (100 Hz). For comparison purposes of the dynamic analysis, the indentation depth of 58 nm was selected (maximum indentation depth observed for highly pigmented areas). Under these conditions, the corresponding storage modulus of non-pigmented areas was approximately 4 GPa. The differences in storage modulus between the non-pigmented and pigmented areas confirm the stiffening of leaflet in the presence of melanocytes. The higher storage modulus (approx. 7 GPa) prevalent in the heavily pigmented region (figure 3) when compared with the scarcely pigmented region (approx. 4 GPa) also confirms local heterogeneity present in the viscoelastic leaflet. In addition, it is expected that stiffening of melanocyte pigmentation will assist load-bearing functionality or will allow the detection of structural alterations, and similar observations have also been reported by Williams et al. (2005) and Stella et al. (2007). Moreover, it has been noted that time-dependent behaviour is complex and often intuitive (Stella et al. 2007). Secondly, the storage modulus values obtained with melanocyte pigmentation will be beneficial in engineering and modelling an artificial leaflet, which could replace a damaged leaflet and take over physiological functionality (Driessen et al. 2007; Liao et al. 2008).
Figure 3.
Modulus variation of wild-type mouse tricuspid valve leaflet with contact depth. Increase in storage stiffness can be observed with heavy pigmentation in the leaflet. Paired t-test was performed since the storage modulus varied with the indentation depth. Assuming a null hypothesis, we obtained p < 0.005 for nine test points (N = 9), which confirms greater than 99.5% confidence in stating that the differences in the mean of different test conditions are statistically extremely significant. Line with filled squares, heavy pigmentation; line with filled circles, moderate pigmentation; line with filled triangles, scarce pigmentation; line with filled inverted triangles, no pigmentation.
The correlation between the levels of pigmentation and the storage moduli of the different areas of the tricuspid leaflet is attributed to the presence of melanocytes. It is possible that melanocytes are stiffer than the endothelial cells that overlie the leaflet or the surrounding ECM. Melanocytes produce melanin, a highly insoluble molecule, within a specialized organelle called the melanosome where it interacts with a proteinaceous matrix. In the skin, melanosomes/melanin are transferred to keratinocytes for ultraviolet radiation photoprotection. The cellular and molecular mechanisms underlying the process of melanosome transfer are not yet fully understood but are starting to be elucidated (Bossche et al. 2006). Because there are no keratinocytes in the heart valves, it is unclear whether the melanocytes retain the melanin or secrete them in the leaflet extracellular environment. Either way, the higher storage modulus (approx. 7–10 GPa) of highly pigmented areas of the leaflet may be a result of the formation of melanin–protein aggregates. Interestingly, a robust cross-linked network of melanin and proteins has been implicated in the mechanical prowess of the jaws of the bloodworm Glycera (Moses et al. 2006). Future work will concentrate on the comparison of the storage modulus of valve leaflets obtained from wild-type and albino mice, which contain melanocytes but are unable to produce melanin, for establishing a more definitive contribution of melanin to the mechanical properties of the leaflets.
It is still possible that the observed variations in stiffness are not directly related to the melanocytes themselves, but, rather, their influence on the ECM with which they are associated. It is well established that cells can alter the ECM around them during various structural rearrangement events. For example, cells with a similar ontogeny to the melanocytes that contribute to the anterior segment of the eye have been shown to secrete enzymes that participate in the remodelling of the ECM for proper corneal differentiation (Hay 1979). This alternative explanation could be evaluated by the comparison of nanodynamic mechanical testing on the valve leaflets of mutant mice with no melanocytes with those that have extra numbers of melanocytes (Brito & Kos 2008).
The proper patterning and functionality of the valves is extensively correlated to the ECM organization, the alignment of endothelial cells and compartmentalization of the interstitial cells. Disruptions in the developmental processes that lead to normal valve histomorphology and the dysfunction of the two major valve cell types have been shown to be responsible for a series of valve pathological conditions (Rabkin et al. 2002; Leask et al. 2003; Armstrong & Bischoff 2004; Rabkin-Aikawa et al. 2004; Hinton et al. 2006). As melanocyte precursors reach the heart during the early stages of valve development and persist into adulthood (Brito & Kos 2008), it is reasonable to expect that their dysfunction might lead to valve abnormalities. Deficits associated with the proper specification, differentiation and migration of neural crest cells and their derivatives, including the melanocytes, lead to a variety of disorders (Hou & Pavan 2008). Melanocyte precursors, and even the differentiated cells, maintain a certain level of plasticity and can trans-differentiate into other cell types (Dupin et al. 2007). This type of plastic behaviour is observed in interstitial cells in developing and diseased valves (Rabkin-Aikawa et al. 2004). Along with the results presented in this study, it will be interesting to further investigate the putative roles for leaflet melanocytes in the organization of the ECM and their interactions with endothelial cells and/or interstitial cells.
In summary, the tricuspid valve leaflets of wild-type mice were found to be very soft (hardness approx. 107 MPa) with a highly viscoelastic nature. Different levels of pigmentation across the leaflets generated a wide variation in the storage modulus ranging between 2.5 and 10 GPa. Highly pigmented areas showed high storage modulus (approx. 7–10 GPa) in comparison with those of lesser pigmentation (approx. 2.5–4.0 GPa) owing to the presence of melanocytes concentration and, consequently, larger melanin content. This study has identified a novel role for melanocytes and/or melanin in the regulation of the mechanical properties of the mammalian cardiac valves. It opens up the possibility of investigating similar melanocyte properties in other relevant tissues such as the skin. It will also aid in a better understanding of valve functionality, with the potential of contributing new insights to the development of tissue engineered heart valves.
Acknowledgments
Animal work was performed according to institutional guidelines established by the National Institutes of Health. Authors (A.A. and K.B.) acknowledge the research funding from the Office of Naval Research under the DURIP (N00014-06-0675) grant to establish Nanomechanics and Nanotribology Laboratory at Florida International University. F. C. B. was supported by a Dissertation Year Fellowship from the FIU Graduate School.
References
- Armstrong E. J., Bischoff J. 2004. Heart valve development: endothelial cell signaling and differentiation. Circ. Res. 95, 459–470. ( 10.1161/01.RES.0000141146.95728.da) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balani K., Agarwal A. 2008. Damping behavior of carbon nanotube reinforced aluminum oxide coatings by nanomechanical dynamic modulus mapping. J. Appl. Phys. 104, 063517 ( 10.1063/1.2978185) [DOI] [Google Scholar]
- Bossche K. V. D., Naeyaert J. M., Lambert J. 2006. The quest for the mechanism of melanin transfer. Traffic 7, 769 ( 10.1111/j.1600-0854.2006.00425.x) [DOI] [PubMed] [Google Scholar]
- Brito F. C., Kos L. 2008. Timeline and distribution of melanocyte precursors in the mouse heart. Pigment Cell Melanoma Res. 21, 464–470. ( 10.1111/j.1755-148X.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- Butcher J. T., Nerem R. M. 2006. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 12, 905 ( 10.1089/ten.2006.12.905) [DOI] [PubMed] [Google Scholar]
- Driessen N. J. B., Mol A., Bouten C. V. C., Baaijens F. P. T. 2007. Modeling the mechanics of tissue-engineered human heart valve leaflets. J. Biomech. 40, 325–334. ( 10.1016/j.jbiomech.2006.01.009) [DOI] [PubMed] [Google Scholar]
- Dupin E., Calloni G., Real C., Gonçalves-Trentin A., Douarin N. M. L. 2007. Neural crest progenitors and stem cells. C. R. Biol. 330, 521–529. ( 10.1016/j.crvi.2007.04.004) [DOI] [PubMed] [Google Scholar]
- Franke O., Durst K., Maier V., Goken M., Birkholz T., Schneider H., Hennig F., Gelse K. 2007. Mechanical properties of hyaline and repair cartilage studied by nanoindentation. Acta Biomater. 3, 873–881. ( 10.1016/j.actbio.2007.04.005) [DOI] [PubMed] [Google Scholar]
- Hay E. D. 1979. Development of the vertebrate cornea. Int. Rev. Cytol. 63, 263–322. ( 10.1016/S0074-7696(08)61760-X) [DOI] [PubMed] [Google Scholar]
- Hinton R. B., Deutsch G. H., Pearl J. M., Hobart H. H., Morris C. A., Benson D. W. 2006. Bilateral semilunar valve disease in a child with partial deletion of the Williams–Beuren syndrome region is associated with elastin haploinsufficiency. J. Heart Valve Dis. 15, 352–355. [PubMed] [Google Scholar]
- Hou L., Pavan W. J. 2008. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf? Cell Res. 18, 1163–1176. ( 10.1038/cr.2008.303) [DOI] [PubMed] [Google Scholar]
- Leask R. L., Jain N., Butany J. 2003. Endothelium and valvular diseases of the heart. Microsc. Res. Tech. 60, 129–137. ( 10.1002/jemt.10251) [DOI] [PubMed] [Google Scholar]
- Lei T. C., Vieira W. D., Hearing V. J. 2002. In vitro migration of melanoblasts requires matrix metalloproteinase-2: implications to vitiligo therapy by photochemotherapy. Pigment Cell Res. 15, 426–432. ( 10.1034/j.1600-0749.2002.02044.x) [DOI] [PubMed] [Google Scholar]
- Liao J., Joyce E. M., Sacks M. S. 2008. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 26, 1065–1074. ( 10.1016/j.biomaterials.2007.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J., Lange A. W., Yutzey K. E. 2006. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev. Biol. 294, 292–302. ( 10.1016/j.ydbio.2006.03.027) [DOI] [PubMed] [Google Scholar]
- Merryman W. D., Youn I., Lukoff H. D., Krueger P. M., Guilak F., Hopkins R. A., Sacks M. S. 2006. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am. J. Physiol. Heart Circ. Physiol. 290, H224 ( 10.1152/ajpheart.00521.2005) [DOI] [PubMed] [Google Scholar]
- Miyamura Y., et al. 2007. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 20, 2–13. ( 10.1111/j.1600-0749.2006.00358.x) [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C. H., Kern C. B., Norris R. A., Fairey S., Cave C. L. 2005. Normal distribution of melanocytes in the mouse heart. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 285, 748 ( 10.1002/ar.a.20210) [DOI] [PubMed] [Google Scholar]
- Moses D. N., Mattoni M. A., Slack N. L., Waite J. H., Zok F. W. 2006. Role of melanin in mechanical properties of Glycera jaws. Acta Biomater. 2, 521–530. ( 10.1016/j.actbio.2006.05.002) [DOI] [PubMed] [Google Scholar]
- Nakamura T., Colbert M. C., Robbins J. 2006. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 98, 1547 ( 10.1161/01.RES.0000227505.19472.69) [DOI] [PubMed] [Google Scholar]
- Rabkin E., Hoerstrup S. P., Aikawa M., Mayer J. E., Jr, Schoen F. J. 2002. Evolution of cell phenotype and extracellular matrix in tissue-engineered heart valves during in-vitro maturation and in-vivo remodeling. J. Heart Valve Dis. 11, 308–314. [PubMed] [Google Scholar]
- Rabkin-Aikawa E., Farber M., Aikawa M., Schoen F. J. 2004. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 13, 841–847. [PubMed] [Google Scholar]
- Rabkin-Aikawa E., Mayer J. E., Jr, Schoen F. J. 2005. Heart valve regeneration. Adv. Biochem. Eng. Biotechnol. 94, 141. [DOI] [PubMed] [Google Scholar]
- Sacks M. S., Yoganathan A. P. 2007. Heart valve function: a biomechanical perspective. Phil. Trans. R. Soc. B 362, 1369 ( 10.1098/rstb.2007.2122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen F. J. 2005. Cardiac valves and valvular pathology: update on function, disease, repair, and replacement. Cardiovasc. Pathol. 14, 189 ( 10.1016/j.carpath.2005.03.005) [DOI] [PubMed] [Google Scholar]
- Seki Y., Schneider M. S., Meyers M. A. 2005. Structure and mechanical behavior of a toucan beak. Acta Mater. 53, 5281–5296. ( 10.1016/j.actamat.2005.04.048) [DOI] [Google Scholar]
- Stella J. A., Liao J., Sacks M. S. 2007. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J. Biomech. 40, 3169–3177. ( 10.1016/j.jbiomech.2007.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. 2001. Cochlear melanocytes and MITF signaling. J. Investig. Dermatol. Symp. Proc. 6, 95–98. ( 10.1046/j.0022-202x.2001.00017.x) [DOI] [PubMed] [Google Scholar]
- Wang J., He S., Xie S., Xu L., Gu N. 2007. Probing nanomechanical properties of nickel coated bacteria by nanoindentation. Mater. Lett. 61, 917–920. ( 10.1016/j.matlet.2006.06.019) [DOI] [Google Scholar]
- Wei G., Bhushan B. 2006. Nanotribological and nanomechanical characterization of human hair using a nanoscratch technique. Ultramicroscopy 106, 742–754. ( 10.1016/j.ultramic.2005.12.009) [DOI] [PubMed] [Google Scholar]
- Williams C., Liao J., Joyce E. M., Wang B., Leach J. B., Sacks M. S., Wong J. Y. 2005. Evaluation of polydimethylsiloxane scaffolds with physiologically relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials 26, 3123–3129. ( 10.1016/j.biomaterials.2004.08.009) [DOI] [PubMed] [Google Scholar]
- Yajima I., Larue L. 2008. The location of heart melanocytes is specified and the level of pigmentation in the heart may correlate with coat color. Pigment Cell Melanoma Res. 21, 471 ( 10.1111/j.1755-148X.2008.00483.x) [DOI] [PubMed] [Google Scholar]
- Yuan Y., Verma R. 2006. Measuring microelastic properties of stratum corneum. Colloids Surf. B, Biointerfaces 48, 6–12. ( 10.1016/j.colsurfb.2005.12.013) [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Robbins J. 2007. Principles of genetic murine models for cardiac disease. Circulation 115, 792 ( 10.1161/CIRCULATIONAHA.106.682534) [DOI] [PubMed] [Google Scholar]