Abstract
Little is known about the role of the endocrine system in financial decision-making. Here, we survey research on steroid hormones and their cognitive effects, and examine potential links to trader performance in the financial markets. Preliminary findings suggest that cortisol codes for risk and testosterone for reward. A key finding of this endocrine research is the different cognitive effects of acute versus chronic exposure to hormones: acutely elevated steroids may optimize performance on a range of tasks; but chronically elevated steroids may promote irrational risk-reward choices. We present a hypothesis suggesting that the irrational exuberance and pessimism observed during market bubbles and crashes may be mediated by steroid hormones. If hormones can exaggerate market moves, then perhaps the age and sex composition among traders and asset managers may affect the level of instability witnessed in the financial markets.
Keywords: testosterone, cortisol, risk, confidence, memory, rationality
1. Introduction
Emotions are commonly viewed as subcortical eruptions impairing the rational guidance of behaviour. However, certain authors (e.g. Damasio 1994; LeDoux 1996; Loewenstein et al. 2001) have disputed this contrast, suggesting that rationality by itself would be overwhelmed and directionless were information not emotionally tagged for significance. Nonetheless, lapses of rationality continue to be blamed on emotional interference. This is especially true of irrational risk-reward choices made during financial market bubbles and crashes, choices considered by many as instances of irrational exuberance and pessimism overwhelming rational economic agency (Shiller 2005). However, there are grounds for believing that the emotions of euphoria and fear displayed in markets may be more accurately described as shifts in confidence and risk preferences, ones caused by elevated levels of steroid hormones.
Steroids are a class of hormone, hormones being chemical messengers sent from one part of the body or brain to another, bringing about a change in the target tissue. The major classes of hormones include amines (such as adrenalin and noradrenalin), peptides and proteins (such as oxytocin and leptin) and steroids (such as testosterone, oestradiol and cortisol). Steroids are lipids cleaved from cholesterol by a series of enzymatic modifications, with the major sites of biosynthesis being the gonads and the adrenal cortex, although some neurosteroids, such as pregnenolone, can be synthesized directly by neurons and glial cells in the brain (Baulieu 1997).
Steroids constitute a particularly influential class of hormones because of their range of action. With receptors in almost every nucleated cell in the body, they affect growth, metabolism, immune function, mood, memory, cognition and behaviour. Steroids are of special interest for the study of emotions and economic behaviour because they help coordinate body and brain in archetypical situations, such as fight, flight, mating, feeding, search and struggle for status. Because they are known to respond powerfully to such behavioural and social situations, steroid hormones may provide an important missing link in the emerging field of neuroeconomics between economic events and brain processes. Here, we review the relevant literature on two steroids that may help provide this link—testosterone and cortisol.
2. Steroid hormones
(a). Testosterone and the hypothalamic–pituitary–gonadal axis
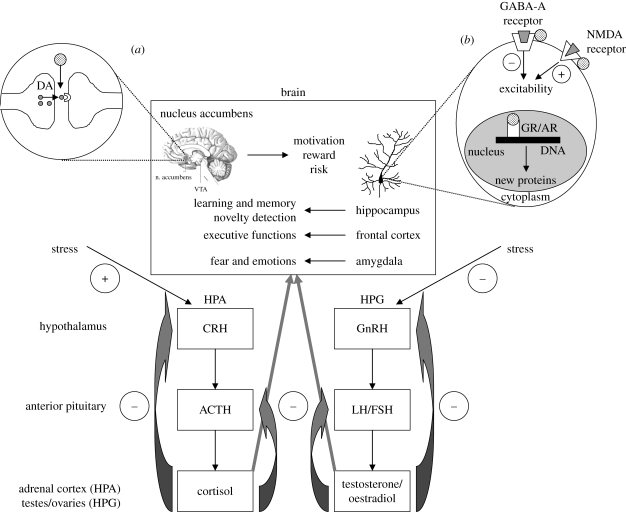
Testosterone is produced by the Leydig cells of the testes, in smaller quantities by the ovaries and by the adrenal cortex in both sexes. The sex steroids, testosterone and oestrogen, are regulated by a series of glands acting in concert—the hypothalamic–pituitary–gonadal (HPG) axis (figure 1). Sex steroids orchestrate reproductive function, regulating spermatogenesis in males, the menstrual cycle in females and sexually relevant and other forms of motivated behaviours in both genders (Reichlin 1998). Gonadotrophin-releasing hormone (GnRH), synthesized by a small group of neurons in the hypothalamus, is transported axonally to the median eminence where it is released in a pulsatile manner into the hypothalamic–pituitary portal circulation (a network of blood vessels connecting the hypothalamus with the pituitary gland). GnRH then acts on the anterior pituitary gonadotrophs—cells responsible for the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). When LH and FSH are released into the bloodstream in response to GnRH stimulation, they travel to the gonads—the ovaries in females and the testes in males.
Figure 1.
Schematic representation of the HPA and HPG axes and their effects on brain function. (a) Effects of steroid hormones on dopaminergic neurotransmisson in the nucleus accumbens; (b) genomic and non-genomic effects of steroids in the brain; for more details see text. GABA, γ-aminobutyric acid; NMDA, N-methyl-d-aspartate; GR, glucocorticoid receptor; AR, androgen receptor; plus, stimulatory effect; minus, inhibitory effect; dotted circles, steroid hormones (either glucocorticoid or testosterone); grey-shaded symbols, cognate ligands for other receptors.
In females, carefully coordinated actions of LH and FSH facilitate follicular maturation and subsequent ovulation in response to rising oestrogen levels. Progesterone levels rise in the second half (luteal phase) of ovulatory cycles, and help maintain the corpus luteum.
In males, FSH is a critical regulator of spermatogenesis, while LH stimulates the production of testosterone. Reactivation of the HPG axis at puberty, and the consequent secretion of testosterone, causes maturation of the reproductive organs and development of secondary sexual characteristics. Testosterone has marked anabolic effects, promoting development of the musculature and increased bone growth, and contributing, with pituitary-derived growth hormone, to a rapid increase in height at puberty (the so-called ‘growth spurt’). Oestrogen, progesterone and testosterone—together with inhibin, which is produced by the gonads in response to FSH action—inhibit the production and release of GnRH, LH and FSH in order to maintain the homeostasis of the system, with the HPG axis being subject to tight feedback control at all levels (Reichlin 1998).
As well as controlling the female menstrual cycle and male spermatogenesis, gonadal steroids also affect sexual behaviour (Vadakkadath Meethal & Atwood 2005). Importantly, they have been shown to exert both organizational and activational effects. The former refers to the fact that sexual differentiation of the brain can be permanently altered by the presence or absence of sex steroids at key stages in development. For example, administration of androgens to female rats within a few days of birth results in long-term virilization of behaviour. Conversely, neonatal castration of male rats causes them to develop behaviourally as females (Phoenix et al. 1959; Breedlove & Hampson 2002). Similar, but less complete, behavioural virilization of female offspring has been demonstrated following androgen administration in non-human primates. Brain development in the absence of sex steroids follows female lines, but is switched to the male pattern by exposure of the hypothalamus to androgen at a key stage of development. After puberty, androgens cause a feeling of well-being, an increase in physical vigour and increased libido. Testosterone's contribution to aggression and other forms of impulsive and risk-taking behaviours remains the subject of intense debate, and we return to this literature below.
(b). Cortisol and the hypothalamic–pituitary–adrenal axis
Cortisol, the main human glucocorticoid, is produced and regulated by the hypothalamic–pituitary–adrenal (HPA) axis (figure 1). This axis is critical to maintaining normal physiological homeostasis, and it regulates diverse processes, including metabolism, cardiovascular biology, immune function/inflammatory responses and cognitive function—indeed disorders of cortisol secretion (e.g. Addison's disease—cortisol deficiency; Cushing's syndrome—cortisol excess) are associated with considerable excess morbidity and mortality if left untreated. The system operates in a hierarchical manner similar to the HPG axis. Corticotropin-releasing hormone (CRH) is produced by neurons in the paraventricular nucleus of the hypothalamus, which project to the base of the hypothalamus, the median eminence. In response to a stressful stimulus, CRH is released from axon terminals into the hypothalamic–pituitary portal circulation, and reaches the anterior pituitary where it promotes the synthesis and secretion of adrenocorticotropic hormone (ACTH) by pituitary corticotrophs. ACTH then travels through the bloodstream to reach the adrenal glands (situated bilaterally above the kidneys) where it stimulates the synthesis and release of adrenal glucocorticoid hormones (cortisol in humans and other primates, corticosterone in rodents; Buckingham 1998) and adrenal androgens (e.g. dehydroepiandrosterone (DHEA)).
Glucocorticoids play a key role in helping the body adapt to changing circumstances in both its internal and external environments. Biologically, glucocorticoids facilitate the mobilization of resources to meet demand, including effects on intermediary metabolism, carbohydrate and protein metabolism, as well as acting as potent regulators of our endogenous ‘defence’ mechanisms, including the innate and adaptive immune responses (Buckingham 1998). Owing to their highly lipophilic nature, they can enter the brain easily and exert widespread effects on emotions, cognition, and the behavioural response to stress (De Kloet 2000).
However, chronic, as opposed to acute, elevation of circulating glucocorticoids may have a number of adverse effects on the body and brain. In its most extreme form (i.e. Cushing's syndrome), hypercortisolism may lead to excessive weight gain (especially abdominal fat), muscle wasting, severe metabolic dysfunction (with resistance to the action of insulin and in some cases overt diabetes mellitus), hypertension, impaired wound healing and enhanced susceptibility to opportunistic infections. Similarly, prolonged supraphysiological glucocorticoid exposure may have deleterious effects on the brain, leading to depression and in extreme cases psychosis, as well as atrophy of the hippocampus, a brain region playing a central role in learning and memory (Sapolsky et al. 2000). Therefore, in order to avoid the undesirable consequences of glucocorticoid excess, the HPA axis is tightly regulated by a sensitive negative feedback loop, similar to that operating in the HPG axis: when glucocorticoid levels are high, CRH and ACTH secretion are downregulated: as cortisol levels subsequently fall, feedback inhibition of hypothalamic–pituitary function is reversed and CRH and ACTH secretion increase, which in turn restores adrenal cortisol production.
(c). Steroid receptors: mechanism of action of steroid hormones
The principles governing the interactions of steroid hormones with their cellular receptors are the same for adrenal and gonadal-derived sex steroids (Gurnell et al. in press) and will be considered together for the purpose of this review. Steroid hormones are highly lipid soluble: they easily enter cells through the outer membrane. Once inside the cell, they bind to high-affinity receptors that belong to the nuclear receptor superfamily of ligand-gated transcription factors. For steroid hormones such as cortisol, oestrogen and testosterone, this process of binding to their receptors occurs outside of the nucleus in the cytoplasm. Hormone-bound receptor then trafficks into the nucleus where it seeks out, and interacts with, specific regions of the DNA to control the rate at which target genes are ‘switched on’ (activation) or ‘switched off’ (repression) (figure 1) (Tsai & O'Malley 1994; Funder 1997). In so doing, steroid hormones are able to increase or decrease the rate at which the cell synthesizes new proteins, and in this way change the structure and/or function of the cell, and the tissues made up of these cells.
These nuclear receptor-mediated events are relatively slow, usually taking several hours, and reflect the need for up- or downregulation of new protein synthesis. However, steroids also exert effects that can be observed within seconds, and these effects cannot be explained by the classic, genomic mechanisms. Instead, steroid hormones appear to act in a non-genomic manner to more rapidly alter cellular function (Falkenstein et al. 2000). Steroid receptors have been found in extranuclear sites in the hippocampus and in many other brain regions (McEwen & Milner 2007). These membrane-associated receptors are connected to a number of intracellular signalling pathways, such as growth factor signalling, kinases and phosphatases, to influence cell function or indirectly alter gene expression in order to support functional and structural plasticity of the nervous system (McEwen & Milner 2007). Furthermore, a particular subclass of steroid hormones, the neuroactive steroids (metabolites of the peripheral steroidogenic pathway, e.g. pregnenolone and DHEA and their sulphated forms (DHEAS)), together with neurosteroids (i.e. those produced by neurons de novo), can rapidly alter neural excitability by acting as allosteric modulators on neurotransmitter-gated ion channels, such as the γ-aminobutyric acid type A (GABA-A) and N-methyl-d-aspartate (NMDA) receptors in the brain (figure 1). In this way, steroids are able to influence emotions and mood within a narrow time frame (Baulieu 1997).
(d). Androgens, glucocorticoids and brain function
Recent work in neuroscience and economics has begun to elucidate how various brain regions process decisions and behaviours that violate the tenets of rational choice theory. Among these are the amygdala, which has been associated with framing effects (De Martino 2006) and ambiguity aversion (Hsu et al. 2005); the nucleus accumbens, associated with irrational risk-seeking (Matthews et al. 2004; Kuhnen & Knutson 2005); and the insula, associated with irrational risk aversion (Kuhnen & Knutson 2005) and the rejection of monetary reward in the ultimatum game (Sanfey et al. 2003). The brain is a major target of steroid hormone action, with cortisol, testosterone and oestradiol (Dreher et al. 2007) regulating neural function in many regions that are now recognized to be involved in economic decision-making (such as the prefrontal cortex and hippocampus) as well as regions implicated in irrational or emotional response to financial cues (such as the amygdala and nucleus accumbens). The powerful effects of steroids on these key brain regions raise the possibility that the irrationality or emotionality displayed in financial decisions may be significantly influenced by the levels of steroid in the body.
Corticosteroids—glucocorticoid and mineralocorticoid produced by the adrenal cortex—have dense receptor fields in the brain, as first demonstrated by McEwen and colleagues, who showed specific accumulation of 3H-corticosterone in the rat hippocampus (McEwen et al. 1968). Glucocorticoids bind to both glucocorticoid (GR) and mineralocorticoid receptors (MR), the latter of which has 10-fold higher affinity for its ligand than the GR (Reul & de Kloet 1985). MRs maintain basal activity of the axis, whereas GRs enhance negative feedback when corticosterone levels rise in response to a stressor. While the GR has a widespread expression pattern throughout the brain, MR expression is mostly restricted to limbic brain regions such as the hippocampus, amygdala, the septum and some cortical areas (de Kloet et al. 1998), regions critically involved in learning and memory, modulation of emotional responses and inhibition of behaviour.
For the purpose of this article, the key neural target regions considered with respect to glucocorticoid action are the hippocampus, amygdala and the prefrontal cortex (McEwen 2007). The hippocampus is essential for novelty detection and for the formation of declarative memory, underlying the conscious acquisition and recollection of facts and events (Scoville & Milner 1957). The prefrontal cortex, on the other hand, plays a key role in working memory, the cognitive mechanism that allows us to keep small amounts of information active for a limited period of time. The amygdala is particularly concerned with fear and emotions and mediates fear-conditioned memories.
The diverse actions of cortisol on human cognitive functions depend, among other factors, on the amount of hormone released, the length of exposure to cortisol, the emotional salience of the situation and the brain areas involved in dealing with the task. Low doses of glucocorticoids impair prefrontal, working memory, whereas high-dose or long-term administration results in an impairment in declarative, hippocampal, memory (Lupien et al. 2007). Furthermore, sustained elevation of corticosterone, or chronic stress, leads to plastic remodelling of neuronal structure in the hippocampus, amygdala and prefrontal cortex, as well as profound changes in functional plasticity, e.g. long-term potentiation (McEwen & Chattarji 2004; Liston et al. 2006). Specifically, chronic stress, through the activation of the HPA axis, decreases the number of apical dendrites of the CA3 pyramidal neurons of the hippocampus and increases the number of dendritic branches in the central nucleus of the amygdala (McEwen & Chattarji 2004). Furthermore, chronic stress induces a selective impairment in attentional set-shifting and a corresponding retraction of apical dendritic arbors in the medial prefrontal cortex (mPFC). In stressed rats, but not in controls, decreased dendritic arborization in the mPFC predicts impaired attentional set-shifting performance (Liston et al. 2006). Consistent with results obtained in rodents, psychosocial stress in humans selectively impairs attentional control and disrupts functional connectivity within a frontoparietal network that mediates attention shifts (Liston et al. 2009). These stress-induced, and perhaps glucocorticoid-mediated, changes in neuroplasticity may underlie altered cognitive functions, such as impaired attention, novelty detection and risk assessment, as well as anxiety and facilitated consolidation of emotionally negative memories typical of chronic stress.
Cortisol, as well as testosterone, may crucially influence economic decision-making through its effects on the nucleus accumbens (or ventral striatum), a main forebrain target of the mesolimbic dopaminergic system. Dopaminergic neurotransmission in the nucleus accumbens underlies motivation and reward-related behaviours such as drug self-administration and reward prediction (Ikemoto & Panksepp 1999; Schultz 2000). One study also found the nucleus accumbens to fire in anticipation of irrational risk-seeking choices in a financial choice task (Kuhnen & Knutson 2005). Both corticosteroids and testosterone profoundly influence dopamine transmission in this region (Piazza & Le Moal 1997; Sarnyai et al. 1998; Frye et al. 2002). Both hormones are self-administered by experimental animals, indicating their reinforcing properties (Piazza et al. 1993; Sato et al. 2008).
Evidence of the ‘rewarding property’ of testosterone is also provided by the finding that it can stimulate a conditioned place preference when administered to rats (Schroeder & Packard 2000; Frye et al. 2002). In humans there is evidence that anabolic steroids are addictive (Kashkin & Kleber 1989). It is thought that the rewarding properties of testosterone derive from the effect it and its metabolites, dihydrotestosterone and 3α-androstanediol, have of increasing dopamine release in the shell of the nucleus accumbens (Frye et al. 2002).
Cortisol has a complex pattern of effects on the nucleus accumbens. The activation of the HPA axis appears to be critically involved, through CRF and glucocorticoids, in different aspects of drug reward (Sarnyai et al. 2001). Acute stress increases extracellular dopamine levels, whereas chronic stress blunts the dopamine response and further inhibits dopamine outflow (Cabib & Puglisi-Allegra 1996). Chronic stress, through elevated corticosterone, appears to result in an increased dopamine D2 receptor density selectively in the shell of the nucleus accumbens (Lucas et al. 2007). D2 receptors are inhibitory autoreceptors that dampen dopamine release from the pre-synaptic terminal. Similarly, we have shown that chronic corticosterone treatment upregulates the binding of the dopamine transporter, which is responsible for the termination of dopamine's effect in the synapse, in the same brain region (Sarnyai et al. 1998). Others have shown long-lasting desensitization of dopamine receptor signalling caused by chronic stress (Choy et al. 2009). Therefore, it can be hypothesized that chronic stress induces an allostatic attenuation of the mesolimbic dopaminergic system, possibly due in part to persistent corticosterone elevation.
3. Steroid hormones and risk-taking
(a). Testosterone and risk-taking
Testosterone mediates sexual behaviour as well as competitive encounters, so there are prima facie reasons for believing it could also affect financial risk-taking. Research into how it may do so is, however, in its infancy. Much of the work on the cognitive and behavioural effects of androgens has instead studied humans taking anabolic steroids, studies that are pharmacological rather physiological because the steroids are taken in supra-physiological doses (Kashkin & Kleber 1989); or the work has studied animal behaviour, thus leaving open the question of the results’ applicability to humans (Sapolsky 1997). The animal studies, besides those examining sexual behaviour, have focused largely on the effects of testosterone on mating–guarding and territorial aggression, and on competitions for rank within a social hierarchy. This research has been elegantly synthesized by the biologist John Wingfield in his highly influential challenge hypothesis.
According to the challenge hypothesis, testosterone in males rises to a minimum level required for sexual behaviour; it will continue to rise beyond this level only when males are confronted with an intruder or a social challenge, the increased testosterone promoting aggressive behaviour (Wingfield et al. 1990). The insights gained from the challenge hypothesis, and from animal hormone studies more generally, have been applied to human behaviour (Archer 2006), but often with questionable success. Many studies, for example, could not determine whether testosterone caused aggression or the other way round; others found testosterone levels were poor predictors of who subsequently became aggressive (Sapolsky 1997; Monaghan & Glickman 2001); still others did not distinguish between aggressive and non-aggressive risk-taking (Vermeersch et al. 2008). One problem with these studies stems from the fact that in humans, as in some non-human primates, higher cognitive functions refract the effects of testosterone, effects which in smaller brained animals are more deterministic. Furthermore, the dependent variables in these studies, such as aggression, dominance, or status seeking, often cannot be defined or measured in humans with any objectivity, leading to marginally significant experimental results and contradictory findings between papers (Archer et al. 1998).
Studies of steroids and financial risk-taking promise to overcome many of these difficulties. To begin with, financial variables, such as profit, variance of returns, volatility of the market, can be defined objectively and measured precisely. Furthermore, the competitive behaviour Wingfield and his colleagues observed in animals may manifest itself in humans, not so much in aggressive encounters as in competitive economic behaviour. Through its known effects on dopamine transmission in the nucleus accumbens, testosterone may well have its most powerful effects in humans by shifting their utility functions, state of confidence or financial risk preferences.
We began testing this hypothesis by setting up a series of experiments on a trading floor in the City of London (Coates & Herbert 2008). We chose to study professional traders because real risk-taking, with meaningful consequences, seemed most likely to trigger large endocrine reactions. Our hypothesis and predictions were based on the challenge hypothesis as well as a closely related model, the winner effect (see below). Biologists working with these models have noticed that two males entering a fight or contest experience androgenic priming in the form of elevated testosterone levels. Moreover, the winning male emerges with even greater levels of testosterone, the loser with lower ones. The orders of magnitude of these hormone swings can be large: Monaghan & Glickman (2001) report that in a competition for rank among recently introduced rhesus monkeys, the winning male emerged with a 10-fold increase in testosterone, while the loser experienced a drop to 10 per cent of baseline levels within 24 h, and these new levels for both winner and loser persisted for several weeks. This reaction may make sense from an evolutionary point of view: in the wild, the loser of a fight is encouraged to retire from the field and nurse his wounds while the winner prepares for new challenges to his recently acquired rank.
A similar result has been found in experiments with humans (Gladue et al. 1989). Athletes, for example, experience the same androgenic priming before a sporting contest, and a further increase in testosterone after a win. This experiment has been repeated for a number of different events, including tennis (Booth et al. 1989) and wrestling (Elias 1981), as well as less physical contests such as chess (Mazur et al. 1992). It has also been found that the rising and falling levels of an athlete's testosterone can be mimicked by fans: Bernhardt et al. (1998) took testosterone samples from fans during a World Cup match in which Brazil defeated Italy. Both sets of fans went into the game with elevated testosterone, but afterwards the Brazilian fans’ testosterone rose while the Italians’ fell.
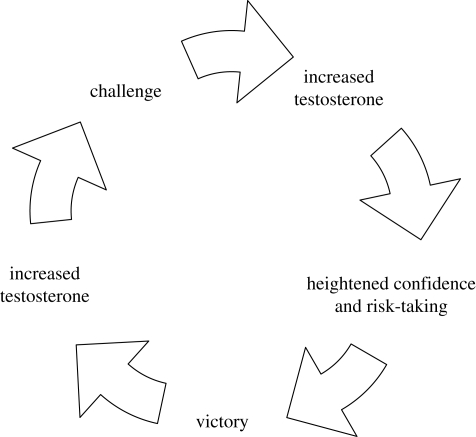
The role of these elevated testosterone levels is further explored in an animal model known as the ‘winner effect’. In this model, winning in an agonistic encounter can itself contribute to a later win (Chase et al. 1994; Oyegbile & Marler 2005), an effect that is independent of (i) an animal's resource-holding potential (RHP), i.e. the physical resources it can draw on in an all-out fight, (ii) its motivation, i.e. the value of the resource in dispute, or (iii) its aggressiveness (Hurd 2006). It is not known if the win imparts information to winner and loser about their respective resources (Hsu & Wolf 2001; Rutte et al. 2006) or whether it has physiological effects. This latter possibility is suggested by experiments in which elevated testosterone has been found to contribute to further wins (Trainor et al. 2004; Oyegbile & Marler 2005). Another possibility not fully considered in the literature is that higher testosterone, through its beneficial effects on the cardiovascular system and muscle mass, may effectively increase an animal's RHP, or, through its effects on confidence and risk-taking, may increase an animal's motivation or aggressiveness (Neat et al. 1998). Whatever the mechanism, a winner, with heightened testosterone levels, may proceed to the next round of competition with an advantage. This positive feedback loop, in which victory raises testosterone which in turn raises the likelihood of later victories (figure 2), may help account for winning and losing streaks in round-robin animal competitions that establish a social hierarchy (Dugatkin & Druen 2004).
Figure 2.
Schematic representation of a winner effect mediated by testosterone.
We examined the relevance of the challenge hypothesis and winner effect models to the financial markets (Coates & Herbert 2008) by looking for evidence that traders experience an increase in testosterone when they enjoy an above-average win in the markets. To do so, we sampled steroids from 17 young male traders, taking saliva samples twice a day, at 11.00 and 16.00, over a period of eight consecutive business days. Hormone readings are notoriously noisy owing to the pulsatile nature of their production and release into the blood stream, hence our protocol of repeated sampling to help separate ‘signal’ from ‘noise’. The traders were engaged in high-frequency trading, meaning that they positioned securities, mostly futures contracts in European and US bond and equity markets, in sizes up to £1 billion, but held their positions for a short period of time—several minutes, and sometimes mere seconds. They rarely positioned trades overnight, and they did not let winning or losing positions run for long.
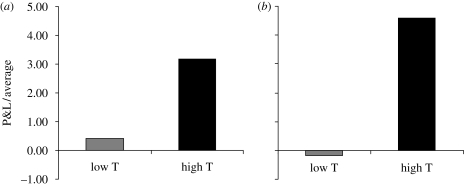
We discovered that these traders did indeed have significantly higher testosterone levels on days when they made an above-average profit. We could not determine from this correlation whether the profits were raising hormone levels or vice versa, but since we took two samples per day, we could examine how morning testosterone levels were related to afternoon profits and losses (P&Ls). To do so, we looked at the days when each trader's 11.00 testosterone levels were above his median level during the study, these days showing testosterone levels a modest 25 per cent higher than on the other days. We found that on days of high morning testosterone, the traders returned an afternoon profit (figure 3a) that was almost a full standard deviation higher than on ‘low-testosterone’ days. Interestingly, this relationship was even stronger among experienced traders (figure 3b), i.e. those who had traded for longer than 2 years, suggesting that testosterone, at moderate levels, was not having its effect by encouraging overly risky behaviour but was instead optimizing performance, at least with respect to high-frequency trading.
Figure 3.
P&L on low- and high-testosterone days. (a) P&L made between 11.00 and 16.00 for 17 traders on days when their testosterone levels were above their median level during the study (‘high T’) and on the rest of the days (‘low T’) (n = 17, paired t-test p = 0.008; Cohen's d = 0.97). P&Ls for each trader were standardized by dividing them by their 1-month average daily P&L. Standardized P&Ls were then averaged across all 17 traders. (b) Afternoon P&L for experienced traders only, i.e. ones with more than 2 years trading experience (n = 10, paired t-test p = 0.005; Cohen's d = 1.37).
The effects of androgens on high-frequency trading were also evident in a second experiment, one that looked at a surrogate marker of pre-natal androgen exposure—the second to fourth digit (finger length) ratio (2D : 4D) (Coates et al. 2009). As mentioned above, there are two distinct periods and types of hormone action—organizational effects of pre-natal steroids on the foetus and activational effects of circulating steroid on the adult. Androgens surge between the ninth and 18th week of gestation, masculinizing the foetus and exerting developmental changes on the body and brain that are permanent (Cohen-Bendahana et al. 2005). After the 19th week, androgen production subsides, spikes again briefly in the neonate and then drops back to low levels until the onset of puberty. At puberty, androgen production increases, activating the circuits created earlier in life by pre-natal hormone exposure. According to the organizational/activational model of hormone action (Phoenix et al. 1959), the sensitivity of adults to changes in circulating testosterone is a function of the amount of pre-natal androgen to which they were exposed (Meaney 1988; Breedlove & Hampson 2002).
Importantly, the amount of pre-natal androgen an individual was exposed to can be estimated because it leaves traces throughout the adult body, traces often measured by paediatricians looking for effects of environmental hormone disruptors on newborn infants. 2D : 4D is the most convenient measure for behavioural studies (McIntyre 2006). A lower 2D : 4D ratio is thought to indicate higher levels of pre-natal testosterone exposure (Manning et al. 1998; Brown et al. 2002). Consistent with this, men on average have lower ratios than women. We sampled 2D : 4D from a total of 44 traders, including 14 from the first study, and found that it predicted both the traders’ P&Ls over a 20-month period and the number of years they had survived in the business. It also predicted, in line with the organizational/activational model, the sensitivity of the trading performance of the original 14 traders to increases in circulating testosterone: the lower the trader's 2D : 4D, the more money he made when his testosterone levels rose.
Pre-natal testosterone appears, therefore, to predict long-term success in high-frequency trading, a style of trading requiring quick physical and cognitive reactions. However, there are grounds for believing that in other types of trading, especially those permitting more time for analysis and a longer holding period, or ones that do not make such physical demands, the correlation may weaken and even reverse sign (Coates et al. 2009). The market, it appears, selects for biological traits but these traits may vary between market segments.
The two trading floor experiments described here raise troubling questions about the efficient markets hypothesis. If, as this hypothesis assumes, markets are random, then we should not be able to predict relative trading performance by means of biological traits. Yet, our results suggest that higher levels of circulating testosterone predict short-term profitability and higher levels of pre-natal testosterone predict long-term profitability, at least in the segment of the market inhabited by high-frequency traders. The implication seems to be that the markets are not efficient or that they select for traits other than rational expectations (De Bondt & Thaler 1987; Shiller 2005; Blume & Easley 2006).
This leads us to another important question: how could testosterone exert its effects on profitability? Field studies such as those reported above do not allow us to establish a causal relationship between testosterone and profits, merely a predictive relationship, albeit a strong one. To establish causality, one needs pharmacological manipulation. Some studies administering testosterone esters to eugonadal males have found significant but weak effects on mood and aggressiveness (Bhasin et al. 2001; O'Connor et al. 2004), although they were not examining financial tasks. However, converging evidence from other lines of research suggests that androgen may affect confidence and risk preferences. For example, administered testosterone promotes confidence and fearlessness in the face of novelty, a result observed in both animals (Boissy & Bouissou 1994) and humans (Hermans et al. 2006). Furthermore, in a between-subjects study of male students playing an investment game, testosterone levels correlated with risk preferences (Apicella et al. 2008). This study also examined 2D : 4D and risk preferences, finding a significant correlation among Swedish Caucasians but not in a more ethnically heterogeneous population, the difference in results being accounted for by the fact that ethnic population is an important confound for 2D : 4D.
Intriguingly, there is another potential path of causation between testosterone and trading profits. Trading, it is not often appreciated, is a physical activity, a demanding one, so the important effects of testosterone may be physical rather than cognitive. High testosterone levels or increased androgenic effects, for example, can increase vigilance and visuomotor skills such as scanning and speed of reactions (Salminen et al. 2004; Falter et al. 2006), qualities that may help traders to spot and trade price discrepancies before others arbitrage them away (Coates et al. 2009). Elevated testosterone levels have also been found to increase an animal's search persistence (Andrew & Rogers 1972) and, during search, to focus visual attention while decreasing distraction by irrelevant stimuli (Andrew 1991). These last traits may be of particular importance in high-frequency trading because this form of trading requires lengthy periods of visuomotor scanning and quick reactions.
An increase in confidence or risk preferences, as found in some studies, would tend to increase a trader's position size; an increase in search persistence the frequency of trading; an increase in reaction times the chances of getting to a trade before others. Given that the traders in our study had a positive expected return, i.e. they usually made money, larger positions or more frequent trades would translate into higher daily profits. However, we cannot at this point say by which route these effects travelled, that is, whether testosterone was having its effect by augmenting the effort, speed, confidence or risk preferences of the traders.
(b). Cortisol and risk-taking
A review of research on cortisol and financial risk-taking is necessarily brief as there is almost no work done on this subject. Van Honk et al. (2003) looked at the cortisol levels of people playing the Iowa Gambling Task and found that they correlated with risk aversion. In our own studies, we hypothesized that cortisol, as a stress hormone, would increase as traders lost money. This seemed a reasonable assumption, but our experiment did not find evidence to support it, as we observed no relationship between trading losses, even above-average ones, and cortisol levels. However, caution is needed before extrapolating these findings, as the style of trading and the risk management practices on this trading floor prevented traders from losing large sums of money. Had they not done so, or had we sampled in a different setting, for example in an investment bank where traders position interest rate or credit risk for longer periods of time, and had these traders entered a sustained losing streak, it is likely they would have experienced high levels of stress and cortisol.
However, we did note a potentially more interesting finding—that cortisol was rising with uncertainty. Early research on stress and cortisol, especially the pioneering work of Hans Selye, focused on how cortisol production reacts to actual bodily harm. But later research found that the HPA axis can respond more robustly to expected harm and that the size of the response is an increasing function of the uncertainty over timing. For example, an animal receiving a shock at regular intervals or after a warning tone may have normal cortisol levels at the end of an experiment; in contrast, an animal receiving the same quantity of shock will experience rising cortisol levels as the timing of the shocks becomes more and more unpredictable, reaching a maximum when the timing becomes random (Levine et al. 1989). Animals can have a similarly elevated HPA response when exposed to situations of novelty (Erikson et al. 2003) or uncontrollability (Swenson & Vogel 1983; Breier et al. 1987). Uncertainty, novelty and uncontrollability can perhaps be reduced to a common denominator of uncertainty; all three describe a situation in which an animal finds it increasingly difficult to predict what may happen and what actions will be required. The necessity of being prepared for the unexpected signals to the body, via cortisol, that catabolic metabolism may be needed. As it transpires, ‘uncertainty’, ‘novelty’ and ‘uncontrollability’ aptly describe the financial markets and the environment in which traders find themselves on a daily basis.
To examine the effect of uncertainty on traders’ HPA axes, we looked at the risk faced by each trader, as measured by the variance of his P&L, over the course of the study (Coates & Herbert 2008). We found a highly significant correlation with cortisol that once again displayed a large effect size. Variance in P&L is a measure of the uncertainty or uncontrollability a trader has just lived through; but we also wanted to measure how uncertain the traders were about upcoming events in the market, such as the release of important economic statistics. To do so, we used the implied volatility of the Bund futures contract (a future on German Government bonds), which was the security most widely traded by the traders in the study. Bond options require for their pricing the market's estimate of the future variance of the underlying asset, so option prices provide an objective measure of the market's collective uncertainty. Here, again we observed a very high and significant correlation between the traders’ daily cortisol levels, averaged from all traders, and the market's uncertainty regarding upcoming market moves. Our results raise the possibility that while testosterone codes for economic return, cortisol codes for risk.
Our experiment represents only the mere beginning of research into the role of cortisol in financial decision-making. To underline our belief in the critical importance of this hormone, we should point out that the cortisol fluctuations we observed were large. In the normal course of a day, cortisol, like testosterone, peaks in the morning and falls over the course of the day. Between our sampling times, cortisol levels would be predicted to fall by approximately 40 per cent, yet in many of our subjects it rose, in some cases by as much as 500 per cent. Similar-sized cortisol fluctuations were also observed between days. What purpose do changes of this magnitude serve? Cortisol, as highlighted above, marshalls glucose for immediate use, and it promotes anticipatory arousal and a focused attention (Erikson et al. 2003). We speculate therefore that traders, when expecting a market move, would benefit from such an acute increase in cortisol, as it prepares them for the money-making opportunities that increased volatility brings.
(c). Steroids and impaired risk-taking
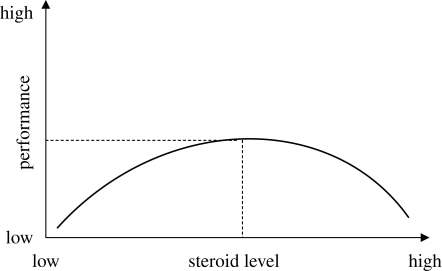
If market volatility or the variance in the traders’ P&L were to remain high, cortisol levels could also remain elevated for an extended period. Chronically elevated cortisol levels, as we have seen, can have the opposite effect on cognitive performance as acute levels. Cortisol displays an inverted U-shaped dose–response curve, according to which performance on a range of cognitive and behavioural tasks is optimized at moderate levels, while being impaired at lower and higher levels (figure 4) (Conrad et al. 1999). As cortisol levels rise past the optimal point on the dose–response curve, they may begin to impair trading performance, specifically by promoting irrational risk aversion. Chronically elevated cortisol levels increase CRH gene transcription in the central nucleus of the amygdala thereby promoting fear (Corodimas et al. 1994), anxiety (Shephard et al. 2000; Korte 2001) and the tendency to find risk where perhaps none exists (Schulkin et al. 1994; McEwen 1998). They may also alter the types of memory recalled, causing a person to selectively recall mostly negative precedents (Erikson et al. 2003). Lastly, chronic stress, as we have seen, downregulates dopamine transporters, receptors and downstream signalling molecules in the nucleus accumbens, and may thereby alter risk-related behaviours. All these effects would tend to decrease a trader's appetite for risk.
Figure 4.
Inverted U-shaped dose–response curve relating cortsol levels to cognitive function, such as performance, on a spatial navigation or declarative memory task.
When might conditions of chronic stress occur in the markets? Bear markets and crashes are notable for their extreme levels of volatility, the protracted subprime mortgage crisis being a notable example, with the VIX, an index of implied volatilities on the New York Stock Exchange, rising from 12 per cent before the crisis to a high of 80 per cent 18 months later. It seems likely that cortisol levels among traders threatened for so long with historic levels of uncertainty would have increased and perhaps remained elevated for a prolonged period of time. Under such circumstances, the steroid may have contributed to the extreme levels of risk aversion observed among traders. Indeed, extended periods of uncertainty and uncontrollable stress can promote a condition known as ‘learned helplessness’, in which persons, and animals, lose all belief in their ability to control or influence their environment (Kademian et al. 2005). Under these circumstances, traders could become price insensitive and fail to respond to lower asset prices or interest rates, thereby rendering monetary policy ineffective. In short, rising cortisol levels among traders and investors may promote risk aversion during a bear market, exaggerating the market's downward move.
Could testosterone work in the opposite direction, encouraging irrational risk-taking during a bull market? This is a difficult question. Moderate levels, as described above, may promote effective risk-taking among animals and high-frequency traders. But higher levels may indeed carry increased costs such as encouraging excessive risk-taking. In studies related to the challenge hypothesis and the winner effect, animal behaviourists have found that the higher a male's testosterone level (either on account of the breeding season, an agonistic encounter or an experimental implant), the more often he fights, the larger the area he patrols or the more often he ventures into the open (Marler & Moore 1988; Beletsky et al. 1995). These habits can lead to loss of fat stores (i.e. nutritional reserves), neglect of parenting duties, frequent wounds and increased predation (Dufty 1989; Wingfield et al. 2001). High-testosterone males end up paying a stiff price for their risk-taking in the form of a higher rate of mortality.
We do not know if traders can experience rises in endogenous testosterone sufficient to encourage analogous forms of over-confidence and irrational risk-taking. The traders we observed experienced only moderate increases, although one trader, who enjoyed a 5-day winning streak during which he made over twice his daily average P&L, experienced a 75 per cent increase in mean daily testosterone. It is known that cortisol can rise to extreme levels, and for extended periods of time; but research on the costs of high physiological levels of testosterone in humans is rare. Nonetheless, some studies have found that physiological levels of testosterone are indeed correlated with risky behaviour (Booth et al. 1999), sensation seeking (Daitzman & Zuckerman 1980) and the size of offers rejected in the Ultimatum Game, rejections often considered as violations of economic rationality (Van den Bergh & Dewitte 2006; Burnham 2007). Other studies with users of anabolic steroids, or subjects administered pharmacological doses of testosterone, have found evidence of manic behaviour (Pope & Katz 1988; Pope et al. 2000). In one study, researchers administered testosterone to a group of women playing the Iowa Gambling Task (van Honk et al. 2004) and found that it shifted risk preferences to such an extent that the women switched from playing the low variance and positive expected-return decks of cards to the high variance but negative expected-return decks. A similar result was found in a physiological study in which the performance of young males on the Iowa Gambling Task was negatively correlated with their testosterone levels (Reavis & Overman 2001). These study results suggest that elevated levels of testosterone could at some point begin to impair rational financial decision-making.
4. Conclusion
Taken together, the findings surveyed in this review suggest the possibility that economic agents are more hormonal than is assumed by theories of rational expectations and efficient markets. These theories assume, for example, that prices in financial markets accurately reflect all available information. But a trader's interpretation of information may not be stable: a trader with high levels of testosterone may see only opportunity in a set of facts; while the same trader with chronically elevated cortisol may find only risk. Furthermore, risk preferences may not be stable. If traders are subject to a financial variant of the winner effect, such that rising levels of testosterone increase their appetite for risk during a bull market, and rising levels of cortisol decrease their appetite for risk during a bear market, then steroid hormones may shift risk preferences systematically across the business cycle. This effect, even if confined to a small number of people, could destabilize the financial markets (Camerer & Fehr 2006). The hypothesis of steroid feedback loops exaggerating market moves raises the further possibility that the emotions of irrational exuberance and pessimism (what the economist John Maynard Keynes called ‘animal spirits’) commonly blamed for financial instability may in fact be steroid-induced shifts in confidence and risk preferences. This is not to say hormones cause bubbles and crashes; advances in technology, for example, caused the bull markets of 1920s and the Dotcom era, but hormones may exaggerate moves once under way.
The study of hormonal influences is, we believe, an important step in the ongoing project, beginning with behavioural economics and continuing with neuroeconomics, of showing how the body influences economic decisions, frequently pushing economic agents, for good or ill, away from rational choice. The research, moreover, carries intriguing policy implications: if hormones affect risk-taking, then perhaps financial markets can be made more stable by having a greater endocrine diversity in the financial industry. How do we achieve endocrine diversity? Hormone levels change over the course of our lives, with testosterone and oestrogen declining, and cortisol increasing; so young and old have markedly different endocrine profiles. The sexes as well have very different endocrine systems. Market stability is served by opinion diversity; so it may be served as well by having more balance in the banks between young and old, men and women. One does not need to argue that one group is better than others for this policy to work; merely different (Dreher et al. 2007). However, there are grounds for thinking that women may be less ‘hormonally reactive’ when it comes to financial risk-taking. For example, women have only 5–10% of the circulating levels of testosterone of men, and they have not been exposed to the same organizing effects of pre-natal androgens. Furthermore, some studies have found that women's HPA axes are less reactive to stressors stemming from a competitive situation (Stroud et al. 2002). Their greater presence in the ranks of money managers may therefore help dampen hormonal swings in the market.
Lastly, the endocrine system may be the missing link in the new field of neuroscience and economics, connecting market events to brain processes (Caldú & Dreher 2007). If research in endocrinology, especially work done with animal models, were to be wedded to recent developments in neuroscience and economics, we could begin to approach a unified scientific subject, from molecule to market (McEwen 2001).
Acknowledgements
J.M.C. and M.G. are supported by an ESRC Programme Grant. M.G. is a Cambridge National Institutes of Health Research Comprehensive Biomedical Research Center Investigator.
Footnotes
One contribution of 12 to a Theme Issue ‘Rationality and emotions’.
References
- Andrew R.1991The development and integration of behaviour. Essays in honour of Robert Hinde (ed. Bateson P.), pp. 171–190 Cambridge, UK: Cambridge University Press [Google Scholar]
- Andrew R., Rogers L.1972Testosterone, search behaviour and persistence. Nature 237, 343–346 (doi:10.1038/237343a0) [DOI] [PubMed] [Google Scholar]
- Apicella C., Dreber A., Campbell B., Gray P., Hoffman M., Little A.2008Testosterone and financial risk preferences. Evol. Hum. Behav. 29, 384–390 (doi:10.1016/j.evolhumbehav.2008.07.001) [Google Scholar]
- Archer J.2006Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 30, 319–345 (doi:10.1016/j.neubiorev.2004.12.007) [DOI] [PubMed] [Google Scholar]
- Archer J., Birring S., Wu F.1998The association between testosterone and aggression among young men: empirical findings and a meta-analysis. Aggress. Behav. 24, 411–420 (doi:10.1002/(SICI)1098-2337(1998)24:6<411::AID-AB2>3.0.CO;2-9) [Google Scholar]
- Baulieu E.1997Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 52, 1–32 [PubMed] [Google Scholar]
- Beletsky L., Gori D., Freeman S., Wingfield J.1995Testosterone and polygyny in birds. Curr. Ornith. 12, 141 [Google Scholar]
- Bernhardt P. C., Dabbs J., Fielden J., Lutter C.1998Changes in testosterone levels during vicarious experiences of winning and losing among fans at sporting events. Physiol. Behav. 65, 59–62 (doi:10.1016/S0031-9384(98)00147-4) [DOI] [PubMed] [Google Scholar]
- Bhasin S., et al. 2001Testosterone dose–response relationships in healthy young men. Am. J. Physiol. Endocrinol. Metab. 281, 1172–1181 [DOI] [PubMed] [Google Scholar]
- Blume L., Easley D.2006If you are so smart why aren't you rich? Belief selection in complete and incomplete markets. Econometrica 74, 929–966 (doi:10.1111/j.1468-0262.2006.00691.x) [Google Scholar]
- Boissy A., Bouissou M.1994Effects of androgen treatment on behavioural and physiological responses of heifers to fear-eliciting situations. Horm. Behav. 28, 66–83 (doi:10.1006/hbeh.1994.1006) [DOI] [PubMed] [Google Scholar]
- Booth A., Shelley G., Mazur A., Tharp G., Kittok R.1989Testosterone, and winning and losing in human competition. Horm. Behav. 23, 556–571 (doi:10.1016/0018-506X(89)90042-1) [DOI] [PubMed] [Google Scholar]
- Booth A., Johnson D., Granger D.1999Testosterone and men's health. J. Behav. Med. 22, 1–19 (doi:10.1023/A:1018705001117) [DOI] [PubMed] [Google Scholar]
- Breedlove S., Hampson E.2002Behavioral endocrinology (eds Becker J., Breedlove S., Crews D., McCarthy M.), pp. 75–114, 2nd edn Cambridge, MA: MIT Press [Google Scholar]
- Breier A., Albus M., Pickar D., Zahn T. P., Wolkowitz O. M., Paul S. M.1987Controllable and uncontrollable stress in humans: alterations in mood and neuroendocrine and psychophysiological function. Am. J. Psychiatry 144, 1419–1425 [DOI] [PubMed] [Google Scholar]
- Brown W., Hines M., Fane B., Breedlove M.2002Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Horm. Behav. 42, 380–386 (doi:10.1006/hbeh.2002.1830) [DOI] [PubMed] [Google Scholar]
- Buckingham J.1998Stress and the hypothalamo-pituitary-immune axis. Int. J. Tissue React. 20, 23–34 [PubMed] [Google Scholar]
- Burnham T.2007High-testosterone men reject low ultimatum game offers. Proc. R. Soc. B 274, 2327–2330 (doi:10.1098/rspb.2007.0546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., Puglisi-Allegra S.1996Different effects of repeated stressful experiences on mesocortical and mesolimbic dopamine metabolism. Neuroscience 73, 375–380 (doi:10.1016/0306-4522(96)00750-6) [DOI] [PubMed] [Google Scholar]
- Caldú X., Dreher J.2007Hormonal and genetic influences on processing reward and social information. Ann. N Y Acad. Sci. 1118, 43–73 (doi:10.1196/annals.1412.007) [DOI] [PubMed] [Google Scholar]
- Camerer C., Fehr E.2006When does ‘economic man’ dominate social behavior? Science 311, 47–52 (doi:10.1126/science.1110600) [DOI] [PubMed] [Google Scholar]
- Chase I. D., Bartolomeo C., Dugatkin L. A.1994Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim. Behav. 48, 393–400 (doi:10.1006/anbe.1994.1253) [Google Scholar]
- Choy K., de Visser Y., van den Buuse M.2009The effect of ‘two-hit’ neonatal and young-adult stress on dopaminergic modulation of prepulse inhibition and dopamine receptor density. Br. J. Pharmacol. 156, 388–396 (doi:10.1111/j.1476-5381.2008.00008.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. M., Herbert J.2008Endogenous steroids and financial risk taking on a London trading floor. Proc. Natl Acad. Sci. USA 105, 6167–6172 (doi:10.1073/pnas.0704025105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. M., Gurnell M., Rustichini A.2009Second-to-fourth digit ratio predicts success among high-frequency financial traders. Proc. Natl Acad. Sci. USA 106, 623–628 (doi:10.1073/pnas.0810907106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahana C., van de Beeka C., Berenbaum S.2005Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci. Biobehav. Rev. 29, 353–384 (doi:10.1016/j.neubiorev.2004.11.004) [DOI] [PubMed] [Google Scholar]
- Conrad C., Lupien S., McEwen B.1999Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol. Learn. Mem. 72, 39–46 (doi:10.1006/nlme.1998.3898) [DOI] [PubMed] [Google Scholar]
- Corodimas K., LeDoux J., Gold P., Schulkin J.1994Corticosterone potentiation of learned fear. Ann. N Y Acad. Sci. 746, 392–393 [DOI] [PubMed] [Google Scholar]
- Daitzman R., Zuckerman M.1980Disinhibitory sensation seeking, personality and gonadal hormones. Pers. Individ. Differ. 1, 103–110 (doi:10.1016/0191-8869(80)90027-6) [Google Scholar]
- Damasio A. R.1994Descartes' error: emotion, reason, and the human brain New York, NY: Grosset/Putnam [Google Scholar]
- De Bondt W., Thaler R.1987Further evidence on investor overreaction and stock market seasonality. J. Finance 42, 557–581 (doi:10.2307/2328371) [Google Scholar]
- de Kloet E. R.2000Stress in the brain. Eur. J. Pharmacol. 405, 187–198 (doi:10.1016/S0014-2999(00)00552-5) [DOI] [PubMed] [Google Scholar]
- de Kloet E. R., Vreugdenhil E., Oitzl M. S., Joels M.1998Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 (doi:10.1210/er.19.3.269) [DOI] [PubMed] [Google Scholar]
- De Martino B., Kumaran D., Seymour B., Dolan R.2006Frames, biases and rational decision-making in the human brain. Science 313, 684–687 (doi:10.1126/science.1128356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J.-C., Schmidt P. J., Kohn P., Furman D., Rubinov D., Berman K. F.2007Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl Acad. Sci. USA 104, 2465–2470 (doi:10.1073/pnas.0605569104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufty A. M.1989Testosterone and survival: a cost of aggressiveness? Horm. Behav. 23, 185–193 (doi:10.1016/0018-506X(89)90059-7) [DOI] [PubMed] [Google Scholar]
- Dugatkin L., Druen M.2004The social implications of winner and loser effects. Proc. Biol. Sci. 271(Suppl. 6), S488–S489 (doi:10.1098/rsbl.2004.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M.1981Serum cortisol, testosterone, and testosterone-binding globulin responses to competitive fighting in human males. Aggress. Behav. 7, 215–224 (doi:10.1002/1098-2337(1981)7:3<215::AID-AB2480070305>3.0.CO;2-M) [Google Scholar]
- Erikson K., Drevets W., Schulkin J.2003Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci. Biobehav. Rev. 27, 233–246 (doi:10.1016/S0149-7634(03)00033-2) [DOI] [PubMed] [Google Scholar]
- Falkenstein E., Tillmann H., Christ M., Feuring M., Wehling M.2000Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol. Rev. 52, 513–556 [PubMed] [Google Scholar]
- Falter C., Arroyo M., Davis G.2006Testosterone: activation or organization of spatial cognition? Biol. Psychol. 73, 132–140 (doi:10.1016/j.biopsycho.2006.01.011) [DOI] [PubMed] [Google Scholar]
- Frye C., Rhodes M., Rosellini R., Svare B.2002The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5alpha-reduced metabolites. Pharmacol. Biochem. Behav. 74, 119–127 (doi:10.1016/S0091-3057(02)00968-1) [DOI] [PubMed] [Google Scholar]
- Funder J. W.1997Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu. Rev. Med. 48, 231–224 (doi:10.1146/annurev.med.48.1.231) [DOI] [PubMed] [Google Scholar]
- Gladue B., Boechler M., McCaul K. D.1989Hormonal response to competition in human males. Aggress. Behav. 15, 409–422 (doi:10.1002/1098-2337(1989)15:6<409::AID-AB2480150602>3.0.CO;2-P) [Google Scholar]
- Gurnell M., Burrin J., Chatterjee K.In press Principles of hormone action. In Oxford textbook of medicine (eds Warrell D., Cox T., Firth J.), 5th edn Oxford, UK: Oxford University Press [Google Scholar]
- Hermans E., Putman P., Baas J., Koppeschaar H., van Honk J.2006A single administration of testosterone reduces fear-potentiated startle in humans. Biol. Psychiat. 59, 872–874 (doi:10.1016/j.biopsych.2005.11.015) [DOI] [PubMed] [Google Scholar]
- Hsu Y., Wolf L.2001The winner and loser effect: what fighting behaviours are influenced? Anim. Behav. 61, 777–786 (doi:10.1006/anbe.2000.1650) [Google Scholar]
- Hsu M., Bhatt M., Adolphs R., Tranel D., Camerer C.2005Neural systems responding to uncertainty in human decision-making. Science 310, 1680–1683 (doi:10.1126/science.1115327) [DOI] [PubMed] [Google Scholar]
- Hurd P.2006Resource holding potential, subjective resource value, and game theoretical models of aggressiveness signaling. J. Theor. Biol. 241, 639–648 (doi:10.1016/j.jtbi.2006.01.001) [DOI] [PubMed] [Google Scholar]
- Ikemoto S., Panksepp J.1999The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 31, 6–41 (doi:10.1016/S0165-0173(99)00023-5) [DOI] [PubMed] [Google Scholar]
- Kashkin K., Kleber H.1989Hooked on hormones? An anabolic steroid addiction hypothesis. J. Am. Med. Assoc. 262, 3166–3170 (doi:10.1001/jama.262.22.3166) [DOI] [PubMed] [Google Scholar]
- Kademian S., Bignante A., Lardone P., McEwen B., Volosin M.2005Biphasic effects of adrenal steroids on learned helplessness behavior induced by inescapable shock. Neuropsychopharm 30, 58–66 (doi:10.1038/sj.npp.1300577) [DOI] [PubMed] [Google Scholar]
- Korte S.2001Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 25, 117–142 (doi:10.1016/S0149-7634(01)00002-1) [DOI] [PubMed] [Google Scholar]
- Kuhnen C., Knutson B.2005The neural basis of financial risk taking. Neuron 47, 763–770 (doi:10.1016/j.neuron.2005.08.008) [DOI] [PubMed] [Google Scholar]
- LeDoux J. E.1996The emotional brain: the mysterious underpinnings of emotional life New York, NY: Simon & Schuster [Google Scholar]
- Levine S., Coe C., Wiener S. G.1989Psychoneuroendocrinology of stress: a psychobiological perspective. In Psychoendocrinology (eds Bush F., Levine S.), pp. 341–377 New York, NY: Academic Press [Google Scholar]
- Liston C., Miller M. M., Goldwater D. S., Radley J. J., Rocher A. B., Hof P. R., Morrison J. H., McEwen B.2006Stress-induced alterations in prefrontal cortical dendritic morphology predicts selective impairments in perceptual attention set-shifting. J. Neurosci. 26, 7870–7874 (doi:10.1523/JNEUROSCI.1184-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., McEwen B., Casey B.2009Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl Acad. Sci. USA 106, 912–917 (doi:10.1073/pnas.0807041106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G., Weber E., Hsee C.2001Risk as feelings. Psychol. Bull. 127, 267–286 (doi:10.1037/0033-2909.127.2.267) [DOI] [PubMed] [Google Scholar]
- Lucas L. R., Wang C. J., McCall T. J., McEwen B.2007Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 1155, 108–115 (doi:10.1016/j.brainres.2007.04.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S. J., Maheu F., Tu M., Fiocco A., Schramek T. E.2007The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 65, 209–237 (doi:10.1016/j.bandc.2007.02.007) [DOI] [PubMed] [Google Scholar]
- Manning J., Scutt D., Wilson D., Lewis-Jones D.19982nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 13, 3000–3004 (doi:10.1093/humrep/13.11.3000) [DOI] [PubMed] [Google Scholar]
- Marler C. A., Moore M. C.1988Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav. Ecol. Sociobiol. 23, 21–26 (doi:10.1007/BF00303053) [Google Scholar]
- Matthews S., Simmons A., Lane S., Paulus M.2004Selective activation of the nucleus accumbens during risk-taking decision making. NeuroReport 15, 2123–2127 (doi:10.1097/00001756-200409150-00025) [DOI] [PubMed] [Google Scholar]
- Mazur A., Booth A., Dabbs J.1992Testosterone and chess competition. Soc. Psychol. Q. 55, 70–77 (doi:10.2307/2786687) [Google Scholar]
- McEwen B.1998Stress, adaptation, and disease: allostasis and allostatic load. Ann. N Y Acad. Sci. 840, 33–44 (doi:10.1111/j.1749-6632.1998.tb09546.x) [DOI] [PubMed] [Google Scholar]
- McEwen B.2001From molecules to mind: stress, individual differences, and the social environment. In Unity of knowledge: the convergence of natural and human science (eds A. Damasio et al.). Ann. N Y Acad. Sci. 935, 42–49 (doi:10.1111/j.1749-6632.2001.tb03469.x) [PubMed] [Google Scholar]
- McEwen B.2007Physiology and neurobiology of stress and adaptation: central role of the brain. Endocr. Rev. 87, 873–904 [DOI] [PubMed] [Google Scholar]
- McEwen B., Chattarji S.2004Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur. Neuropsychopharmacol. 14, S497–S502 (doi:10.1016/j.euroneuro.2004.09.008) [DOI] [PubMed] [Google Scholar]
- McEwen B., Milner T.2007Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 55, 343–355 (doi:10.1016/j.brainresrev.2007.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B., Weiss J. M., Schwartz L. S.1968Selective retention of corticosterone by limbic structures in rat brain. Nature 220, 911–912 (doi:10.1038/220911a0) [DOI] [PubMed] [Google Scholar]
- McIntyre M.2006The use of digit ratios as markers for perinatal androgen action. Reprod. Biol. Endocrinol. 4, 10 (doi:10.1186/1477-7827-4-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.1988The sexual differentiation of social play. Trends Neurosci. 11, 54–58 (doi:10.1016/0166-2236(88)90164-6) [DOI] [PubMed] [Google Scholar]
- Monaghan E. P., Glickman S. E.2001Hormones and aggressive behavior. In Behavioural endocrinology (eds Becker J. B., Breedlove S. M., Crews D.), pp. 261–287 Cambridge, MA: MIT Press [Google Scholar]
- Neat F., Huntingford F., Beveridge M.1998Fighting and assessment in male cichlid fish: the effects of asymmetries in gonadal state and body size. Anim. Behav. 55, 883–891 (doi:10.1006/anbe.1997.0669) [DOI] [PubMed] [Google Scholar]
- O'Connor D., Archer J., Wu F.2004Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J. Clin. Endo. Metabol. 89, 2837–2845 (doi:10.1210/jc.2003-031354) [DOI] [PubMed] [Google Scholar]
- Oyegbile T., Marler C.2005Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267 (doi:10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- Phoenix C., Goy R., Gerall A., Young W.1959Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382 (doi:10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- Piazza P. V., Le Moal M.1997Glucocorticoids as biological substrate of reward: physiological and pathophysiological implications. Brain Res. Rev. 25, 259–372 (doi:10.1016/S0165-0173(97)00025-8) [DOI] [PubMed] [Google Scholar]
- Piazza P., Deroche V., Deminière J. M., Maccari S., Le Moal M., Simon H.1993Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc. Natl Acad. Sci. USA 90, 11 738–11 742 (doi:10.1073/pnas.90.24.11738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope H., Katz D.1988Affective and psychotic symptoms associated with anabolic steroid use. Am. J. Psychiatry 145, 487–490 [DOI] [PubMed] [Google Scholar]
- Pope H., Kouri E., Hudson J.2000Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch. Gen. Psychiatry 57, 133–140 (doi:10.1001/archpsyc.57.2.133) [DOI] [PubMed] [Google Scholar]
- Reavis R., Overman W.2001Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav. Neurosci. 115, 196–206 (doi:10.1037/0735-7044.115.1.196) [DOI] [PubMed] [Google Scholar]
- Reichlin S.1998Neuroendocrinology. In Williams textbook of endocrinology (eds Nelson J. D., Kronenberg H. M., Larson P. P.), pp. 165–248, 10th edn Philadelphia, PA: N. B. Saunders [Google Scholar]
- Reul J. M., de Kloet E. R.1985Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511 (doi:10.1210/endo-117-6-2505) [DOI] [PubMed] [Google Scholar]
- Rutte C., Taborsky M., Brinkhof M.2006What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21 (doi:10.1016/j.tree.2005.10.014) [DOI] [PubMed] [Google Scholar]
- Salminen E., Portin R., Koskinen A., Helenius H., Nurmi M.2004Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin. Can. Res. 10, 7575–7582 (doi:10.1158/1078-0432.CCR-04-0750) [DOI] [PubMed] [Google Scholar]
- Sanfey A., Rilling J. K., Aronson J. A., Nystrom L. E., Cohen J. D.2003The neural basis of economic decision-making in the ultimatum game. Science 13, 1755–1758 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.1997The trouble with testosterone: and other essays on the biology of the human predicament New York, NY: Simon & Schuster [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U.2000How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Sarnyai Z., McKittrick C. R., McEwen B., Kreek M. J.1998Selective regulation of dopamine transporter binding in the shell of the nucleus accumbens by adrenalectomy and corticosterone replacement. Synapse 30, 334–337 (doi:10.1002/(SICI)1098-2396(199811)30:3<334::AID-SYN11>3.0.CO;2-#;) [DOI] [PubMed] [Google Scholar]
- Sarnyai Z., Shaham Y., Heinrichs S. C.2001The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 53, 209–243 [PubMed] [Google Scholar]
- Sato S. M., Schulz K., Sisk C., Wood R.2008Adolescents and androgens, receptors and rewards. Horm. Behav. 53, 647–658 (doi:10.1016/j.yhbeh.2008.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J., Packard M.2000Role of dopamine receptor subtypes in the acquisition of a testosterone conditioned place preference in rats. Neurosci. Lett. 282, 17–20 (doi:10.1016/S0304-3940(00)00839-9) [DOI] [PubMed] [Google Scholar]
- Schulkin J., McEwen B. S., Gold P. W.1994Allostasis, amygdala, and anticipatory angst. Neurosci. Biobehav. Rev. 18, 385–396 (doi:10.1016/0149-7634(94)90051-5) [DOI] [PubMed] [Google Scholar]
- Schultz W.2000Multiple reward signals in the brain. Nat. Rev. Neurosci. 1, 199–207 (doi:10.1038/35044563) [DOI] [PubMed] [Google Scholar]
- Scoville W. B., Milner B.1957Loss of recent memory after bilateral hippocampal lesions. J. Neurochem. 20, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard J. D., Barron K. W., Myers D. A.2000Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 861, 288–295 [DOI] [PubMed] [Google Scholar]
- Shiller R.2005Irrational exuberance New York, NY: Doubleday [Google Scholar]
- Stroud L., Salovey P., Epel E.2002Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry 319, 318–327 (doi:10.1016/S0006-3223(02)01333-1) [DOI] [PubMed] [Google Scholar]
- Swenson R., Vogel W.1983Plasma catecholamine and corticosterone as well as brain catecholamine changes during coping in rats exposed to stressful footshock. Pharmacol. Biochem. Behav. 18, 689–693 (doi:10.1016/0091-3057(83)90007-2) [DOI] [PubMed] [Google Scholar]
- Trainor B. C., Bird I. M., Marler C. A.2004Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 45, 115–121 (doi:10.1016/j.yhbeh.2003.09.006) [DOI] [PubMed] [Google Scholar]
- Tsai M.-J., O'Malley B. W.1994Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63, 451–486 (doi:10.1146/annurev.bi.63.070194.002315) [DOI] [PubMed] [Google Scholar]
- Vadakkadath Meethal S., Atwood C. S.2005The role of hypothalamic–pituitary–gonadal hormones in the normal structure and functioning of the brain. Cell Mol. Life Sci. 62, 257–270 (doi:10.1007/s00018-004-4381-3) [DOI] [PubMed] [Google Scholar]
- Van den Bergh B., Dewitte S.2006Digit ratio (2D : 4D) moderates the impact of sexual cues on men's decisions in ultimatum games. Proc. R. Soc. B 273, 2091–2095 (doi:10.1098/rspb.2006.3550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J., Schutter D., Hermans E., Putman P.2003Low cortisol levels and the balance between punishment sensitivity and reward dependency. NeuroReport 14, 1993–1996 [DOI] [PubMed] [Google Scholar]
- van Honk J., Schuttera D. J. L. G., Hermansa E. J., Putmana P., Tuitena A., Koppeschaar H.2004Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinol. 29, 937–943 (doi:10.1016/j.psyneuen.2003.08.007) [DOI] [PubMed] [Google Scholar]
- Vermeersch H., T'sjoen G., Kaufman J. M., Vincke J.2008The role of testosterone in aggressive and non-aggressive risk-taking in adolescent boys. Horm. Behav. 53, 463–471 (doi:10.1016/j.yhbeh.2007.11.021) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Hegner R. E., Dufty A. M., Ball G. F.1990The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 (doi:10.1086/285134) [Google Scholar]
- Wingfield J. C., Lynn S., Soma K.2001Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav. Evol. 57, 239–251 (doi:10.1159/000047243) [DOI] [PubMed] [Google Scholar]