Abstract
Background
Persistent MRSA bacteremia (PB) represents an important subset of Staphylococcus aureus infections and correlates with poor clinical outcomes.
Methods
We profiled relevant in vitro phenotypic and genotypic characteristics of MRSA isolates from 39 persons with bacteremia (21 had PB and 18 had resolving bacteremia [RB]). We also compared the intrinsic virulence and responsiveness to vancomycin of selected PB and RB strains in an experimental endocarditis model (IE).
Results
PB and RB isolates differed significantly with regard to several in vitro characteristics that are believed to impact endovascular infections. PB isolates exhibited significantly more resistance to the cationic defensin hNP-1, enhanced membrane fluidity, and substantially greater adhesion to fibronectin, fibrinogen, and endothelial cells. Genotypically, PB isolates had higher frequency of SCCmec II, CC30, and spa 16; and higher rates of agr type III, cap8, tst-1, and cna carriage. Finally, a prototypic PB strain was more resistant to vancomycin treatment in the infective endocarditis model than a RB comparator strain, despite equivalent virulence profiles.
Conclusions
Our findings indicate that PB isolates may have specific virulence signatures that distinguish them from RB isolates. These data suggest that methods might be developed to identify patients at higher risk for PB in real-time, thereby optimizing the effectiveness of anti-MRSA therapeutic strategies.
The persistent bacteremia (PB) syndrome is well represented in large clinical series of persons with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection (PB prevalence, 20%–30%), and it is especially relevant to patients with endovascular infection [1, 2]. However, in up to one-third of cases, no readily identifiable cause of PB is identified, despite extensive clinical evaluation [3]. Therefore, understanding the molecular mechanisms and determinants of PB is essential to optimize prevention and therapy against life-threatening S. aureus infections.
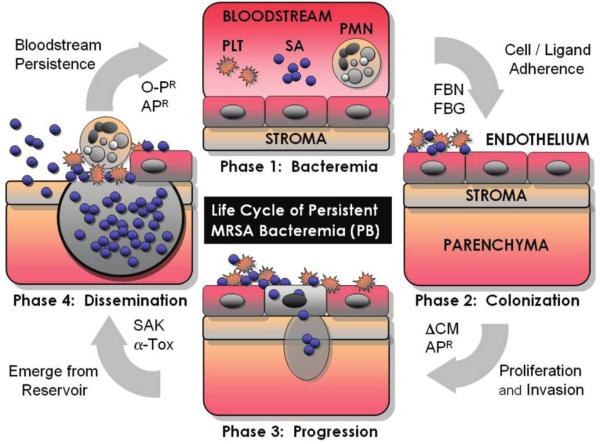
We hypothesize that S. aureus uses specific virulence determinants to persist in the bloodstream and cause PB in the context of endovascular infection. For example, the organism must avoid immediate killing by host-defense peptides liberated by platelets at sites of endovascular infection. Next, the organism must evade or survive phagocytosis and intracellular killing by neutrophil-associated oxidative and nonoxidative killing mechanisms (including those associated with antimicrobial peptides, such as α-defensins). During this phase, the pathogen must adhere to host cells and ligands to colonize vascular endothelium and avoid clearance by the reticuloendothelial system. Following adherence, the organism proliferates and invades tissues, creating reservoir foci. Finally, it deploys exotoxins and exoenzymes to reemerge from these reservoir sites, reenter the bloodstream, and hematogenously seed metastatic target organs (figure 1).
Figure 1.
Hypothesized life cycle of methicillin-resistant Staphylococcus aureus (SA) isolates that cause persistent bacteremia in the context of endovascular infection. AP, antimicrobial peptides; α-Tox, α-toxin; CM, cell membrane; FBG, fibrinogen; FBN, fibronectin; O-P, PMN opsonophagocytosis; PLT, platelets; PMN, neutrophils; SAK, staphylokinase.
Because distinct infection foci are not synchronized, organisms are continuously reemerging into the bloodstream, accounting for persistent bacteremia. On the basis of this hypothesized life cycle of PB isolates, we investigated a cadre of in vitro phenotypic and genotypic characteristics considered to be involved in these phases of endovascular pathogenesis. In addition, we examined the in vivo virulence and antibiotic responsiveness of MRSA isolates associated with PB (hereafter, “PB isolates”) and those associated with resolving bacteremia (here-after, “RB isolates”) in a rabbit model of infective endocarditis.
PATIENTS, MATERIALS, AND METHODS
Collection of strains
All MRSA isolates were recovered from bacteremic patients at Duke University Medical Center during 1994–1999. All isolates were susceptible to vancomycin (minimum inhibitory concentration [MIC] range, 0.25–1μg/mL), with no significant differences in MICs between PB and RB strains.
Two groups of MRSA isolates were studied. The first group was collected from 21 patients with PB, all of whom had MRSA-positive blood cultures for ≥7 days while receiving an antibiotic to which the isolate was susceptible. The second group was collected from 18 patients with RB who had an initial blood culture that yielded MRSA and subsequent blood cultures that yielded no MRSA 2–4 days after therapy initiation. Patients in the 2 cohorts were matched on the basis of demographic characteristics. Both patient groups had similar initial clinical characteristics and laboratory findings but differed significantly with respect to characteristics associated with clinical course and outcome [1, 4, 5].
In vitro assay for susceptibility to hNP-1
In a recent report, we showed that PB isolates were significantly more resistant than RB isolates to mammalian platelet microbicidal proteins in vitro [1]. To evaluate whether the disparity in the in vitro susceptibility profiles of PB and RB isolates extended to other important host-defense effectors, we assessed their in vitro responses to hNP-1, a 3.8-kDa cationic α-defensin peptide from human neutrophils [6-8]. Nonoxidative neutrophil-mediated killing related to this peptide family is likely important during the initial bacteremic clearance stage of endovascular pathogenesis [6, 9]. Purified hNP-1 was purchased from Peptide International. Bacterial susceptibility to hNP-1 was assayed in vitro, as described in detail elsewhere [7]. Results are expressed as the percentage of colony-forming units in the initial inoculum (105 cfu) that survived exposure to hNP-1 after a 2-h incubation period.
In vitro measurement of adherence to host endovascular ligands (fibrinogen and fibronectin) and host cells (endothelial cell and platelets)
Tissue culture plates were coated with purified human fibrinogen (50 μg/mL; Sigma Chemical) or fibronectin (50 μg/mL; Sigma Chemical) and washed with phosphate-buffered saline (PBS; pH 7.2) [10, 11]. Plates were then treated with 3% bovine serum albumin (Sigma Chemicals) to prevent nonspecific adhesion and were washed again with PBS before organism seeding.
Human umbilical cord veins were obtained and maintained as previously described [12]. Endothelial cell monolayer plates were washed twice with prewarmed Hanks balanced salt solution before organism seeding.
In assays to detect fibrinogen, fibronectin, and endothelial cell binding, PB or RB isolates that had been cultured overnight were added at a final inoculum of 5 × 103 cfu/mL to control plates without either matrix ligands or endothelial cells, to fibrinogen-coated or fibronectin-coated plates, or to endothelial cell monolayer-coated plates. The plates were incubated for 1 hunder static conditions, as previously described [10-12]. Unbound bacteria were removed by washing with PBS, and tryptic soy agar was added. Adherence was expressed as the percentage of the initial inoculum bound.
Bacterial adherence to platelets was tested as previously described [13]. Fresh rabbit platelets (109 platelets/mL) were specifically labeled with CellTracker Red (2.5 mmol/L). Overnight-cultured PB or RB isolates (109 cfu/mL) were labeled with a fluorescent dye, Syto13 (2 mmol/L). Binding studies were done by mixing Syto13-labeled S. aureus cells (108 cfu/mL) and Cell-Tracker Red–labeled platelets (107 platelets/mL). The percentage of S. aureus cells bound to platelets was calculated as previously described [13].
Measurement of S. aureus cell membrane fluidity
We previously showed that cationic peptide-resistant S. aureus strains (e.g., strains resistant to tPMP-1) exhibited significantly higher degrees of membrane fluidity [14]. Such resistance is believed to contribute to both initial bloodstream and progressive stages of endovascular infection [15]. Therefore, we tested the membrane fluidity of the strain sets, using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH) [7, 14]. Protocols for DPH incorporation into target cell membranes, measurement of fluorescence polarization, and calculation of the degree of fluorescence polarization index are described in detail elsewhere [14]. As the polarization index decreases, the degree of membrane fluidity increases [14].
E-test
Macro E-tests (AB Biodisk) were performed to screen for hetero–vancomycin-intermediately resistant S. aureus (hetero-VISA). The assays were done as recommended by the manufacturer. Strains with vancomycin MICs of ≥8 μg/mL were defined as hetero-VISA [16].
Multilocus sequence typing (MLST) and clonal complexes (CCs)
MLST is a powerful typing tool for defining the molecular epidemiology of S. aureus because it can identify specific MLST clones and group strains into unique CCs [17]. It has recently been shown that such bacterial genotypes are associated with distinct clinical outcomes [18]. In addition, CC5 and CC30 have been shown to be significantly associated with more-severe S. aureus infection [19]. The protocol for MLST has been published previously [19].
Staphylococcal protein A (spa) typing
Sequence analysis of the tandem repeat region in the spa subtypes of MRSA helps further discriminate the MLST profiles into distinct lineages [20]. We have previously shown that spa type 16 is associated with more-complicated outcomes in patients with S. aureus bacteremia [19]. The spa typing of the current strain set was performed by means of previously published methods [21].
Staphylococcal cassette chromosome mec (SCCmec) classification
SCCmec typing distinguishes MRSA strains into 5 major classes (types I–V) and provides another genotypic assessment to further refine strain differentiation on the basis of MLST and spa typing. SCCmec typing was performed for several important reasons: (1) our prior pulsed-field gel electrophoresis profiling suggested that PB isolates were of 2 predominant and related lineages (A1 and A2) [1]; (2) because isolates were obtained from a single referral center (Duke University Medical Center), it was likely that clonal bias would be identified; and (3) specific SCCmec types may be associated with specific clinical syndromes (e.g., skin and soft-tissue infections related to SCCmec type IV) [19]. SCCmec typing was performed on the 39 MRSA strains, using methods described elsewhere [22].
Multiplex PCR for S. aureus virulence genes
Coordinated sets of virulence genes or gene networks are believed to be involved in ≥1 pathogenic step during MRSA infection (table 1) [23, 24]. To compare the endovascular virulon of PB and RB strains, we used multiplex PCR to determine the presence or absence of 33 such virulence genes, representing a range of regulatory, adhesin, exotoxin, antiphagocytic, and exoenzyme genes. The PCR-based assays were performed by use of previously published techniques [25].
Table 1.
Findings of multiplex PCR profiling of select prototypic virulence genes in methicillin-resistant Staphylococcus aureus isolates that cause persistent bacteremia (PB).
| Virulence gene(s) |
Description | Hypothesized role in life cyclea |
|---|---|---|
| cna | Adhesin, binds collagen | Colonization |
| fnbA | Adhesin, binds fibronectin | Colonization |
| clfB | Adhesin, binds fibrinogen | Colonization |
| sdrD | Adhesin, binds fibrinogen | Colonization |
| sdrE | Adhesin, binds fibrinogen | Colonization |
| ica | Polysaccharide intercellular adhesion (involved in biofilm formation) | Colonization |
| pvl | Exotoxin, putative virulence factor | Dissemination |
| tst | Exotoxin, toxic shock syndrome toxin-1 | Dissemination |
| ssp | Enzyme; serine protease | Dissemination |
| cap5, cap8 | Capsular polysaccharide– antiphagocytic factor | Clearance |
A detailed account of the life cycle of PB isolates is presented in figure 1.
Rabbit infective endocarditis model
A well-characterized model of catheter-induced infective endocarditis in rabbits was used to study the following 4 phasic outcomes of infection due to PB and RB isolates in vivo [15, 24]: early bacteremia clearance, initial vegetation colonization, composite virulence, and responsiveness to vancomycin therapy. Infective endocarditis was produced by intravenous injection of select PB isolates (from strain 420) or RB isolates (from strain 1507) 24 h after catheterization. Strains 420 and 1507 were selected because of their highly distinguishable in vitro phenotypic and genotypic profiles (table 2). Phenotypically, the PB strain differed from the RB strain in several features likely to impact endovascular pathogenesis: the PB strain has greater resistance to killing by a representative platelet antimicrobial peptide, tPMP-1 [4]; greater adherence to endothelial cells, fibronectin, and fibrinogen in vitro; and substantially more fluidic cell membranes (table 2). Genotypically, the PB strain was CC30 and spa type 16 (both of which were associated with more-severe MRSA clinical infections in other studies [19]) and harbored SCCmec type II; in addition, this strain was agr type III and positive for cna and tst-1. In contrast, the RB strain was CC8 and spa type 7, contained SCCmec type IV, and was negative for agr type I, cna, and tst-1 (table 2). Both strains were pvl negative and had vancomycin MICs of 1 μg/mL. Neither strain exhibited a hetero-VISA phenotype (data not shown).
Table 2.
Phenotypic and genotypic characteristics of methicillin-resistant Staphylococcus aureus strains used to induce persistent bacteremia (PB) or resolving bacteremia (RB) in an infective endocarditis model.
| Characteristic | PB strain 420 | RB strain 1507 |
|---|---|---|
| Phenotypica | ||
| tPMP-1 susceptibilityb | 61 ± 11 | 9 ± 3.5 |
| Fibrinogen binding | 15.2 ± 2.3 | 7.4 ± 1.6 |
| Fibronectin binding | 17.0 ± 0.2 | 1.3 ± 0.1 |
| Platelet binding | 16.6 ± 3.7 | 17.7 ± 2.2 |
| Endothelial cell binding | 12.7 ± 0.4 | 7.3 ± 0.2 |
| Membrane fluidity, polarization index ± SD | 0.319 ± 0.005 | 0.333 ± 0.016 |
| Genotypic | ||
| MLST | CC30 | CC8 |
| spa type | 16 | 7 |
| SCCmec type | II | IV |
| agr type | III | I |
| pvl | Absent | Absent |
| cna | Present | Absent |
| cap type | 8 | 5 |
| tst | Present | Absent |
NOTE. MLST, multilocus sequence type; SCCmec, staphylococcal cassette chromosome mec.
Data are percentage of isolates (±SD), unless otherwise indicated.
Data are percentage of isolates that survived exposure to 1 μg/mL of tPMP-1.
Each animal was challenged intravenously with 109 cfu of PB strain 420 or RB strain 1507 24 h after catheterization. Blood samples were obtained for quantitative culture 1 min and 30 min after challenge. Bacterial blood density was expressed in mean log10 cfu/mL (±SD). In addition, animals were sacrificed 30 min after challenge, and all vegetations were removed for quantitative culture. The in vivo adherence of each strain to vegetations was expressed in mean log10 cfu per g of vegetation (±SD).
The intrinsic virulence of infective endocarditis strains can be measured as a composite of infective endocarditis induction rates over an inoculum challenge range and of target tissue bacterial densities 24 h after receipt of the ID95 inoculum. Thus, 24 h after catheterization, aortic-catheterized animals were challenged intravenously with 104, 105, or 106 cfu of PB or RB strains, the inoculum range that encompasses the ID95 for most S. aureus strains in this model [26]. Twenty-four hours after inoculation, all animals were euthanized, and their cardiac vegetations, kidneys, and spleen were removed and quantitatively cultured.
Because PB isolates were obtained from patients with no response to vancomycin therapy, we postulated that the in vivo response of the PB and RB strains to vancomycin therapy would differ in the infective endocarditis model. Twenty-four hours after receipt of the infective endocarditis-inducing inoculation (105 cfu), animals were randomized to receive either no therapy (controls) or vancomycin (15 mg/kg intravenously twice per day for 3 days). This vancomycin dose strategy is associated with slow and incomplete clearance of most susceptible S. aureus strains from vegetations and other target organs over a 3-day treatment period [27]. Twenty-four hours after the last antibiotic dose, all animals were sacrificed, and target tissues were removed and quantitatively cultured as described above.
Statistical analysis
Data were analyzed by Kruskal-Wallis analysis of variance, with corrections made for multiple comparisons when appropriate. P values of ≤.05 were considered statistically significant.
RESULTS
In vitro susceptibility to hNP-1
Nearly all strains were resistant to a 20-μg/mL dose of hNP-1. However, at a 40-μg/mL dose of hNP-1, the percentage of isolates that survived was significantly higher in the PB group, compared with the RB group (P = .04) (table 3).
Table 3.
Phenotypic and genotypic characteristics of methicillin-resistant Staphylococcus aureus strains used to induce persistent bacteremia (PB) or resolving bacteremia (RB).
| Characteristic | PB isolates (n = 21) |
RB isolates (n = 18) |
P |
|---|---|---|---|
| Phenotype | |||
| hNP-1 susceptibilitya | 52 ± 5 | 36 ± 10 | <.05 |
| Fibrinogen bindingb | 21 ± 4 | 14 ± 6 | <.05 |
| Membrane fluidity, polarization index | 0.276 (more fluid) | 0.290 (less fluid) | .11 |
| Genotype | |||
| MLST CC30 | 19 (90) | 8 (44) | <.05 |
| spa16 | 12 (57) | 7 (39) | .16 |
| SCCmec type II | 20 (95) | 13 (72) | <.05 |
| agr type III | 17 (81) | 8 (44) | <.05 |
| cna | 18 (85) | 10 (56) | .07 |
| cap5 | 3 (14) | 11 (61) | <.05 |
| cap8 | 18 (86) | 7 (39) | <.05 |
| pvl | 0 (0) | 0 (0) | NS |
| tst | 18 (86) | 8 (44) | <.05 |
NOTE. Data are no. (%) of isolates, unless otherwise indicated. The following genes were also tested, but there were no significant differences between PB and RB isolates: bsaA1, sea-e, sej, sem, sei, seg, seo, sen, sdrC-E, bbp, ebpS, icaA, efb, sbl, ssp, clfB, splB, fnbA, lukDE, lukM, and EDIN. CC30, clonal complex 30; MLST, multilocus sequence type; NS, not signficant; SCCmec, staphylococcal cassette chromosome mec.
Data are percentage of isolates (±SD) that survived exposure to 40 μg/mL of hNP-1.
Data are percentage of isolates (±SD) that bound to fibrinogen.
In vitro adherence to host endovascular ligands (fibrinogen and fibronectin) and host cells (endothelial cells and platelets)
In general, PB isolates bound better than RB isolates to matrix ligands and host cells relevant to endovascular infection. For example, the PB group had substantially greater mean adherence to fibrinogen and fibronectin than the RB group (figure 2; P < .05 for fibrinogen binding). Results also indicated that PB isolates selected for infective endocarditis studies bound substantially better to human endothelial cell monolayers than their RB counterparts (figure 2). In contrast, there was no difference in the capacity of the PB and RB strain sets overall to bind to platelets (figure 2).
Figure 2.
Adherence of methicillin-resistant Staphylococcus aureus isolates causing persistent bacteremia (PB; black bars) and those causing resolving bacteremia (RB; white bars) to fibrinogen, fibronectin, platelets, and endothelial cells. Fibrinogen, fibronectin, and platelet binding values represent comparisons of the entire strain set cohort; endothelial cell binding represents comparison of the 2 strains used for in vivo analyses. Data represent findings from at least 2 independent assays performed on different days. *P < .05, compared with RB isolates.
S. aureus cell membrane fluidity
The PB strain set exhibited higher cell membrane fluidity than RB isolates. The mean polarization indices of membrane fluidity (±SD) for PB and RB isolates were 0.276 ± 0.03 and 0.292 ± 0.02, respectively. However, these differences did not reach statistical significance (P = .11).
PB and RB isolates did not differ in other relevant phenotypic characteristics
No PB or RB isolate had a hetero-VISA phenotype detected by means of a macro E-test.
MLST and CCs
A total of 9 sequence types contained in 5 CCs were represented among the 39 isolates. The most common CC was CC30, present in 27 (69%) of 39 isolates. PB isolates were significantly more likely than RB isolates to be CC30 (90% vs. 44%; P < .05) (table 3).
Staphylococcal protein A (spa) typing
Consistent with findings in the recent study by Fowler et al. [19], there was an association trend of spa type 16 (W-G-K-A-K-A-O-M-Q-Q-Q-Q) with the PB strain set. Thus, 57% of PB isolates were spa type 16, compared with 39% of RB isolates (P = .16) (table 3).
SCCmec classification
SCCmec II was the most commonly observed type (85% of isolates). In addition, 20 PB isolates (95%) contained the SCCmec II element, compared with 13 RB isolates (72%; P < .05) (table 3).
Multiplex PCR for S. aureus virulence genes
A total of 81% of PB isolates were agr type III, compared with only 44% of RB isolates (P = .017) (table 3). All isolates possessed the fnbA and clfB surface adhesin genes. Of note, 85% of PB isolates were cna positive, compared with only 56% of RB isolates (P = .07) (table 3). Also, only 15% of PB isolates carried sdrD and sdrE, compared with 47% of RB isolates (P < .05). Carriage of the tst-1 endotoxin gene was significantly over-represented among PB isolates, compared with RB isolates (86% vs. 44%; P = .03). Panton-Valentine leukocidin locus was absent in all strains (table 3). A total of 86% of PB isolates had the cap type 8 genotype (cap8); among RB isolates, 39% had the cap8 genotype, and 61% had the cap5 genotype (table 3). Last, all strains carried the biofilm-associated ica gene, as well as the V8 protease gene ssp. There were no significant differences between the PB and RB strain sets with regard to the presence or absence of other virulence genes studied (table 3).
Infective endocarditis model
No significant differences between the rate of early clearance of the PB isolate and the rate of early clearance of the RB isolate were observed 1 and 30 min after infection (data not shown). In addition, no significant differences were observed between the PB and RB strains with respect to the extent of initial colonization of vegetations (data not shown).
At the 104, 105 and 106 cfu inocula, all catheterized animals developed infective endocarditis. At inocula of 104 cfu and 105 cfu (table 4), bacterial densities in the 3 target tissues were not significantly different between animals infected with the RB isolate and those infected with the PB isolate. The majority of animals infected with either the PB or RB strain at an inoculum of 106 cfu died ≤24 h after infection.
Table 4.
Densities of Staphylococcus aureus strains that induced persistent bacteremia (PB) or resolving bacteremia (RB) in target tissues of an infective endocarditis model, with or without vancomycin therapy.
| Group, isolate type |
Animals, no. |
Inoculum, cfu | Received 15 mg/kg vancomycin |
MRSA density, log10 cfu/g of specimen, mean ± SD |
||
|---|---|---|---|---|---|---|
| Vegetations | Kidneys | Spleen | ||||
| Group 1 | ||||||
| PB | 7 | 104 | No | 7.21 ± 0.72 | 5.31 ± 0.11 | 4.76 ± 0.51 |
| RB | 6 | 104 | No | 6.28 ± 1.18 | 5.41 ± 1.83 | 4.88 ± 1.68 |
| Group 2 | ||||||
| PB | 9 | 105 | No | 7.46 ± 1.53 | 5.23 ± 0.91 | 4.83 ± 1.54 |
| RB | 9 | 105 | No | 7.61 ± 1.34 | 5.53 ± 0.73 | 5.12 ± 0.50 |
| Group 3 | ||||||
| PB | 8 | 105 | Yes | 4.23 ± 1.41a | 2.19 ± 0.23a | 2.38 ± 0.50a |
| RB | 7 | 105 | Yes | 2.31 ± 0.92b | 1.81 ± 0.63c | 1.96 ± 0.38c |
NOTE. The PB isolate was strain 420, and the RB isolate was strain 1507.
P < .005, compared with the density in corresponding specimens from PB controls from group 2.
P < .01, compared with the density in corresponding specimens from RB controls from group 2.
P < .005, compared with the density in corresponding specimens from RB controls in group 2.
As shown in table 4, vancomycin therapy resulted in significant reductions of all target tissue MRSA densities in animals infected with either PB or RB isolates, compared with respective untreated controls (P < .005). Of note, for cardiac vegetations, the residual MRSA density after vancomycin treatment in animals infected with the PB strain was ~2.0 log10 cfu per g of vegetation greater than that observed for animals infected with the RB strain (P = .01) (table 4).
DISCUSSION
A number of interesting observations emerged from the present investigations. Phenotypically, the current data extend upon past findings regarding antimicrobial peptide resistance; PB strains tend to be more resistant to key innate cationic host defense molecules from both neutrophils (e.g., hNP-1) and platelets (e.g., tPMP-1) [1]. These findings suggest that PB isolates have an intrinsic capacity to survive interactions with 2 predominant host defense cells early in the course of bloodstream invasion (figure 1, phase 1 bacteremia). The ability of PB strains to circumvent such innate immune defenses likely enhance their subsequent pathogenic potential [15, 28]. Furthermore, since these 2 innate defense molecules may be important in multiple stages of endovascular pathogenesis (e.g., tPMP-1–resistance fostering infective endocarditis progression) [28], the ability of a strain to resist their microbicidal actions probably contributes to PB.
The present findings also demonstrated that PB isolates adhere better than RB isolates to host cells (i.e., endothelium) and matrix ligands relevant to endovascular pathogenesis (i.e., fibrinogen and fibronectin). Such capabilities may facilitate the colonization phases of PB infection (phase 2 colonization) (figure 1). Also, since fibronectin and fibrinogen binding are now considered integral to endothelial cell and vegetation persistence in experimental infective endocarditis [29], increased binding of PB isolates to these ligands, compared with binding of RB isolates, would theoretically provide an advantage for PB pathogenesis.
Importantly, PB isolates exhibited substantially more fluidic membranes than RB isolates. A fluidic membrane phenotype has previously been linked to cationic antimicrobial peptide resistance in S. aureus laboratory strains [14]. This characteristic may facilitate persistent and progressive infective endocarditis due to PB isolates [14]. The mechanism (or mechanisms) by which enhanced fluidity causes increased resistance to such peptides is not well understood but is postulated to be associated with reduced membrane binding or intramembrane organization of these cationic molecules.
Genotypically, a greater percentage of PB isolates were associated with SCCmec II (95%, compared with 72% of RB isolates), CC30 (90% vs. 44%), and spa type 16 (57% vs. 39%). This observation is in line with recent findings by Fowler et al. [19], who demonstrated a significant trend toward more frequent hematogenous complications in strains exhibiting these genotypic profiles. Multiplex PCR revealed that PB isolates differed from RB isolates in terms of overrepresentation of capsule type 8 and cna and tst-1 gene carriage. The increased presence of the adhesin gene, cna, would theoretically allow PB strains to exploit specific anatomic targets, such as bones, joints, or endothelial substrata [30]. This role could potentially contribute to enhancement of the colonization and/or persistence phases in the life cycle of PB isolates. In addition, overrepresentation of the tst-1 gene could ostensibly increase the incidence of “cytokine storm”-associated sepsis syndromes, leading to worse clinical outcomes for patients with PB [31].
The net intrinsic virulence properties of these 2 strains were not different in the context of infective endocarditis induction or progression. In contrast, vancomycin therapy clearly divulged significant outcome differences between groups. Thus, isolates from the PB strain set were able to persist within cardiac vegetations to a greater extent than those from the RB strain set during vancomycin therapy. This result occurred despite identical vancomycin MICs and no hetero-VISA subpopulations in the PB and RB strain sets. A similar observation by Fowler et al. [1] and Hawkins et al. [32] demonstrated that vancomycin susceptibility was not decreased among PB isolates. However, these findings contrast with those of other studies in which the PB phenotype has been associated with reduced susceptibility to vancomycin [33, 34]. These in vivo data regarding vancomycin-induced disclosure of the PB outcome suggest several interesting possibilities. For example, PB and RB isolates may differ in other features not assessed in our profiling that may impact net responsiveness to vancomycin (e.g., cell wall perturbations, cell surface charge, and global metabolic pathway abnormalities) [7]. Also, it is possible that vancomycin may itself differentially impact virulence pathways in PB isolates, compared with RB isolates.
Our study has several potential limitations. First, all strains were obtained from Duke University Medical Center, raising the possibility of single-center and/or geographic bias. Second, these strains emanated from 1994–1999 and therefore do not represent recent shifts. Third, we compared only the initial PB and RB isolates and did not screen for virulence signatures that may have adaptively evolved during treatment among follow-up blood isolates. Finally, we only examined a single PB-RB strain pair in vivo. Current studies are being designed to address these limitations.
In summary, the present data support our hypothesis that there are significant phenotypic and genotypic profiles that can distinguish PB isolates from RB isolates. Characterization of PB isolates may afford breakthrough discoveries in the treatment of life-threatening MRSA infections.
Acknowledgments
Financial support:
National Institutes of Health (grants AI-039108 to A.S.B., AI-059111 and AI-068804 to V.G.F., and AI-039001 to M.R.Y.); American Heart Association (grant 0465142Y to Y.Q.X.]).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Fowler VG, Jr, Sakoulas G, McIntyre LM, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190:1140–9. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 2.El-Ahdab F, Benjamin DK, Jr, Wang A, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225–9. doi: 10.1016/j.amjmed.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006;38:7–14. doi: 10.1080/00365540500372846. [DOI] [PubMed] [Google Scholar]
- 4.Fowler VG, Jr, McIntyre LM, Yeaman MR, et al. In vitro resistance to thrombin-induced platelet microbicidal protein in isolates of Staphylococcus aureus from endocarditis patients correlates with an intravascular device source. J Infect Dis. 2000;182:1251–4. doi: 10.1086/315812. [DOI] [PubMed] [Google Scholar]
- 5.Fowler VG, Jr, Kong LK, Corey GR, et al. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients. J Infect Dis. 1999;179:1157–61. doi: 10.1086/314712. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 7.Xiong YQ, Mukhopadhyay K, Yeaman MR, Adler-Moore J, Bayer AS. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Anti-microb Agents Chemother. 2005;49:3114–21. doi: 10.1128/AAC.49.8.3114-3121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White SH, Wimley WC, Selsted ME. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521–7. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 10.Kupferwasser LI, Yeaman MR, Nast CC, et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J Clin Invest. 2003;112:222–33. doi: 10.1172/JCI16876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong YQ, Bayer AS, Yeaman MR, Van Wamel W, Manna AC, Cheung AL. Impacts of sarA and agr in Staphylococcus aureus strain Newman on fibronectin-binding protein A gene expression and fibronectin adherence capacity in vitro and in experimental infective endocarditis. Infect Immun. 2004;72:1832–6. doi: 10.1128/IAI.72.3.1832-1836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Ibrahim AS, Sheppard DC, et al. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002;44:61–72. doi: 10.1046/j.1365-2958.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- 13.Yeaman MR, Sullam PM, Dazin PF, Norman DC, Bayer AS. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis. 1992;166:65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Bayer AS, Prasad R, Chandra J, et al. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect Immun. 2000;68:3548–53. doi: 10.1128/iai.68.6.3548-3553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhawan VK, Bayer AS, Yeaman MR. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect Immun. 1998;66:3476–9. doi: 10.1128/iai.66.7.3476-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–15. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 17.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–30. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melles DC, Gorkink RF, Boelens HA, et al. Natural population dynamics and expansion of pathogenic clones of Staphylococcus aureus. J Clin Invest. 2004;114:1732–40. doi: 10.1172/JCI23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler VG, Jr, Nelson CL, McIntyre LM, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007;196:738–47. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 20.Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–8. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koreen L, Ramaswamy SV, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42:792–9. doi: 10.1128/JCM.42.2.792-799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuma K, Iwakawa K, Turnidge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Entenza JM, Foster TJ, Ni Eidhin D, Vaudaux P, Francioli P, Moreillon P. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect Immun. 2000;68:5443–6. doi: 10.1128/iai.68.9.5443-5446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong YQ, Willard J, Yeaman MR, Cheung AL, Bayer AS. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J Infect Dis. 2006;194:1267–75. doi: 10.1086/508210. [DOI] [PubMed] [Google Scholar]
- 25.Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau-Remington F. Roles of 34 virulence genes in the evolution of hospital-and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2006;193:1495–503. doi: 10.1086/503777. [DOI] [PubMed] [Google Scholar]
- 26.Bayer AS, Coulter SN, Stover CK, Schwan WR. Impact of the high-affinity proline permease gene (putP) on the virulence of Staphylococcus aureus in experimental endocarditis. Infect Immun. 1999;67:740–4. doi: 10.1128/iai.67.2.740-744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhawan VK, Yeaman MR, Bayer AS. Influence of in vitro susceptibility phenotype against thrombin-induced platelet microbicidal protein on treatment and prophylaxis outcomes of experimental Staphylococcus aureus endocarditis. J Infect Dis. 1999;180:1561–8. doi: 10.1086/315063. [DOI] [PubMed] [Google Scholar]
- 28.Dhawan VK, Yeaman MR, Cheung AL, Kim E, Sullam PM, Bayer AS. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–9. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Que YA, Haefliger JA, Piroth L, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med. 2005;201:1627–35. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elasri MO, Thomas JR, Skinner RA, et al. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone. 2002;30:275–80. doi: 10.1016/s8756-3282(01)00632-9. [DOI] [PubMed] [Google Scholar]
- 31.Galperine T, Neau D, Lina G, et al. Menstrual staphylococcal toxic shock is still a reality [in French] Presse Med. 2003;32:1121–2. [PubMed] [Google Scholar]
- 32.Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med. 2007;167:1861–7. doi: 10.1001/archinte.167.17.1861. [DOI] [PubMed] [Google Scholar]
- 33.Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448–51. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- 34.Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis. 2004;38:521–8. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]