Abstract
OBJECTIVE
An association of the C-857T polymorphism of the tumor necrosis factor-α (TNF-α) gene promoter region with LDL cholesterol levels has been reported. This study was designed to evaluate the relationship between the TNF-α-C-857T polymorphism and LDL cholesterol levels according to statin treatment in subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
DNA was obtained from 322 Japanese subjects (160 male and 162 female) with type 2 diabetes, and TNF-α-C-857T polymorphisms were determined by direct sequencing. Serum LDL cholesterol was measured by a direct method.
RESULTS
Although serum LDL cholesterol levels were significantly higher in the T carriers (C/T + T/T) than in the non–T carriers (C/C) (3.14 ± 0.86 vs. 2.89 ± 0.75 mmol/l, P < 0.05), there was no difference in LDL cholesterol levels between the non–T carriers and the T carriers in statin-untreated subjects (2.87 ± 0.73 vs. 2.89 ± 0.76 mmol/l, NS), whereas in statin-treated subjects, LDL cholesterol levels were significantly higher in the T carriers than in the non–T carriers (3.43 ± 0.89 vs. 2.90 ± 0.78 mmol/l, P = 0.0007). There were no differences in HDL cholesterol and triglyceride levels between the non–T carriers and the T carriers in both statin-treated and -untreated subjects. The percent decrease in LDL cholesterol levels after administration of statins was significantly smaller in the T carriers compared with the non–T carriers (27.6 vs. 36.4%, P = 0.031).
CONCLUSIONS
The mutant allele of the C-857T promoter polymorphism of the TNF-α gene may predispose to resistance to the LDL cholesterol–lowering effect of statins and could be one of the markers used to predict the efficacy of statins.
Tumor necrosis factor-α (TNF-α) is a potent immunomodulator and proinflammatory cytokine with multiple functions and plays a variety of roles in pathological and physiological conditions. There have been many reports on relationships between TNF-α gene polymorphisms and various diseases including infectious and metabolic disorders (1,2). Regarding lipid metabolism, there have been a few reports on an association of TNF-α gene polymorphism with serum lipids including cholesterol levels, the most potent risk factor for cardiovascular diseases (3–5). Shiau et al. (4) have shown that TNF-α-G-238A is associated with LDL cholesterol levels in Taiwanese patients with type 2 diabetes. We have recently reported that TNF-α-C-857T, a functional TNF-α gene promoter polymorphism with higher transcriptional activity (6), was associated with higher LDL cholesterol levels and carotid plaques in Japanese subjects with type 2 diabetes (5). In the course of this study, our preliminary analysis indicated that an association of TNF-α-C-857T with higher LDL cholesterol levels was observed only in subjects treated with the 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors (statins), but not in those without statin treatment (7), implying that this polymorphism is resistant to the effect of statins. We therefore performed a study to confirm that the C-857T promoter polymorphism of the TNF-α gene is associated with resistance to the cholesterol-lowering effect of statins in type 2 diabetic subjects.
RESEARCH DESIGN AND METHODS
After obtaining approval from the ethics committee of Iwate Medical University and informed consent from all subjects, blood samples were collected from 322 type 2 diabetic subjects (160 male and 162 female). All subjects were Japanese. The present study was performed in accordance with the guidelines expressed in the Declaration of Helsinki.
Identification of polymorphisms
Genomic DNAs were obtained from peripheral blood leukocytes by standard phenol-chloroform extraction and ethanol precipitation methods or by the Biomek 3000 Laboratory Automation System (Beckman-Coulter, Fullerton, CA). The 5′-flanking region of the TNF-α gene, spanning from −188 to −1,229, relative to the TNF-α transcription start site, was amplified by PCR using a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). The PCR primers were as follows (6): sense 5′-GCTTGTGTGTGTGTGTCTGG-3′ and antisense 5′-GGACACACAAGCATCAAGG-3′. PCR conditions were as follows (6): denaturing at 94°C for 1 min, annealing at 55°C for 2 min, extension at 72°C for 3 min, for 40 cycles, final incubation at 72°C for 10 min, and cooling to 4°C. The PCR products were purified using NucleoSpin Extract (Macherey-Nagel, Duren, Germany). Sequence analysis was performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (PerkinElmer, Norwalk, CT) with the sequence primer 5′-TGTGGCCATATCTTCTTAAA-3′ to analyze the sequence from −782 to −1,209 for polymorphisms at −857, −863, and −1,031. Finally, the cycle sequencing products were purified again with a Dye Terminator Removal Kit (ABgene House, Epsom, Surrey, U.K.) and analyzed by a Prism 3100 Genetic Analyzer (Applied Biosystems), according to the manufacturer's instructions.
Laboratory examinations
For all subjects, blood was obtained after fasting for ≥12 h, and blood cell counts, fasting plasma glucose levels, fasting insulin (immunoreactive insulin) levels, A1C, total cholesterol, triglyceride, HDL cholesterol, and LDL cholesterol were measured at the Central Laboratory in our hospital.
Statistics
Data are expressed as means ± SD. Statistical significance was analyzed by unpaired t test and χ2 test using StatView-J5.0 (Abacus Concepts, Berkeley, CA). Significance was considered at P < 0.05.
RESULTS
Serum LDL cholesterol levels are higher in diabetic subjects with the T allele of the TNF-α-C-857T promoter gene polymorphism than in those with the C allele
The frequencies of C and T alleles of TNF-α-C-857T were 85.1 and 14.9%, respectively, which are not significantly different from those reported in the Japanese population (5,6,8). Hardy-Weinberg equilibrium was maintained in this population. We reconfirmed our previous observation (5) for double the number of instances in which the T carriers (C/T and T/T) displayed significantly higher serum LDL cholesterol levels than the noncarriers (C/C) (3.14 ± 0.86 vs. 2.89 ± 0.75 mmol/l, P < 0.05) (Table 1). Clinical backgrounds, such as sex and age distributions, physiques, blood pressures, A1C, and serum lipid levels other than LDL cholesterol showed no difference between these groups (Table 1) nor did the baseline characteristics of medication for dyslipidemia, diabetes, and hypertension (Table 2). Other promoter polymorphisms of the TNF-α gene, including C-863A and T-1031C, were not associated with the serum LDL cholesterol levels (data not shown).
Table 1.
Comparison of clinical characteristics of diabetic subjects according to the TNF-α-C-857T polymorphism and statin treatment
| All subjects |
Subjects without statin treatment |
Subjects with statin treatment |
||||
|---|---|---|---|---|---|---|
| C/C | C/T,T/T | C/C | C/T,T/T | C/C | C/T,T/T | |
| Sex (male/female) | 116/115 | 44/47 | 77/59 | 29/20 | 39/56 | 15/27 |
| Age (years) | 62.9 ± 10.9 | 62.2 ± 13.6 | 62.3 ± 11.7 | 62.3 ± 15.8 | 63.4 ± 9.6 | 61.9 ± 11.0 |
| Height (cm) | 158.6 ± 8.8 | 156.7 ± 15.4 | 159.3 ± 9.2 | 159.1 ± 8.7 | 157.5 ± 8.4 | 156.3 ± 8.7 |
| Body wt (kg) | 62.5 ± 11.8 | 61.5 ± 12.4 | 61.2 ± 11.6 | 60.9 ± 12.6 | 63.4 ± 13.9 | 62.5 ± 12.4 |
| BMI (kg/m2) | 24.8 ± 4.0 | 24.1 ± 3.5 | 23.8 ± 4.1 | 23.8 ± 3.6 | 25.9 ± 4.2 | 24.8 ± 3.4 |
| Systolic blood pressure (mmHg) | 134.8 ± 19.9 | 134.1 ± 17.7 | 136.2 ± 21.5 | 132.3 ± 18.1 | 134.9 ± 18.1 | 136.8 ± 17.6 |
| Diastolic blood pressure (mmHg) | 78.0 ± 13.3 | 74.9 ± 11.6 | 78.8 ± 13.9 | 73.4 ± 10.6 | 76.1 ± 12.4 | 76.5 ± 12.9 |
| A1C (%) | 7.64 ± 1.73 | 7.39 ± 1.65 | 7.72 ± 1.91 | 7.52 ± 1.70 | 7.59 ± 1.46 | 7.30 ± 1.60 |
| Total cholesterol (mmol/l) | 5.05 ± 0.92 | 5.21 ± 1.02 | 4.95 ± 0.82 | 4.83 ± 0.86 | 5.18 ± 1.05 | 5.68 ± 1.01* |
| Triglycerides (mmol/l) | 1.51 ± 0.95 | 1.52 ± 0.83 | 1.39 ± 0.94 | 1.34 ± 0.88 | 1.51 ± 0.84 | 1.58 ± 0.68 |
| HDL cholesterol (mmol/l) | 1.45 ± 0.45 | 1.48 ± 0.45 | 1.44 ± 0.46 | 1.46 ± 0.49 | 1.46 ± 0.43 | 1.53 ± 0.40 |
| LDL cholesterol (mmol/l) | 2.89 ± 0.75 | 3.14 ± 0.86* | 2.87 ± 0.73 | 2.89 ± 0.76 | 2.90 ± 0.78 | 3.43 ± 0.89† |
Data are means ± SD.
*P < 0.05 (vs. C/C);
†P < 0.0001 (vs. C/C).
Table 2.
Comparison of medications in diabetic subjects between the TNF-α-C-857T polymorphisms
| C/C | C/T, T/T | P | |
|---|---|---|---|
| Statins (without/with) | 136/95 | 49/42 | NS |
| Fibrates (without/with) | 228/3 | 90/1 | NS |
| Oral hypoglycemic drugs (without/with) | 50/181 | 22/69 | NS |
| Insulin (without/with) | 136/95 | 49/42 | NS |
| Hypotensive drugs (without/with) | 115/72 | 26/29 | NS |
Higher LDL cholesterol level in the T carriers of the TNF-α-C-857T polymorphism is observed in subjects treated with statins but not in those without statins
Because statins affect LDL cholesterol levels, we divided subjects according to statin treatment and compared the serum LDL cholesterol levels between the T carriers and the non–T carriers in those with and without statin treatment. As shown in Table 1, the T carriers with statin treatment displayed significantly higher LDL cholesterol levels than the non–T carriers (3.43 ± 0.89 vs. 2.90 ± 0.78 mmol/l, P = 0.0007), whereas in subjects without statin treatment the LDL cholesterol levels did not differ irrespective of genotype. Other clinical characteristics did not differ between the T carriers and non–T carriers in statin-treated and -untreated subjects (Table 1). The distribution of use of statin subclasses including atorvastatin, pitavastatin, pravastatin, fluvastatin, and rosuvastatin was not different between the T carriers and non–T carriers in the statin-treated subjects (data not shown).
T carriers of the TNF-α-C-857T polymorphism are more resistant to the LDL cholesterol–lowering effect of statin treatment than non–T carriers
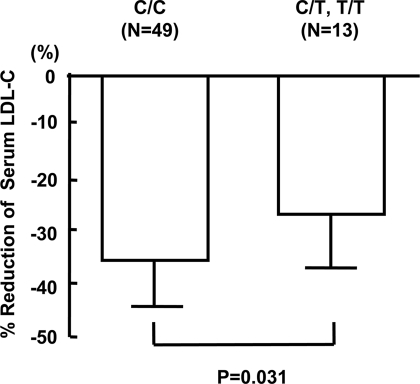
Table 1 indicates the possibility of a difference in the LDL cholesterol–lowering effect of statins between the T carriers and the non–T carriers. To see whether the T carriers are resistant to the statin treatment, we retrospectively analyzed the LDL cholesterol levels before and 3–6 months after statin administration among a subset of the diabetic subjects with hypercholesterolemia, whose complete datasets were available for analysis. As shown in Fig. 1, the percent reduction in LDL cholesterol levels after statin administration was significantly smaller in the T carriers than in the non–T carriers (−27.6 vs. −36.4%, P = 0.031).
Figure 1.
Percent reduction of serum LDL cholesterol (LDL-C) levels after statin treatment according to the TNF-α-C-857T polymorphism. Percent reduction = ([LDL cholesterol levels before statin treatment] − [LDL cholesterol levels 3–6 months after statin treatment])/[LDL cholesterol levels before statin treatment] × 100. Percent reductions in C/C and C/T,T/T were −36.4 and −27.6%, respectively (P = 0.031).
CONCLUSIONS
In this clinical observation, our data indicate for the first time that the C-857T polymorphism in the promoter region of the TNF-α gene is associated with serum LDL cholesterol levels in statin-treated subjects with type 2 diabetes and the T carrier is resistant to statin treatment.
We have confirmed that T carriers displayed higher serum LDL cholesterol levels consistent with our previous report (5), after doubling the number of subjects. The T allele of TNF-α-C-857T generates significantly higher transcriptional promoter activity than the C allele does, possibly leading to elevated TNF-α production (6,9). The administration of TNF-α in rodents is followed by an increase in serum concentrations of total cholesterol and hepatic cholesterol synthesis (10), probably by stimulating the activity of HMG-CoA reductase (11). However, in our human subjects, the increase in HMG-CoA reductase activity is not a mechanism of the increased serum LDL cholesterol in the T carriers of TNF-α-C-857T, because there was no difference in LDL cholesterol levels between the T carriers and non–T carriers, who were not treated with statins (Table 1).
A further analysis revealed that the C-857T promoter polymorphism affected the cholesterol-lowering effect of statins but did not have a direct effect on cholesterol synthesis. T carriers taking statins displayed significantly higher serum LDL cholesterol levels but those who were not taking statins did not (Table 1), indicating that TNF-α productivity possibly affects sensitivity to the LDL cholesterol–lowering effects of statins. Indeed, the T carriers exhibited a significantly smaller LDL cholesterol–lowering rate in response to statin treatment than the non–T carriers (Fig. 1). Fibrates, other drugs affecting the lipid profile, probably did not influence our results, because very few subjects were treated with fibrates (Table 2).
Statins are substrates of several drug transporters (12), including the influx transporter solute carrier organic anion transporter family, member 1B1 (SLCO1B1, previously known as OATP1B1/OATP-C/OATP2/LST- 1) (13–15). It is reasonable to assume that impaired function or expression of the transporters would result in reduced hepatic uptake of statins and then in reduced cholesterol-lowering efficacy because of lower intracellular statin concentrations of hepatocytes. TNF-α reportedly suppressed protein expression and transport activity of SLCO1B1 (16), which is located on the sinusoidal membrane of hepatocytes (17). This molecule plays a pivotal role as a major transporter of various statins, including atorvastatin, simvastatin, pitavastatin, pravastatin, fluvastatin, and rosuvastatin (13–15), from the portal blood into hepatocytes. In fact, a retrospective study in Caucasian subjects suggested a weaker effect of pravastatin on inhibition of cholesterol synthesis among carriers of the SLCO1B1*17 haplotype, which is associated with impaired OATPB1B function and/or expression (18). Therefore, T alleles of the TNF-α-C-857T polymorphism with the higher promoter transcriptional activities (6,9) possibly result in a reduced LDL cholesterol–lowering effect of statins, which may be in line with our present observation. However, the serum concentration of TNF-α was not significantly higher in the T carriers of the C-857T than in the non–T carriers (5), probably because of dilution of TNF-α in circulation compared with those in local region. The lower hepatic statin concentrations in the T carriers associated with defective function and/or expression of OATPB1B remains to be proven. We could not find differences in the proportion of the medicines used such as hydrophilic (pravastatin and rosuvastatin) and lipophilic (atorvastatin, simvastatin, pitavastatin, and fluvastatin) statins or statins with weak or strong cholesterol-lowering effect between the T carriers and non–T carrier, although the number of patients was small (data not shown).
It is possible, as mentioned above, that the TNF-α gene polymorphism is involved in the sensitivity to the LDL cholesterol–lowering effect of statins via TNF-α productivity. However, other possibilities including linkage disequilibrium of this polymorphism with a susceptibility gene to the effect of statins cannot be ruled out.
In summary, these results strongly suggest that the mutant allele of the C-857T promoter polymorphism of the TNF-α gene may predispose to resistance to the LDL cholesterol–lowering effect of statins and then could be one of markers to predict the efficacy of statins.
Acknowledgments
This research was supported by the Open Research Center Project for Private Universities with a matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology, Japan, 2004–2008, and by a research fund from Iwate Prefecture.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
We thank Dr. Paul Langman for assistance with English usage.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Sookoian SC, González C, Pirola CJ: Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res 2005;13:2122–2131 [DOI] [PubMed] [Google Scholar]
- 2. Elahi MM, Asotra K, Matata BM, Mastana SS: Tumour necrosis factor α-308 gene locus promoter polymorphism: an analysis of association with health and disease. Biochim Biophys Acta 2009;1792:163–172 [DOI] [PubMed] [Google Scholar]
- 3. Fontaine-Bisson B, Wolever TM, Chiasson JL, Rabasa-Lhoret R, Maheux P, Josse RG, Leiter LA, Rodger NW, Ryan EA, El-Sohemy A: Tumor necrosis factor α-238G>A genotype alters postprandial plasma levels of free fatty acids in obese individuals with type 2 diabetes mellitus. Metabolism 2007;56:649–655 [DOI] [PubMed] [Google Scholar]
- 4. Shiau MY, Wu CY, Huang CN, Hu SW, Lin SJ, Chang YH: TNF-α polymorphisms and type 2 diabetes mellitus in Taiwanese patients. Tissue Antigens 2003;61:393–397 [DOI] [PubMed] [Google Scholar]
- 5. Yamashina M, Kaneko Y, Maesawa C, Kajiwara T, Ishii M, Fujiwara F, Taneichi H, Takebe N, Ishida W, Takahashi K, Masuda T, Satoh J: Association of TNF-α gene promoter C-857T polymorphism with higher serum LDL cholesterol levels and carotid plaque formation in Japanese patients with type 2 diabetes. Tohoku J Exp Med 2007;211:251–258 [DOI] [PubMed] [Google Scholar]
- 6. Higuchi T, Seki N, Kamizono S, Yamada A, Kimura A, Kato H, Itoh K: Polymorphism of the 5′-flanking region of the human tumor necrosis factor (TNF)-α gene in Japanese. Tissue Antigens 1998;51:605–612 [DOI] [PubMed] [Google Scholar]
- 7. Takahashi T, Yamashina M, Maesawa C, Honma H, Kakino S, Ishii M, Fujiwara F, Kajiwara T, Takebe N, Taneichi H, Miura M, Ishida W, Takahashi K, Kaneko Y, Masuda T, Satoh J: Subjects with type 2 diabetes with TNF-α-C-857T polymorphism is resistant to cholesterol-lowering effect of statin (Abstract). Diabetes 2008;57(Suppl. 1):A25 [Google Scholar]
- 8. Negoro K, Kinouchi Y, Hiwatashi N, Takahashi S, Takagi S, Satoh J, Shimosegawa T, Toyota T: Crohn's disease is associated with novel polymorphisms in the 5′-flanking region of the tumor necrosis factor gene. Gastroenterology 1999;117:1062–1068 [DOI] [PubMed] [Google Scholar]
- 9. Hohjoh H, Tokunaga K: Allele-specific binding of the ubiquitous transcription factor OCT-1 to the functional single nucleotide polymorphism (SNP) sites in the tumor necrosis factor-α gene (TNFA) promoter. Genes Immun 2001;2:105–109 [DOI] [PubMed] [Google Scholar]
- 10. Grunfeld C, Soued M, Adi S, Moser AH, Dinarello CA, Feingold KR: Evidence for two classes of cytokines that stimulate hepatic lipogenesis: relationships among tumor necrosis factor, interleukin-1 and interferon-α. Endocrinology 1990;127:46–54 [DOI] [PubMed] [Google Scholar]
- 11. Nishimura F, Taniguchi A, Yamaguchi-Morimoto M, Soga Y, Iwamoto Y, Kokeguchi S, Kuroe A, Fukushima M, Nakai Y, Seino Y: Periodontal infection and dyslipidemia in type 2 diabetics: association with increased HMG-CoA reductase expression. Horm Metab Res 2006;38:530–535 [DOI] [PubMed] [Google Scholar]
- 12. Neuvonen PJ, Niemi M, Backman JT: Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther 2006;80:565–581 [DOI] [PubMed] [Google Scholar]
- 13. Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL: Substantially elevated levels of atorvastatin and metabolites in cyclosporine-treated renal transplant recipients. Clin Pharmacol Ther 2004;76:388–391 [DOI] [PubMed] [Google Scholar]
- 14. Matsushima S, Maeda K, Kondo C, Hirano M, Sasaki M, Suzuki H, Sugiyama Y: Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein J Pharmacol Exp Ther 2005;314:1059–1067 [DOI] [PubMed] [Google Scholar]
- 15. Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB: Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 2006;130:1793–1806 [DOI] [PubMed] [Google Scholar]
- 16. Vee ML, Lecureur V, Stieger B, Fardel O: Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-α or interleukin-6. Drug Metab Dispos 2009;37:685–693 [DOI] [PubMed] [Google Scholar]
- 17. König J, Cui Y, Nies AT, Keppler D: A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 2000;278:G156–G164 [DOI] [PubMed] [Google Scholar]
- 18. Niemi M, Neuvonen PJ, Hofmann U, Backman JT, Schwab M, Lütjohann D, von Bergmann K, Eichelbaum M, Kivistö KT: Acute effects of pravastatin on cholesterol synthesis are associated with SLCO1B1 (encoding OATP1B1) haplotype *17. Pharmacogenet Genomics 2005;15:303–309 [DOI] [PubMed] [Google Scholar]