Abstract
OBJECTIVE
Initial treatment with antidepressant medication is insufficiently effective in some patients with type 2 diabetes, and factors predicting treatment outcome are poorly understood.
RESEARCH DESIGN AND METHODS
Aggregate data from two published trials were analyzed to determine the rates and predictors of response to antidepressant pharmacotherapy in adults with type 2 diabetes using conventional markers of initial treatment outcome (improvement, response, partial remission, and remission). Three hundred eighty-seven patients who received up to 16 weeks of open-label, acute-phase treatment using bupropion (n = 93) or sertraline (n = 294) were studied. Logistic regression was used to identify predictors of poor treatment outcome. Candidate predictors included age, race, sex, initial Beck Depression Inventory (iBDI) score, treatment received (sertraline or bupropion), family history of depression, extant diabetes complications (eDC), and A1C level.
RESULTS
Of 387 patients initiated on treatment, 330 (85.3%) met criteria for improvement, 232 (59.9%) for response, 207 (53.5%) for partial remission, and 179 (46.3%) for full remission. Significant independent predictors of poor outcome included eDC (for no improvement); sertraline treatment, eDC, and younger age (for nonresponse); sertraline treatment, eDC, and higher iBDI (for failure to partially remit); and younger age and higher iBDI (for failure to fully remit). Higher pain scores predicted three of the four markers of poor outcome in the subset with pain data.
CONCLUSIONS
In patients with type 2 diabetes, poor initial response to antidepressant medication is predicted by multiple factors. Auxiliary treatment of pain and impairment may be required to achieve better outcomes.
Clinically significant depression often occurs in the context of medical illness. It is present in one of every four patients with diabetes (1), is highly recurrent (2), and imposes additional risks of poor diabetes self-care, hyperglycemia (3), diabetes complications (4), and death (5). Randomized controlled trials demonstrate the efficacy of pharmacotherapy (6–8), cognitive behavior therapy (CBT) (9), and stepped, collaborative care treatments (10) for major depressive disorder (MDD) in patients with diabetes. Relief of depression symptoms, however, often is incomplete and leaves the patient at risk of relapse or recurrence of MDD. Improvement in mood is associated with improvement in glycemic control in many but not all of these trials. Small depression treatment effects limit the ability to study this question, and this has fueled interest in efforts to improve the potency of depression treatment.
Research to identify predictors of response to antidepressant treatment in psychiatric samples has been inconclusive. Methodological issues including small sample size, sample heterogeneity, variable definitions of depression treatment response, inadequate diagnostic specificity, and suboptimal statistical methods have limited the reproducibility and generalizability of previous findings (11). Research of this kind in individuals with diabetes is scant. In a controlled trial of CBT for MDD in 42 adults with type 2 diabetes, Lustman et al. (12) found that factors pertaining to diabetes management (poor adherence to glucose monitoring instructions) and severity (higher glycosylated hemoglobin levels, presence of diabetes complications) were independent predictors of poor treatment outcome.
Recent antidepressant trials in nondiabetic samples suggest that up to 70% of patients do not respond adequately to initial pharmacotherapy (13). Similarly, the prognosis of depression in diabetes samples is guarded, even following successful treatment. Of those who achieve recovery, one-third suffer a recurrence within a year (8) and <10% remain depression free for 5 years (2). As a step toward the goal of improving depression treatment outcome, the aim of the present study was to identify the rates and predictors of initial response to antidepressant pharmacotherapy in people with diabetes.
RESEARCH DESIGN AND METHODS
This report presents a secondary analysis of acute-phase data aggregated from two previously published treatment trials of pharmacotherapy for MDD in adults with diabetes. The purpose, design, and findings from these two studies are described in detail elsewhere (8,14) and are summarized here. The first study was a multicenter, randomized, placebo-controlled trial of sertraline for prevention of MDD recurrence in adults with type 1 or type 2 diabetes (8). Collaborating sites included Washington University in St. Louis, the University of Arizona in Tucson, and the University of Washington in Seattle. The second study was a single-site (Washington University in St. Louis) trial of bupropion for MDD in adults with type 2 diabetes (14). Inclusion in the aggregate analysis was limited to patients with type 2 diabetes, as the bupropion trial had included only those patients and excluded those with type 1 diabetes. Open treatment with antidepressant medication was provided to subjects in both studies during the initial (acute) phase, the goal of acute treatment being induction of depression remission. Written informed consent was obtained from all subjects before evaluation, and the institutional review board at each participating site reviewed and approved the trials. Both studies were two-phase depression treatment trials that included an acute (induction) and maintenance phase. Analysis and discussion in the current report is based upon the acute-phase findings. The length of the acute phase was 16 weeks for the sertraline trial and 10 weeks for the bupropion trial.
The two studies utilized similar eligibility criteria and enrolled patients who were aged 18–80 years, had diagnoses of type 1 or type 2 diabetes and MDD, and had depression symptoms of at least moderate severity, as indicated by a total score of >14 on the Beck Depression Inventory (version I) (BDI) (15). Patients were excluded from participation if they had active suicidal or homicidal ideation, a prior suicide attempt, current substance abuse, a history of psychotic or bipolar disorder, or a contraindication to the study medication.
Assessment and monitoring during treatment
The presence of MDD was determined by structured psychiatric interview and conformed to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (16). The BDI (15) and Hamilton Depression Rating Scale (HDRS) (17) were used to assess the severity of depression symptoms. Demographic information (age, sex, race, marital status, and education level), history of depression treatment, family history of depression, and diabetes characteristics (age of onset, method of treatment, and the presence of diabetes complications including neuropathy, retinopathy, nephropathy, hyperlipidemia, and atherosclerosis [coronary artery disease, cerebrovascular disease, peripheral vascular disease]) were obtained using a combination of patient self-report, physical exam, and a review of clinical records.
Eligible, consenting participants were enrolled and open treatment with a standardized dosage of antidepressant medication (50 mg/day for sertraline and 150 mg/day for bupropion) was initiated following completion of the baseline evaluation. The dosage was adjusted biweekly (to a maximum of 200 mg/day for sertraline and 450 mg/day for bupropion) depending on clinical response and adverse events. Patients taking an antidepressant at the time of study enrollment were tapered off the medication over an interval of ≤2 weeks while sertraline or bupropion was introduced.
Patients were seen every 2 weeks at office visits. The BDI and HDRS were administered at each visit. Glycemic control (A1C) (18) was measured at baseline and the end of the acute phase. Health-related quality of life was assessed at these points with the Short Form-36 Health Survey (SF-36) (19). The SF-36 includes a subscale that measures the severity and impact of bodily pain.
Definitions of treatment outcome
Rates of response were calculated according to the intention-to-treat principle and to conventional depression treatment outcome descriptors (improvement, response, partial remission, and full remission). Definitions of these outcomes were made in accordance with the DSM-IV section on episode specifiers for mood disorders (16) and operational criteria recommendations by Riso et al. (20) and the American College of Neuropsychopharmacology Task Force on Conceptualizing Response and Remission in MDD (21). Response was defined as a ≥50% reduction in BDI total score from baseline to the end of the acute phase. Partial remission was defined as a BDI score of ≤9 at the end of the acute phase. Full remission was defined as a BDI score of ≤9 for a period of 4 weeks prior to the end of the acute phase. No improvement categorized those whose depression severity did not change or worsened during the course of treatment and was defined as an end of acute phase BDI score ≥100% of the baseline BDI score. The categories are not mutually exclusive; a given patient may meet criteria for any one, any combination, or all four favorable outcomes. For example, a patient having a BDI score of 15 at entry and 8 at the end of acute phase (with that degree of improvement limited to the preceding 2 weeks) would meet criteria for improvement and partial remission.
Statistical analysis
Logistic regression analysis was used to determine significant (P ≤ 0.05) predictors of poor depression treatment outcome (i.e., no improvement, nonresponse, nonpartial remission, and nonfull remission) in order to identify factors that might themselves be targets of intervention. Candidate predictors were derived from the depression treatment literature in diabetes (12) and psychiatric samples (11). A correlational matrix was constructed to identify highly correlated (r ≥ 0.40) variables; in one instance when this occurred (baseline BDI and HDRS total scores, r = 0.59), initial BDI (iBDI) was included in the main regression model. The final model included demographic (age, race, and sex), depression (treatment type [sertraline versus bupropion], iBDI, and family history of depression), and diabetes (A1C, presence of any diabetes complications) variables. While the study lacked the statistical power to parse the unique contribution of each diabetes complication to depression treatment outcome, the presence of increasing complications is a proxy for severity of symptomatic diabetes. An “Enter” method was used for the variable input in all regression analyses. For those patients who dropped out before completing the acute phase, a last-observation-carried-forward method was used. Positive predictive values for nonremission and nonimprovement were calculated as the proportion of true positives over the sum of true positives plus false-positives.
A logistic regression analysis was also performed in the subgroup (n = 267) with data on pain severity and impact (SF-36 bodily pain scale score), sleep dysfunction (aggregate score from the three HDRS sleep questions), and self-reported history of depression treatment. This subgroup analysis added the variables of pain, sleep, and prior depression treatment to those used in the main model to determine additional predictors of poor treatment outcome.
RESULTS
Three hundred eighty-seven patients received up to 16 weeks of open-label, acute-phase treatment for MDD. Two hundred ninety-four (76.0%) patients were treated with sertraline, and 93 (24.0%) received bupropion. Demographic, diabetes, depression, and other clinical characteristics of the sample are presented in Table 1. Overall, 330 (85.3%) of 387 patients evidenced some degree of symptomatic improvement and 232 (59.9%) met criteria for response. Two hundred and seven (53.5%) patients achieved partial remission and 179 (46.3%) achieved full remission.
Table 1.
Demographic, diabetes, depression, and other clinical characteristics of the sample
| n | 387 |
| Age (years) | 53.0 ± 11.1 |
| Female sex | 234 (60.5) |
| Caucasian race | 272 (70.3) |
| Married | 209 (54.0) |
| Education (years) | 13.5 ± 2.9 |
| Age of diabetes onset (years) | 45.2 ± 11.6 |
| Duration of diabetes (years) | 7.1 ± 7.3 |
| Any diabetes complications | 247 (63.8) |
| Neuropathy | 157 (40.6) |
| Nephropathy | 32 (8.3) |
| Retinopathy | 63 (16.3) |
| Atherosclerosis | 60 (15.5) |
| Hyperglycemia | 146 (37.7) |
| Diabetes management | — |
| Diet only | 42 (10.9) |
| Insulin | 76 (19.6) |
| Oral agent | 197 (50.9) |
| Insulin and oral agent | 61 (15.8) |
| A1C at baseline (%) | 8.31 ± 2.1 |
| A1C at end of acute phase (%) | 7.70 ± 1.8 |
| No improvement | 57 (14.7) |
| Responders | 232 (59.9) |
| Partial remitters | 207 (53.5) |
| Full remitters | 179 (46.3) |
| Family history of depression | 203 (52.5) |
| Prior depression treatment | 209 (54.0) |
| BDI at baseline | 24.6 ± 8.2 |
| BDI at end of acute phase | 9.2 ± 8.7 |
| Bodily pain* | 6.5 ± 2.4 |
| Sleep† | 2.7 ± 1.8 |
Data are means ± SD or n (%).
*Mean SF-36 scale score.
†Mean aggregate score from the three HDRS questions on sleep dysfunction.
The results of the logistic regression analyses are presented in Table 2. The significant predictor of no improvement was extant diabetes complications (odds ratio [OR] 2.75 [95% CI 1.24–6.11]; P = 0.01). Predictors of nonresponse were treatment with sertraline (2.47 [1.39–4.41]; P = 0.002), extant diabetes complications (eDC) (2.27 [1.37–3.76]; P = 0.001), and younger age (1.03 [1.01–1.05]; P = 0.009). Predictors of nonpartial remission included sertraline treatment (2.80 [1.59–4.94]; P > 0.001), eDC (2.04 [1.25–3.34]; P = 0.005), and higher iBDI (1.06 [1.03–1.09]; P < 0.001), while predictors of nonfull remission were younger age (1.02 [1.00–1.05]; P = 0.04) and higher iBDI (1.06 [1.03–1.09]; P < 0.001).
Table 2.
Predictors of no improvement, nonresponse, nonpartial remission, and nonfull remission to pharmacotherapy of MDD (n = 352)
| No improvement (R2 = 0.06)* |
Nonresponse (R2 = 0.09) |
Nonpartial remission (R2 = 0.15)* |
Nonfull remission (R2 = 0.10) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | OR† (95% CI)‡ | P | β | OR† (95% CI)‡ | P | β | OR† (95% CI)‡ | P | β | OR† (95% CI)‡ | P | |
| Sertraline treatment | −0.24 | 0.79 (0.37–1.69) | 0.54 | 0.91 | 2.47 (1.39–4.41) | 0.002 | 1.03 | 2.80 (1.59–4.94) | <0.001 | 0.26 | 1.29 (0.77–2.18) | 0.33 |
| Higher iBDI | 0.02 | 1.02 (0.98–1.06) | 0.32 | −0.01 | 1.00 (0.97–1.02) | 0.74 | 0.05 | 1.06 (1.03–1.09) | <0.001 | 0.06 | 1.06 (1.03–1.09) | <0.001 |
| Family history of depression | −0.25 | 0.78 (0.40–1.51) | 0.46 | −0.13 | 0.88 (0.55–1.40) | 0.59 | 0.02 | 1.02 (0.64–1.63) | 0.93 | −0.12 | 0.89 (0.56–1.40) | 0.60 |
| eDC | 1.01 | 2.75 (1.24–6.11) | 0.01 | 0.82 | 2.27 (1.37–3.76) | 0.001 | 0.71 | 2.04 (1.25–3.34) | 0.005 | 0.40 | 1.49 (0.93–2.38) | 0.10 |
| Higher baseline A1C | 0.04 | 1.04 (0.90–1.20) | 0.61 | 0.01 | 1.01 (0.91–1.13) | 0.86 | −0.03 | 0.97 (0.87–1.08) | 0.55 | 0.02 | 1.02 (0.92–1.14) | 0.72 |
| Younger age | 0.02 | 1.02 (0.99–1.06) | 0.19 | 0.03 | 1.03 (1.01–1.05) | 0.009 | 0.02 | 1.02 (1.00–1.04) | 0.06 | 0.02 | 1.02 (1.00–1.05) | 0.04 |
| Non-Caucasian race | −0.30 | 0.74 (0.34–1.62) | 0.45 | 0.15 | 1.16 (0.68–1.97) | 0.59 | 0.05 | 1.05 (0.61–1.78) | 0.87 | 0.09 | 1.09 (0.65–1.83) | 0.73 |
| Female sex | 0.05 | 1.06 (0.53–2.10) | 0.88 | 0.10 | 1.11 (0.68–1.80) | 0.68 | 0.11 | 1.12 (0.69–1.81) | 0.65 | 0.04 | 1.04 (0.66–1.66) | 0.86 |
*Nagelkerke R2 value.
†Coefficient for the constant.
‡OR/exponential β value (95% CI).
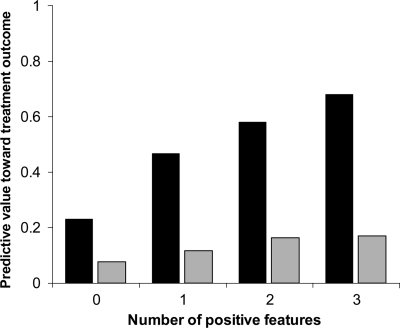
The cumulative contribution of any eDC, younger age (<50 years), and higher iBDI score (>23) on the predictive value of nonimprovement and nonremission was examined (Fig. 1). With an increasing number of these features, the likelihood of poor depression treatment outcome increased linearly. Whereas patients with none of the features had <25% likelihood of failing depression treatment, subjects with any one feature had a nearly 50% chance of not remitting. Patients with all three features were at the highest risk of poor treatment outcome as 68% of them did not remit, and nearly 20% did not improve.
Figure 1.
Positive predictive value of poor depression treatment outcome (nonremission [■] or nonimprovement [ ]) by cumulative number of the three features most often found to be significant in the regression analyses (presence of any diabetes complications, younger age [<50 years], and higher iBDI score [>23]). Patients without any of these features had a 23% chance of not remitting and an 8% chance of not improving. Presence of all three features had a positive predictive value of 68% for nonremission and 17% for nonimprovement.
]) by cumulative number of the three features most often found to be significant in the regression analyses (presence of any diabetes complications, younger age [<50 years], and higher iBDI score [>23]). Patients without any of these features had a 23% chance of not remitting and an 8% chance of not improving. Presence of all three features had a positive predictive value of 68% for nonremission and 17% for nonimprovement.
Subgroup analysis
Logistic regression analysis was performed in the subgroup (n = 267) with data on pain severity and impact, sleep dysfunction, and prior depression treatment. Of these three factors, higher pain scores emerged as the only significant predictor, and it predicted three of four measures of poor outcome: nonresponse (OR 1.18 [95% CI 1.04–1.35]; P = 0.009), nonpartial remission (1.23 [1.08–1.39]; P = 0.001), and nonremission (1.25 [1.11–1.42]; P < 0.001). The other predictors found in the main analysis remained the same for the subgroup, except that younger age was no longer significant in the nonresponse and nonfull remission models.
CONCLUSIONS
The primary purpose of this study was to identify predictors of initial depression treatment outcome in patients with type 2 diabetes, information that could help clinicians identify and manage those at greatest risk of nonresponse. A multivariate set of predictors comprised of epidemiological, diabetes, and depression characteristics were identified. These included extant diabetes complications, sertraline treatment, higher iBDI, younger age, and, in subgroup analyses, higher pain scores.
Our findings affirm reports that more severe diabetes portends poor response to depression treatment (12). The presence of complications predicted three of four measures of nonresponse and was the only predictor of treatment resistance defined as no decrease in depression symptom severity during treatment. In the present report, baseline A1C (mean = 8.3 ± 2.1%) did not predict depression treatment outcome as it had in a previous study (12), a finding potentially attributable to greater variance in baseline A1C (mean = 10.3 ± 3.1%) in the previous study. Poor compliance with blood glucose monitoring has been shown to predict nonremission of MDD with cognitive behavior therapy, but unfortunately, it was not measured in the current trial.
This study was designed to identify predictors of initial depression treatment response and was not designed to assess the efficacy or effectiveness of specific antidepressants. Nonetheless, treatment (sertraline or bupropion) was one of the variables included in the regression model, and sertraline emerged as a significant predictor of poor outcome. A randomized, controlled trial would be required to truly determine the comparative effectiveness of these medications. More severe depression at trial entry (higher iBDI) predicted failure to achieve partial remission and full remission, a finding reported in some (22), but not all, depression treatment trials (11). A history of prior episodes or treatment of depression, while predictive of poor outcomes in psychiatric samples (11), did not predict any of the four treatment outcomes in our study. While reliable methods for determination of MDD have been available for some time, less attention has been paid to measurement of MDD history and duration. Precise calculation of cumulative time spent in MDD may be an important element in the assessment. In a 10-year prospective study of children with type 1 diabetes, Kovacs et al. (23) found that cumulative time spent in depression and in poor glycemic control each independently predicted development of retinopathy.
Age has not been a reliable predictor of depression treatment outcome in psychiatric samples. In diabetic patients, Williams et al. (24) reported that younger patients were more likely to relapse during the maintenance phase of depression treatment. Younger age also predicted poor outcome (nonresponse and nonremission) in the present study. Patients with chronic medical illnesses tend to be older than those typically enrolled in antidepressant trials, and this may help to explain the discrepant findings in the diabetes and psychiatric literatures.
In the subgroup analysis, higher pain scores predicted three of the four measures of poor treatment outcome. Chronic pain is commonly comorbid with depression, and our analysis adds to mounting evidence in nondiabetic samples that pain has a negative impact on depression treatment course (25). Pain, measured in this study with the SF-36, is a composite score reflecting both severity and functional limitations associated with pain. The presence of pain and functional impairment may help identify those at risk for poor depression treatment outcome.
Our study is subject to the limitations of secondary analyses with combined datasets, and the findings warrant prospective replication. While methodologically similar in most regards (e.g., methods for diagnosing and measuring depression and change in depression severity), the interval covering acute treatment was longer in the sertraline versus bupropion trial (16 vs. 10 weeks, respectively). While this would appear to favor sertraline by allowing subjects more time to respond, rates of response were uniformly lower in sertraline- versus bupropion-treated patients. The extent to which our findings may be generalized to patients with type 1 diabetes is unclear. The aggregate analysis was limited to patients with type 2 diabetes because of the small number and disproportionate distribution of type 1 diabetic patients in the two trials (n = 57, all from the sertraline trial). In exploratory analyses that included both patients with type 1 as well as those with type 2 diabetes (n = 444), the factors that predicted treatment response were unchanged from those found when the analyses were limited to patients with type 2 diabetes. While the study design is desirable from the standpoint of external validity, it cannot apportion response as related to nonspecific versus direct medication effect. Finally, the modest proportion of variance explained by the logistic regression models (6–15%) underscores the need to study novel predictors and predictive models.
In summary, our results indicate that response to depression treatment in type 2 diabetes is influenced by a multidimensional set of factors. Physical disease markers, initial depression severity, demographic, and other clinical variables are relevant to the course of depression treatment, as is perhaps the choice of antidepressant agent. These predictors may serve as targets for intervention and avenues for further research, steps toward personalized mental health care and improved patient outcomes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
This article was presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ: The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 2. Lustman PJ, Griffith LS, Clouse RE: Depression in adults with diabetes: results of a 5-year follow-up study. Diabetes Care 1988;11:605–612 [DOI] [PubMed] [Google Scholar]
- 3. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM: Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:434–442 [DOI] [PubMed] [Google Scholar]
- 4. de Groot M, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ: Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–630 [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS: Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol 2005;161:652–660 [DOI] [PubMed] [Google Scholar]
- 6. Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, Carney RM, McGill JB: Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med 1997;59:241–250 [DOI] [PubMed] [Google Scholar]
- 7. Lustman PJ, Freedland KE, Griffith LS, Clouse RE: Fluoxetine for depression in diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Care 2000;23:618–623 [DOI] [PubMed] [Google Scholar]
- 8. Lustman PJ, Clouse RE, Nix BD, Freedland KE, Rubin EH, McGill JB, Williams MM, Gelenberg AJ, Ciechanowski PS, Hirsch IB: Sertraline for prevention of depression recurrence in diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry 2006;63:521–529 [DOI] [PubMed] [Google Scholar]
- 9. Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE: Cognitive behavior therapy for depression in type 2 diabetes: a randomized controlled trial. Ann Intern Med 1998;129:613–621 [DOI] [PubMed] [Google Scholar]
- 10. Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T: The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042–1049 [DOI] [PubMed] [Google Scholar]
- 11. Esposito K, Goodnick P: Predictors of response in depression. Psychiatr Clin North Am 2003;26:353–365 [DOI] [PubMed] [Google Scholar]
- 12. Lustman PJ, Freedland KE, Griffith LS, Clouse RE: Predicting response to cognitive behavior therapy of depression in type 2 diabetes. Gen Hosp Psychiatry 1998;20:302–306 [DOI] [PubMed] [Google Scholar]
- 13. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M: Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163:28–40 [DOI] [PubMed] [Google Scholar]
- 14. Lustman PJ, Williams MM, Sayuk GS, Nix BD, Clouse RE: Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care 2007;30:459–466 [DOI] [PubMed] [Google Scholar]
- 15. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571 [DOI] [PubMed] [Google Scholar]
- 16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision. Washington, D.C., American Psychiatric Association, 2000. [Google Scholar]
- 17. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM: American Diabetes Association. Tests of glycemia in diabetes. Diabetes Care 2004;27:S91–S93 [DOI] [PubMed] [Google Scholar]
- 19. Stewart AL, Hays RD, Ware JE, Jr: The MOS short-form general health survey: reliability and validity in a patient population. Med Care 1988;26:724–735 [DOI] [PubMed] [Google Scholar]
- 20. Riso LP, Thase ME, Howland RH, Friedman ES, Simons AD, Tu XM: A prospective test of criteria for response, remission, relapse, recovery, and recurrence in depressed patients treated with cognitive behavior therapy. J Affect Disord 1997;43:131–142 [DOI] [PubMed] [Google Scholar]
- 21. Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF: Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006;31:1841–1853 [DOI] [PubMed] [Google Scholar]
- 22. Kilts CD, Wade AG, Andersen HF, Schlaepfer TE: Baseline severity of depression predicts antidepressant drug response relative to escitalopram. Expert Opin Pharmacother 2009;10:927–936 [DOI] [PubMed] [Google Scholar]
- 23. Kovacs M, Mukerji P, Drash A, Iyengar S: Biomedical and psychiatric risk factors for retinopathy among children with IDDM. Diabetes Care 1995;18:1592–1599 [DOI] [PubMed] [Google Scholar]
- 24. Williams MM, Clouse RE, Nix BD, Rubin EH, Sayuk GS, McGill JB, Gelenberg AJ, Ciechanowski PS, Hirsch IB, Lustman PJ: Efficacy of sertraline in prevention of depression recurrence in older versus younger adults with diabetes. Diabetes Care 2007;30:801–806 [DOI] [PubMed] [Google Scholar]
- 25. Bair MJ, Robinson RL, Katon W, Kroenke K: Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163:2433–2445 [DOI] [PubMed] [Google Scholar]