Abstract
OBJECTIVE
To determine if glucose and C-peptide values obtained as part of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study could be used to estimate insulin sensitivity during late pregnancy.
RESEARCH DESIGN AND METHODS
A total of 78 women enrolled in the HAPO study were recruited for this ancillary study. Venous plasma samples were drawn after an 8- to 10-h fast (time 0) and at 30, 60, 90, and 120 min after a 75-g glucose challenge, which was performed at 24–32 weeks' gestation. Samples were analyzed for plasma glucose, insulin, and C-peptide. Insulin sensitivity was estimated using the established Matsuda and DeFronzo insulin sensitivity index for oral glucose tolerance tests (ISOGTT). Insulin sensitivity was also calculated from two other commonly used indexes of insulin sensitivity (that for homeostasis model assessment [ISHOMA] and that for quantitative insulin sensitivity check index [ISQUICKI]). A new insulin sensitivity index was calculated using the glucose and C-peptide concentrations at 0 and 60 min to derive ISHOMA C-pep, ISQUICKI C-pep, and ISOGTT C-pep. These indexes were then correlated with insulin sensitivity estimated from the ISOGTT.
RESULTS
The strongest correlation with the ISOGTT was obtained for ISOGTT C-pep (r = 0.792, P < 0.001). Further, the correlations of ISHOMA C-pep and ISQUICKI C-pep with ISOGTT were also significant (r = 0.676, P < 0.001 and r = 0.707, P < 0.001, respectively).
CONCLUSIONS
These data suggest that calculated ISOGTT C-pep is an excellent predictor of insulin sensitivity in pregnancy and can be used to estimate insulin sensitivity in over 25,000 women participating in the HAPO study.
Gestational diabetes mellitus (GDM) is a common metabolic disorder in developed countries occurring in 2–10% of pregnancies (1). GDM is associated with an increased risk of maternal and perinatal complications such as preeclampsia, macrosomia, shoulder dystocia, and neonatal hypoglycemia. Furthermore, the offspring of GDM pregnancies have an increased risk of obesity and type 2 diabetes in later life (2,3). In a recent study, Crowther et al. (4) reported that treatment of GDM in the form of dietary advice, blood glucose monitoring, and insulin therapy as required for glycemic control reduces the rate of serious perinatal complications. Although the risks associated with GDM are well recognized, there has been no general agreement about which glucose criteria should be used for diagnosis of GDM based primarily on maternal perinatal and neonatal outcomes.
The recently completed Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study was a prospective observational study during which >25,000 pregnant women in 10 different countries were recruited to determine the levels of glycemia, less severe than diabetes, associated with risks of large for gestational age, clinical neonatal hypoglycemia, cord-blood C-peptide, and primary cesarean delivery (5) as well as neonatal adiposity (6). The results confirm that there is a strong continuous association between maternal glucose concentrations below those currently used to diagnose GDM and adverse perinatal outcomes.
Normal human pregnancy is characterized by a significant decrease in insulin sensitivity (7). Furthermore, women developing GDM in addition to an inadequate insulin response have decreased insulin sensitivity compared with women with normal glucose tolerance (8). In many of these studies, insulin sensitivity has been estimated using a euglycemic-hyperinsulinemic clamp, which is considered the gold standard method. However, the clamp is a complicated, high-cost, and labor-intensive procedure and is not suitable for large studies.
Various investigators have validated indexes of insulin sensitivity derived from an oral glucose tolerance test (ISOGTT) (9) or fasting glucose and insulin levels (that for homeostasis model assessment [ISHOMA] and that for quantitative insulin sensitivity check index [ISQUICKI]) (10,11): in all these studies, only nonpregnant adults were evaluated. However, we recently reported a study of normal glucose tolerant and GDM pregnant women, comparing different indexes of insulin sensitivity derived from OGTTs with hyperinsulinemic-euglycemic clamps throughout pregnancy (12). We concluded that during pregnancy, the ISOGTT was the index that best correlated with insulin sensitivity when compared with the euglycemic-hyperinsulinemic clamp (r = 0.86, P < 0.001). Hence, the purpose of this study was to investigate if insulin sensitivity could be reasonably estimated using glucose and C-peptide measures obtained during a 75-g OGTT in HAPO study subjects.
RESEARCH DESIGN AND METHODS
The protocol was approved by the MetroHealth's Institutional Review Board, and the Scientific Review Committee of the General Clinical Research Center (now the Clinical Research Unit of the Clinical and Translational Science Award) subjects were recruited from women who had already formally agreed to participate in the HAPO study. Each subject signed a written consent form describing the ancillary research protocol. None of the subjects or investigators was made aware of the results of the HAPO OGTT so as not to affect the outcome of the primary project, unless fasting or 2-h glucose values exceeded predefined thresholds. After completion of the HAPO study and publication of the primary results, a post hoc analysis was performed to assess the number of women in this ancillary study who would have been diagnosed as having GDM using criteria established by the Fourth International GDM Workshop criterion (13).
A total of 78 women enrolled in the HAPO study were evaluated as part of this ancillary study. None of the patients had medical or obstetrical problems in a previous or the index pregnancy. A 75-g HAPO OGTT was performed in all subjects as close as possible to the 28th week of gestation according to standardized procedures (14): mean ± SD gestational age at recruitment was 27.6 ± 1.2 weeks. The OGTT was performed after an 8- to 10-h overnight fast. The HAPO OGTT consisted of fasting and 30-, 60-, 90-, and 120-min glucose measures and a fasting and 60-min C-peptide determination. To calculate the ISOGTT, plasma insulin was also obtained at fasting and at 30, 60, 90, and 120 min.
Plasma glucose concentrations were measured by the glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Blood samples for insulin measurements were centrifuged at 4°C and stored at −70°C. Insulin and C-peptide determinations were subsequently performed in duplicate on all samples by a double-antibody radioimmunoassay (Insulin: Linco, St. Louis, MO; C-peptide: Diagnostic Products, Los Angeles, CA) as previously described (7). The glucose values are expressed in milligrams per deciliter, insulin as microunits per milliliter, and C-peptide as picomoles per liter.
The insulin sensitivity index was calculated from the OGTT according to three different equations. The first was the equation described by Matsuda and DeFronzo (ISOGTT) (9): insulin sensitivity was calculated as follows:
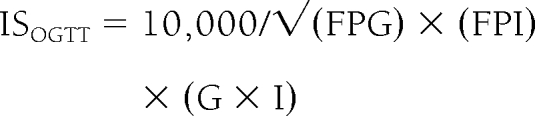 |
FPG and FPI are fasting plasma glucose and fasting plasma insulin, respectively, whereas G and I are mean glucose and mean insulin from 0 to 120 min.
The second equation was described by Matthews et al. (10) (ISHOMA), and insulin sensitivity was calculated as follows:
The last considered equation was proposed by Katz et al. (11) and is called ISQUICKI (Quantitative Insulin Sensitivity Check Index):
Insulin sensitivity indexes were then calculated using glucose and C-peptide concentrations available as part of the standard HAPO OGTT, i.e., C-peptide instead of insulin, at 0 and 60 min (ISOGTT C-pep, ISHOMA C-pep, and ISQUICKI C-pep). To have the units from the estimates of insulin sensitivity from the ISOGTT C-pep resemble those of the ISOGTT, 500,000 was used as the numerator rather than 10,000.
All data are presented as means ± SDs. Because ISOGTT is the index that best correlated with insulin sensitivity calculated from the euglycemic-hyperinsulinemic clamp (12), we evaluated the correlation between ISOGTT C-pep, ISHOMA C-pep, and ISQUICKI C-pep with ISOGTT using Pearson correlations and linear regression analyses. Comparisons between the normal glucose tolerant and GDM groups were made using Mann-Whitney U tests because of non-normal distribution of the data. All analyses were performed with Statistix, version 8.0 (Abacus Concepts, Berkeley, CA). A P value ≤0.05 was considered significant.
RESULTS
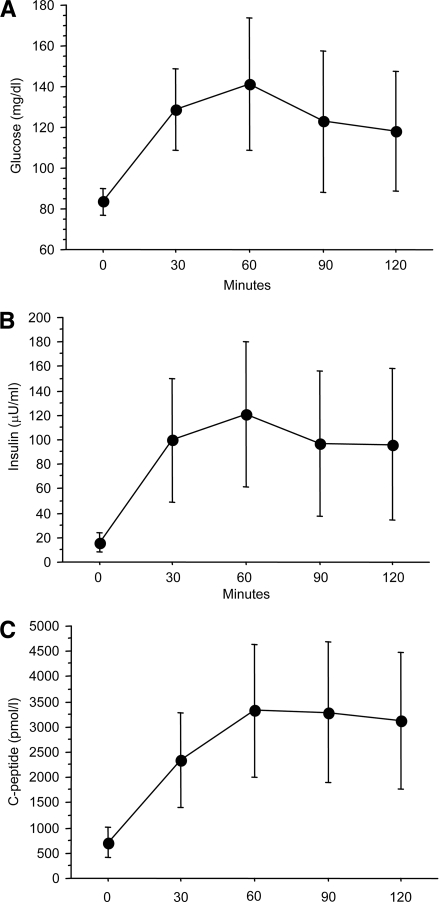
Maternal demographic characteristics of the study population are presented in Table 1. Mean maternal pregravid BMI was >26 kg/m2, reflecting a tendency toward our study subjects being overweight and obese. The results of the 75-g OGTT are shown in Fig. 1: fasting glucose, post-OGTT glucose, insulin, and C-peptide levels are shown in Fig. 1A, B, and C, respectively. Mean fasting glucose was 83.5 ± 6.5 mg/dl and mean fasting insulin and C-peptide concentrations were 16.0 ± 8.1 μU/ml and 710 ± 304 pmol/l, respectively. Six of the 78 (7.7%) subjects had GDM as defined by the Fourth International Workshop Conference on GDM criteria (13). The mean ± SD estimates of insulin sensitivity for the ISOGTT, ISOGTT C-pep, ISHOMA C-pep, and ISQUICKI C-pep are shown in Table 2.
Table 1.
Demographics of study population
| Maternal | |
| Age (years) | 27.6 ± 5.3 |
| Height (cm) | 163 ± 7 |
| Pre-pregnancy weight (kg) | 73.7 ± 20.3 |
| Pre-pregnancy BMI (kg/m2) | 27.5 ± 7.0 |
| Parity | |
| 0 | 37 |
| 1 | 23 |
| >1 | 18 |
| Race/ethnicity | |
| Caucasian | 58 |
| African American | 11 |
| Hispanic | 7 |
| Asian | 2 |
Data are means ± SD or n.
Figure 1.
The results of the 75-g OGTT: A: Glucose. B: Insulin. C: C-peptide. Data are presented as means ± SD. n = 78.
Table 2.
Estimates of insulin sensitivity
| ISOGTT | 3.456 ± 1.678 |
| ISOGTT C-pep | 5.016 ± 1.907 |
| ISHOMA C-pep | 2,680 ± 1,293 |
| ISQUICKI C-pep | 0.212 ± 0.009 |
Data are means ± SD.
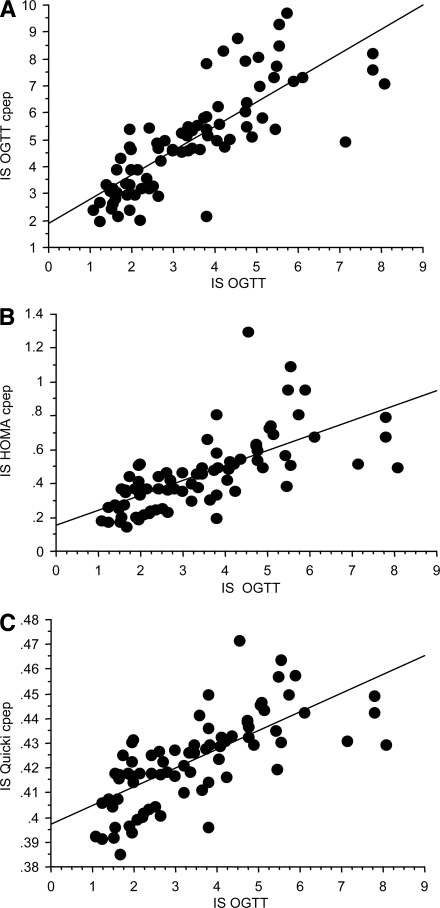
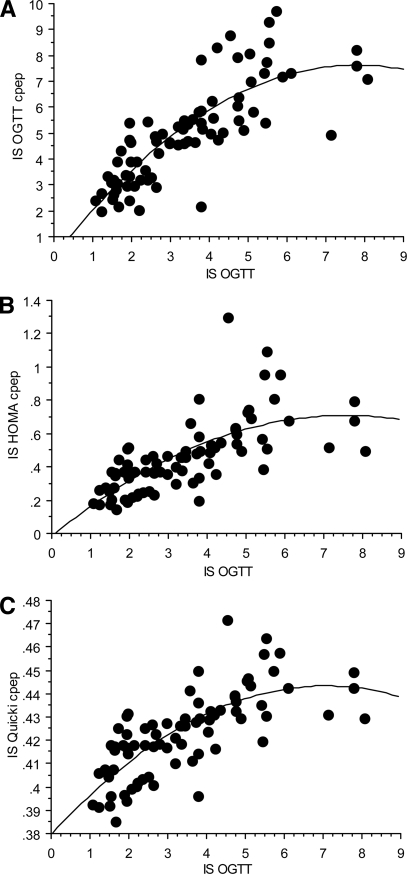
The correlations between insulin sensitivity indexes (ISOGTT C-pep, ISHOMA C-pep, and ISQUICKI C-pep) and ISOGTT are shown in Fig. 2. ISOGTT C-pep (r = 0.792, P < 0.001) had the strongest correlation with the standard ISOGTT. In contrast, using the fasting glucose and C-peptide data, the correlations of ISHOMA C-pep and ISQUICKI C-pep with ISOGTT were r = 0.676 (P < 0.001) and r = 0.707 (P < 0.001), being weaker compared with ISOGTT C-pep (Fig. 2). There was a stronger association (multiple r) when a squared term was added to the regression model; ISOGTT C-pep (r = 0.821, P < 0.0001), ISHOMA C-pep (multiple r = 0.705, P < 0.001), and ISQUICKI C-pep (r = 0.748, P < 0.001), but the relative strength of the relationships to ISOGTT was similar to the correlations from the simple linear models (Fig. 3).
Figure 2.
The regression models for ISOGTT and ISOGTT C-pep (A): y = 1.906 + 0.9x, r = 0.792, P < 0.001; ISHOMA C-pep (B): y = 0.156 + 0.088x, r = 0.676, P < 0.001; and ISQUICKI C-pep (C): y = 0.397 + 0.008x, r = 0.707, P < 0.001, n = 78.
Figure 3.
The regression models for ISOGTT and ISOGTT C-pep (A): y = 0.261 + 1.894x − 0.122x2, multiple r = 0.821; ISHOMA C-pep (B): y = −0.016 + 0.192x − 0.013x2, multiple r = 0.705; and ISQUICKI C-pep (C): y = 0.38 + 0.018x − 0.001x2, multiple r = 0.748, n = 78.
Last, there was a significant inverse correlation between maternal prepregnancy BMI and ISOGTT C-pep (r = −0.385, P < 0.001), and the six women with GDM had decreased insulin sensitivity compared with women with normal glucose tolerance (ISOGTT C-pep 2.86 ± 1.16 vs. 5.20 ± 1.85, P = 0.0025).
CONCLUSIONS
Measurement of insulin sensitivity in >25,000 HAPO subjects using either the euglycemic-hyperinsulinemic clamp or the intravenous minimal model technique was not possible. We therefore elected to use the ISOGTT as our reference standard, based on previous work by Matsuda and DeFronzo showing that the ISOGTT correlated well with the euglycemic clamp in a large number of study subjects having a wide range of age and body size (9). Furthermore, we have previously shown (12) that during pregnancy, the ISOGTT had the strongest correlation with insulin sensitivity as measured by the euglycemic clamp, adjusted for residual hepatic glucose production. In this earlier study, the correlation of the ISOGTT with insulin sensitivity in pregnancy measured by clamp was r = 0.86 (P < 0.001). The correlation during early pregnancy (12–14 weeks) was r = 0.89 (P < 0.001) and during late pregnancy (34–36 weeks) was r = 0.80 (P < 0.001). The correlation of the ISOGTT with the clamp was also highly significant for both women with normal glucose tolerance and women with GDM. One of the strengths of the ISOGTT as a measure of insulin sensitivity is that it incorporates both basal and hepatic measures of insulin sensitivity as well as post-absorptive or peripheral insulin sensitivity.
In the present study, the ISOGTT C-pep proved to be the best predictor of insulin sensitivity when we used the standard ISOGTT as the reference measure of insulin sensitivity. These data give us the confidence that the ISOGTT C-pep can provide important data regarding insulin sensitivity in the greater HAPO population and opens the possibility of developing large-scale metabolic studies from the data generated in this cohort. The ISOGTT C-pep may also be a useful tool because it includes the C-peptide response to glucose and hence provides an important measure of insulin secretion. As noted by Matsuda and DeFronzo (9), the ISOGTT provides a more robust measure of insulin sensitivity in normoglycemic individuals compared with individuals with type 2 diabetes. This may be related to the greater insulin secretory capacity in normal glucose tolerant individuals. Because normal pregnancy is associated with an increase in insulin response (7), the inclusion of a measure of insulin secretion such as in the ISOGTT C-pep may be advantageous in assessing metabolic function in the HAPO cohort.
ISHOMA C-pep and ISQUICKI C-pep were also used to estimate insulin sensitivity in this study. ISHOMA is frequently used to estimate insulin sensitivity in nonpregnant individuals. The index is based on the use of the fasting glucose and insulin. The index assumes that the circulating glucose and insulin are determined by a feedback loop between the liver and pancreatic β-cells. ISQUICKI is based on a logarithmic and reciprocal transformation of a single fasting glucose and insulin value. The model is similar to a HOMA model and differs only in the treatment of the data. In contrast to ISOGTT, ISHOMA and ISQUICKI incorporate only basal or fasting glucose and insulin measures and may be more reflective of only hepatic insulin sensitivity. Therefore, it is not surprising that ISHOMA C-pep and ISQUICKI C-pep provided a less robust estimate of insulin sensitivity compared with ISOGTT C-pep. Supplementary Table 1, showing the correlations between ISHOMA C-pep and ISQUICKI C-pep in comparison with ISHOMA, ISQUICKI, and ISOGTT, is provided in an online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1463/DC1. We observed similar results when ISHOMA and ISQUICKI were compared with the clamp measures during pregnancy in our previous study.
A limitation of our protocol is that we estimated HAPO pancreatic β-cell function using C-peptide at time 0 and 60 rather than insulin. The reason for using C-peptide rather than insulin was that with over 25,000 maternal samples to be transported across continents, there was a risk that some samples might be hemolyzed. Hemolysis is known to increase insulin degradation but not to affect C-peptide (15). Therefore, because insulin and C-peptide are secreted in equimolar levels, we elected to use C-peptide rather than insulin to estimate insulin sensitivity.
In conclusion, this study confirms that among the three proposed indexes, the best estimate of insulin sensitivity during pregnancy in the HAPO study subjects can be obtained from the ISOGTT C-pep. The index provides a reproducible and accurate measure of maternal metabolism during pregnancy. The ability to assess insulin sensitivity in a large and diverse subject population evaluated in the HAPO study may aid in the assessment of the short- and long-term affect of maternal metabolism on the offspring and the potential of fetal programming outcomes. Although these indexes cannot replace more direct measures of insulin sensitivity such as the euglycemic clamp, ISOGTT C-pep can be considered a reasonable low-cost clinical parameter for assessing insulin sensitivity during pregnancy in the 25,000 subjects who participated in the HAPO study.
Supplementary Material
Acknowledgments
The work was supported by National Institutes of Health Clinical Research Unit, National Center for Research Resources, Clinical and Translational Science Award (CTSA) UL1-RR-024989, RO1-HD34243, RO-1 HD22965, and HD34242.
No potential conflicts of interest relevant to this article were reported.
The authors wish to thank the research nurses in the General Clinical Research Center (CTSA) for their invaluable assistance to conduct this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008. [Google Scholar]
- 2. Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC: Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 1983;308:242–245 [DOI] [PubMed] [Google Scholar]
- 3. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC: Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 4. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS: Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–2486 [DOI] [PubMed] [Google Scholar]
- 5. HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 6. HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA: Longitudinal changes in insulin release and insulin resistance in non-obese pregnant women. Am J Obstet Gynecol 1991;165:1667–1672 [DOI] [PubMed] [Google Scholar]
- 8. Buchanan TA, Xiang AH: Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 10. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 11. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ: Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 12. Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM: Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001;24:1602–1607 [DOI] [PubMed] [Google Scholar]
- 13. Metzger BE, Coustan DR: Summary and recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21(Suppl. 2):B161–B167 [PubMed] [Google Scholar]
- 14. HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet 2002;78:69–77 [DOI] [PubMed] [Google Scholar]
- 15. HAPO Study Cooperative Research Group. Nesbitt GS, Smye M, Sheridan B, Lappin TR, Trimble ER: Integration of local and central laboratory functions in a worldwide multicenter study: experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials 2006;3:397–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.