Abstract
OBJECTIVE
To update and validate a diabetes-specific screening tool for disordered eating (the Diabetes Eating Problem Survey [DEPS]) in contemporary youth with type 1 diabetes.
RESEARCH DESIGN AND METHODS
A total of 112 youth with type 1 diabetes, ages 13–19 years, completed the DEPS. Higher scores on the DEPS indicate more disordered eating behaviors. Youth and their parents also completed additional surveys to examine diabetes-specific family conflict, negative affect related to blood glucose monitoring, youth quality of life, and diabetes burden. Clinicians provided data on height, weight, A1C, and insulin dosing. The DEPS was revised into a shorter, updated measure and validated.
RESULTS
The revised 16-item DEPS (DEPS-R) displayed excellent internal consistency (Cronbach's α = 0.86). Construct validity was demonstrated by positive correlations with zBMI (P = 0.01), A1C (P = 0.001), diabetes-specific family conflict (P < 0.005), youth negative affect around blood glucose monitoring (P = 0.001), parental diabetes-specific burden (P = 0.0005), and negative correlations with frequency of blood glucose monitoring (P = 0.03) and quality of life (P ≤ 0.002). External validity was confirmed against clinician report of insulin restriction.
CONCLUSIONS
The DEPS-R is a 16-item diabetes-specific self-report measure of disordered eating that can be completed in <10 min. It demonstrated excellent internal consistency, construct validity, and external validity in this contemporary sample of youth with type 1 diabetes. Future studies should focus on using the DEPS-R to identify high-risk populations for prevention of and early intervention for disordered eating behaviors.
Clinical and subclinical disordered eating behaviors such as binge eating disorder and eating disorder–not otherwise specified (ED-NOS) are more common in adolescent girls with type 1 diabetes than age-matched control subjects (1–3). Both comorbid disordered eating (subclinical as well as clinical) and elevated BMI can have a negative influence on glycemic control and health outcomes in type 1 diabetes (1,2,4–7). While the majority of research in this area has been conducted with females, a large population-based study found that boys with diabetes were twice as likely to report concerns about body development as boys without chronic illness (8). In the same study, boys with diabetes were more likely to report vomiting for weight control and dieting than boys without diabetes.
For individuals with type 1 diabetes, insulin restriction is a unique disordered eating behavior that is used to induce weight loss. The percentage of females who admit to insulin restriction varies with age and by study, from ∼15% of girls in their mid-teen years (2,9) to ∼30% of older teenagers and adult females (6,7,10–12). Disordered eating is not uncommon and can be detrimental to both short- and long-term health for patients with type 1 diabetes. As confirmation of the deleterious effects of disordered eating behaviors in type 1 diabetes, a recently published retrospective follow-up study found self-reported insulin restriction at baseline resulted in a 3.2-fold increased risk of death during the 11 years of follow-up (13).
Although there are many measures of disordered eating, there is currently no validated screening tool for disordered eating in people with diabetes for use in a clinical population (14). General measures of disordered eating may not be appropriate for use in individuals with type 1 diabetes for several reasons. First, diabetes management necessitates an emphasis on food intake and carbohydrate counting. General measures of disordered eating may misidentify what is an appropriate level of attention to food intake for a person with type 1 diabetes as a disordered eating behavior. Second, general measures of disordered eating do not identify disordered eating behaviors that are unique to individuals with type 1 diabetes, such as insulin restriction or omission. Therefore, it is important to use a screening measure designed specifically for people with diabetes when assessing disordered eating in this population. A short, self-report measure designed explicitly for this purpose would likely be useful for clinicians and researchers, as it would be a way to screen quickly and efficiently to identify need for further evaluation or intervention.
One measure designed for this purpose, the Diabetes Eating Problem Survey (DEPS) (15), was created before the current era of extensive penetration of intensive insulin therapy and availability of multiple insulin analogs. In addition, the DEPS was originally validated in an adult sample. The current study aims to update and validate the DEPS in a contemporary pediatric sample of males and females by assessing its internal consistency and external validity and to optimize its clinical utility for routine use.
RESEARCH DESIGN AND METHODS
Study participants were adolescents with type 1 diabetes, aged 13–19 years, and their parents, followed at a tertiary care center. Patient records were reviewed for the following eligibility criteria: duration of type 1 diabetes ≥1 year, residence in the northeastern U.S., no other major medical or psychiatric disorder, stable living environment, and English speaking. Written informed consent was obtained from parents, and assent was obtained from youth. The study procedures were approved by the Committee on Human Studies at the Joslin Diabetes Center.
During a regularly scheduled medical visit, each child, along with a parent, met with a trained research assistant who gathered demographic and diabetes management information and administered surveys. The validated questionnaires administered in this cross-sectional study included the DEPS (15), the Diabetes Family Conflict Scale (16), the Blood Glucose Monitoring Communication questionnaire (17), the Pediatric Quality of Life Inventory (18), the Problem Areas in Diabetes Survey–Parent version (19), and the Diabetes Quality of Life for Youth questionnaire (20). Frequency of blood glucose monitoring, A1C, height, weight, Tanner staging, total daily insulin dose, and mode of insulin delivery were extracted from the medical record, and the patient's clinician completed a brief diabetes adherence scale (21). The inter-rater reliability of data extraction from chart review exhibited >95% concordance.
Measures
DEPS.
Youth completed the 28-item DEPS (15), a diabetes-specific self-report measure of disordered eating behaviors. The DEPS was previously validated against a clinical diagnosis of eating disorders in patients with type 1 diabetes (15). Items are answered on a 6-point Likert scale (0 = never, 1 = rarely, 2 = sometimes, 3 = often, 4 = usually, 5 = always). Higher scores indicate more disordered eating behaviors.
Diabetes Family Conflict Scale.
Youth and parents completed the validated Diabetes Family Conflict Scale (16) to assess the level of family conflict around 19 diabetes-specific tasks. Items are answered on a 3-point Likert scale (1 = never argue, 2 = sometimes argue, 3 = always argue). Total scores could range from 19 to 57, with higher scores indicating greater conflict.
Blood Glucose Monitoring Communication questionnaire.
Youth completed the eight-item, validated, Blood Glucose Monitoring Communication questionnaire (17) to assess negative affect related to blood glucose monitoring. Items are answered on a 3-point Likert scale (1 = almost never; 3 = almost always). Total scores could range from 8 to 24, with higher scores indicating a greater degree of negative affect surrounding blood glucose monitoring.
Pediatric Quality of Life Inventory–Generic Core Scales.
Youth and parents completed the 23-item Pediatric Quality of Life Inventory–Generic Core Scales (PedsQL) (18), which measures the child's and parent's perceptions of the child's quality of life. The PedsQL includes two subscales related to physical and psychosocial functioning and is scored using a 5-point Likert scale (0 = never a problem; 4 = almost always a problem). Responses are scored as follows: 0 scored as 100, 1 as 75, 2 as 50, 3 as 25, and 4 as 0. Total quality of life score results from averaging all items. Total scores could range from 0 to 100, with higher scores indicating higher quality of life.
Diabetes Quality of Life for Youth survey.
Youth completed the Diabetes Quality of Life for Youth (DQOLY) (20) survey, a measure of diabetes-specific quality of life. We formed a three-item eating subscale with the following three items from the full measure: 1) How often do you feel restricted by your diet? 2) How often do you find you eat something you shouldn't rather than tell someone that you have diabetes? and 3) How often do you find that your diabetes prevents you from going out to eat with your friends? Items are answered on a 5-point Likert scale (1 = never; 5 = always).
Problem Areas in Diabetes Survey–Parent version.
Parents completed the 20-item Problem Areas in Diabetes Survey–Parent version (PAID-P) (19) to assess perceived burden of care by parents associated with taking care of a child with diabetes. The PAID-P was adapted from the adult version by Polonsky et al. (22). Items are answered on a 5-point Likert scale (1 = agree; 5 = disagree) with higher scores indicating higher perceived burden.
Clinician-rated adherence scale
To assess treatment adherence, the participant's clinician completed a modified adherence scale based on Jacobson et al. (21). Clinician-reported blood glucose monitoring frequency was based on data from patient logbooks and/or meter downloads. To rate adherence to insulin therapy, clinicians indicated whether or not they thought the patient was “skipping shots, misusing insulin, or ‘forgetting’ to bolus on the pump.” Such patients are categorized as those missing or restricting insulin. This rating of insulin restriction was based on clinician assessment and lab results, but did not specify that insulin restriction was for purposes of weight management.
Glycemic control
Blood samples were obtained at the time of the medical visit to measure A1C. Values were assayed by automated high-performance liquid chromatography (reference range 4.0–6.0%; Tosoh 2.2, Tosoh Corp., Foster City, CA).
Formation of the revised DEPS
The original 28-item DEPS previously demonstrated excellent internal consistency (Cronbach's α = 0.95) and was significantly correlated with diabetes-specific distress (r = 0.83, P < 0.001) in an adult population (15). In revising the DEPS for use with a pediatric population in the current era of diabetes management, we first eliminated any items with low face validity (items that did not appear to measure disordered eating). We then examined the remaining questions for redundancy (Table 1). When duplicate questions were found, we included the item with higher item-to-total correlation. For example, the original DEPS included seven questions about insulin use and feelings about insulin. Four of these questions were eliminated, due primarily to the lack of face validity and low item-to-total correlation. The resulting scale, the Diabetes Eating Problem Survey–Revised (DEPS-R), has 16 items, is rated on the same 6-point Likert scale as the original measure, and is scored by summing all 16 items (Table 1).
Table 1.
Formation of the DEPS-R: omitted items and revised measure
| Correlation with total | |
|---|---|
| Items omitted because of lack of face validity | |
| Controlling my diabetes is very important to me* | 0.38 |
| I forget to take my insulin | 0.36 |
| Before exercising, I eat carbohydrates to avoid going low* | 0.24 |
| When my blood sugar is low, I eat something immediately* | 0.28 |
| When my blood sugar is high, I take extra insulin* | 0.02 |
| I take less insulin than what my doctor tells me | 0.13 |
| I exercise to control my blood sugars* | 0.02 |
| Items omitted because of redundancy | |
| I eat in private when no one else is around | 0.47 |
| I check my blood sugar less frequently than my doctor tells me | 0.28 |
| I feel comfortable eating in front of others* | 0.31 |
| I adjust my insulin dose based on the results of my blood sugar checks* | 0.18 |
| I like to have ketones in my urine because that means that I am burning fat | −0.15 |
|
| |
| Items retained in DEPS-R† | |
| Losing weight is an important goal to me | |
| I skip meals and/or snacks | |
| Other people have told me that my eating is out of control | |
| When I overeat, I don't take enough insulin to cover the food | |
| I eat more when I am alone than when I am with others | |
| I feel that it's difficult to lose weight and control my diabetes at the same time | |
| I avoid checking my blood sugar when I feel like it is out of range | |
| I make myself vomit | |
| I try to keep my blood sugar high so that I will lose weight | |
| I try to eat to the point of spilling ketones in my urine | |
| I feel fat when I take all of my insulin | |
| Other people tell me to take better care of my diabetes | |
| After I overeat, I skip my next insulin dose | |
| I feel that my eating is out of control | |
| I alternate between eating very little and eating huge amounts | |
| I would rather be thin than to have good control of my diabetes | |
*Reverse-scored items.
†Items are answered on a 6-point Likert scale: 0 = never, 1 = rarely, 2 = sometimes, 3 = often, 4 = usually, 5 = always.
Statistical analysis
Analyses used SAS (version 9.2 for Windows; SAS Institute, Cary, NC). All data are presented as means ± SD or percent as indicated. Statistics included unpaired t tests and Pearson and Spearman correlations. Internal consistency of the survey was assessed using Cronbach's α. Analyses included the entire sample of males and females, as well as separate analyses performed with females only. A P value of <0.05 conveyed statistical significance.
RESULTS
The study sample consisted of 112 youth with diabetes (56% female). Average age of participants was 15.1 ± 1.2 years (mean ± SD), and average diabetes duration was 7.5 ± 3.7 years. Mean zBMI (age- and sex-adjusted BMI) (23) was 0.8 ± 0.7. Participants had an average A1C of 8.7 ± 1.7%. The majority of participants (62%) were treated with multiple daily injections; 13% received two injections daily, and 26% received insulin pump therapy. See Table 2 for further participant characteristics.
Table 2.
Participant characteristics
| All | Males | Females | |
|---|---|---|---|
| n | 112 | 49 | 63 |
| Age (years) | 15.1 ± 1.2 | 15.3 ± 1.4 | 14.9 ± 1.0 |
| Type 1 diabetes duration (years) | 6.8 ± 3.4 | 6.7 ± 3.4 | 6.8 ± 3.4 |
| Developmental stage | |||
| Prepubertal (Tanner 1) (%) | 0 | 0 | 0 |
| Pubertal (Tanner 2–4) (%) | 38 | 49 | 29 |
| Postpubertal (Tanner 5) (%) | 63 | 51 | 71 |
| zBMI (SDS) | 0.8 ± 0.7 | 0.7 ± 0.8 | 0.9 ± 0.7 |
| Insulin dose (units · kg−1 · day−1) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Blood glucose monitoring frequency (checks/day) | 3.6 ± 1.2 | 3.5 ± 1.1 | 3.7 ± 1.2 |
| Insulin treatment plan | |||
| 2 injections/day (%) | 13 | 10 | 14 |
| ≥3 injections/day (%) | 62 | 73 | 52 |
| Insulin pump (%) | 26 | 16 | 33 |
| A1C (%) | 8.7 ± 1.7 | 8.9 ± 1.8 | 8.6 ± 1.7 |
| Missing or restricting insulin (%) | 27 | 24 | 29 |
Data are means ± SD, unless otherwise indicated. SDS, SD score.
Internal consistency
The 16-item DEPS-R demonstrated excellent internal consistency in this sample of youth with type 1 diabetes, with a Cronbach's α of 0.86 (in females only, Cronbach's α was 0.87).
Construct validity
The DEPS-R demonstrated construct validity through comparison with areas that would be influenced by the presence of disordered eating. The DEPS-R correlated positively with youth zBMI (r = 0.24, P = 0.01), age (r = 0.25, P = 0.01), A1C (r = 0.30, P = 0.001), youth and parent report of diabetes-specific family conflict (r = 0.37, P < 0.0001), youth report of negative affect related to blood glucose monitoring (r = 0.36, P = 0.001), youth score on the eating subscale of the DQOLY (r = 0.59, P < 0.0001), and parent report of diabetes-specific burden (r = 0.39, P = 0.0005). The DEPS-R correlated negatively with frequency of blood glucose monitoring (r = −0.21, P = 0.03) and youth and parent-proxy report of youth quality of life (youth: r = −0.30, P = 0.002; parent: r = −0.35, P = 0.0002) (Table 3).
Table 3.
Correlations with DEPS-R
| r | P | |
|---|---|---|
| Age | 0.24 | 0.01 |
| zBMI | 0.24 | 0.01 |
| Blood glucose monitoring frequency (checks/day)* | −0.21 | 0.03 |
| A1C | 0.30 | 0.001 |
| Youth report | ||
| Diabetes-specific family conflict | 0.37 | <0.0001 |
| Negative affect related to blood glucose monitoring (n = 76) | 0.36 | 0.001 |
| Youth quality of life* | −0.30 | 0.002 |
| Eating subscale of DQOLY (n = 76) | 0.59 | <0.0001 |
| Parent report | ||
| Diabetes-specific family conflict | 0.20 | 0.04 |
| Diabetes-related burden (n = 76) | 0.39 | 0.0005 |
| Youth quality of life* | −0.35 | 0.0002 |
*Negative correlations with DEPS-R.
External validity
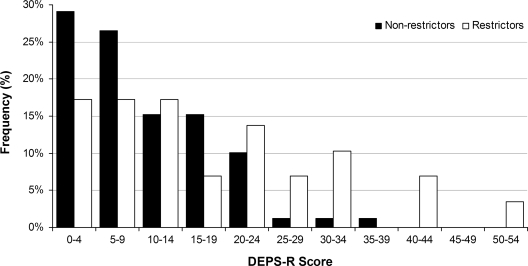
Youth were classified as “missing or restricting insulin” if their clinician answered “yes” or “possibly” to the question about insulin restriction on the clinician adherence rating scale. Youth who were classified as missing or restricting insulin scored significantly higher on the DEPS-R (17.7 ± 13.7) than other youth (10.2 ± 8.1, P = 0.009) (Fig. 1). More than half of youth (52%) who scored ≥20 on the DEPS-R were identified by their provider as missing or restricting insulin. In addition, youth categorized as missing or restricting insulin had significantly higher A1C levels than youth who were not classified as missing or restricting insulin (10.2 ± 2.1 vs. 8.1 ± 1.2%, P < 0.0001).
Figure 1.
DEPS-R scores by insulin restrictor category. Significantly more youth identified by their clinician as insulin restrictors scored ≥20 on the DEPS-R compared with nonrestrictors; 41% of insulin restrictors scored ≥20 on the DEPS-R compared with 14% of nonrestrictors (P = 0.002).
Among all participants, the highest A1C (11.3 ± 1.9%) was observed in individuals missing or restricting insulin and also scoring ≥20 on the DEPS-R. Furthermore, participants who scored ≥20 on the DEPS-R and were classified as missing or restricting insulin had lower zBMI scores (0.9 ± 0.7) than those who scored ≥20 on the DEPS-R and were not identified as insulin restrictors (1.3 ± 0.7). While this difference is not statistically significant, it may be clinically significant.
There was no difference between males and females on rates of clinician-determined insulin restriction or omission. However, females scored significantly higher on the DEPS-R (14.1 ± 11.0) than males (9.3 ± 8.7, P = 0.02). All analyses were repeated with females only and results were consistent with those reported above.
CONCLUSIONS
The DEPS-R is a 16-item diabetes-specific self-report screening measure for disordered eating that can be completed in <10 min during a routine clinical encounter. The DEPS-R demonstrated excellent internal consistency and external validity in this contemporary sample of youth with type 1 diabetes. Construct validity was demonstrated through significant correlations with variables that may be influenced by the presence of disordered eating in youth with diabetes. It is expected that older age, higher A1C, less frequent blood glucose monitoring, greater negative affect around blood glucose monitoring, greater diabetes-specific family conflict, poorer quality of life, and greater perceived diabetes burden would be associated with disordered eating attitudes and behaviors.
External validity was assessed by the association between clinician-determined insulin restriction or omission and scores on the DEPS-R. Youth who were classified by their clinician as missing or restricting insulin scored higher on the DEPS-R and had less optimal glycemic control than other youth. This indicates that youth who omit insulin are more likely to endorse disordered eating behaviors than those who do not omit insulin. In this study, males and females had similar rates of clinician-reported insulin restriction. This is likely due to the wording of the question, which did not specify that the purpose of the insulin restriction was weight management. Some youth/families with suboptimal diabetes management may be included among those classified as missing or restricting insulin. In the pediatric population, it is not uncommon for youth to miss insulin, and this behavior is not necessarily indicative of disordered eating.
Despite the similarity in clinician-reported insulin restriction or omission in females and males, females scored significantly higher on the DEPS-R, which is not surprising, given the increased rates of disordered eating in females. Thus, insulin omission in females might raise a clinician's suspicion of disordered eating behaviors. However, clinician-reported insulin restriction or omission does not appear to be sufficient for identifying disordered eating behaviors, unless supplemented by a more extensive clinical interview and assessment. A screening tool such as the DEPS-R could assist in helping the clinician determine whether a more extensive assessment is necessary. In the group of participants who scored ≥20 on the DEPS-R, there were more insulin restrictors than nonrestrictors. In addition, participants who were classified as missing or restricting insulin who also scored ≥20 on the DEPS-R had the highest A1C of all participants.
Participants who scored ≥20 on the DEPS-R and were classified as missing or restricting insulin had clinically, although not statistically, lower zBMI scores than those who scored ≥20 but were not classified as missing or restricting insulin. This may be explained by the use of insulin omission for weight control resulting in a lower zBMI. In addition, it is possible for adolescents with type 1 diabetes to engage in disordered eating behaviors that do not include insulin omission, which could potentially result in a higher zBMI.
As discussed earlier, the DEPS-R correlates positively with age, which is supported by the diagnostic criteria for eating disorders, that states that the onset of both bulimia nervosa and binge eating disorder most often occurs in late adolescence and early adulthood (anorexia nervosa usually begins in mid to late adolescence) (24). As an exploratory analysis, we examined the stability of the DEPS-R by age-group, comparing youth <15 years old to individuals ≥15 years old. Although the item-to-total correlations were in the same direction for both age-groups, the correlations were predominately stronger in the older age-group, providing further support for the construct validity of the DEPS-R, as it is likely identifying behaviors related to disordered eating.
A major limitation of this study is its inability to validate the DEPS-R against an established measure of disordered eating. In addition, the sample size is relatively small, and the age range of the population limits the generalizability of the results beyond adolescence. Despite these limitations, we demonstrated that the DEPS-R correlates significantly with variables related to disordered eating behaviors in youth with type 1 diabetes. In addition, the age range targeted in the study focused on a vulnerable population in which preventive measures are needed.
A short, self-administered screening tool for disordered eating such as the DEPS-R can be used routinely in the clinical care of youth with diabetes. Future research should focus on confirming the survey's validity in varied populations and against established measures of disordered eating such as the Eating Disorder Examination (25). In addition, continued work is needed to prevent and to treat disordered eating behaviors in youth with diabetes.
Acknowledgments
This work was supported in part by National Institutes of Health Training Grant T32 DK007260, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-46887, the Charles H. Hood Foundation, the Maria Griffin Drury Pediatric Fund, the Katherine Adler Astrove Youth Education Fund, and the Diabetes and Endocrinology Research Center (P30DK036836).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in poster form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Nielsen S: Eating disorders in females with type 1 diabetes: an update of a meta-analysis. Eur Eat Disord Rev 2002;10:241–254 [Google Scholar]
- 2. Jones JM, Lawson ML, Daneman D, Olmsted MP, Rodin G: Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ 2000;320:1563–1566 [PMC free article] [PubMed] [Google Scholar]
- 3. Colton P, Olmsted M, Daneman D, Rydall A, Rodin G: Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes: a case-controlled study. Diabetes Care 2004;27:1654–1659 [DOI] [PubMed] [Google Scholar]
- 4. Nielsen S, Emborg C, Mølbak AG: Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care 2002;25:309–312 [DOI] [PubMed] [Google Scholar]
- 5. Domargård A, Särnblad S, Kroon M, Karlsson I, Skeppner G, Aman J: Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr 1999;88:1223–1228 [DOI] [PubMed] [Google Scholar]
- 6. Bryden KS, Neil A, Mayou RA, Peveler RC, Fairburn CG, Dunger DB: Eating habits, body weight, and insulin misuse: a longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care 1999;22:1956–1960 [DOI] [PubMed] [Google Scholar]
- 7. Peveler RC, Bryden KS, Neil HA, Fairburn CG, Mayou RA, Dunger DB, Turner HM: The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005;28:84–88 [DOI] [PubMed] [Google Scholar]
- 8. Neumark-Sztainer D, Story M, Resnick MD, Garwick A, Blum RW: Body dissatisfaction and unhealthy weight-control practices among adolescents with and without chronic illness: a population-based study. Arch Pediatr Adolesc Med 1995;149:1330–1335 [DOI] [PubMed] [Google Scholar]
- 9. Peveler RC, Fairburn CG, Boller I, Dunger D: Eating disorders in adolescents with IDDM: a controlled study. Diabetes Care 1992;15:1356–1360 [DOI] [PubMed] [Google Scholar]
- 10. Rydall AC, Rodin GM, Olmsted MP, Devenyi RG, Daneman D: Disordered eating behavior and microvascular complications in young women with insulin-dependent diabetes mellitus. N Engl J Med 1997;336:1849–1854 [DOI] [PubMed] [Google Scholar]
- 11. Ackard DM, Vik N, Neumark-Sztainer D, Schmitz KH, Hannan P, Jacobs DR, Jr: Disordered eating and body dissatisfaction in adolescents with type 1 diabetes and a population-based comparison sample: comparative prevalence and clinical implications. Pediatr Diabetes 2008;9:312–319 [DOI] [PubMed] [Google Scholar]
- 12. Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF: Insulin omission in women with IDDM. Diabetes Care 1994;17:1178–1185 [DOI] [PubMed] [Google Scholar]
- 13. Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K: Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care 2008;31:415–419 [DOI] [PubMed] [Google Scholar]
- 14. Criego A, Crow S, Goebel-Fabbri AE, Kendall D, Parkin C: Eating disorders and diabetes: screening and detection. Diabetes Spectrum 2009;22:143–146 [Google Scholar]
- 15. Antisdel J, Laffel LMB, Anderson BJ: Improved detection of eating problems in women with type 1 diabetes using a newly developed survey (Abstract). Diabetes 2001;50 (Suppl. 2):A47 [Google Scholar]
- 16. Hood KK, Butler DA, Anderson BJ, Laffel LM: Updated and revised Diabetes Family Conflict Scale. Diabetes Care 2007;30:1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hood KK, Butler DA, Volkening LK, Anderson BJ, Laffel LM: The Blood Glucose Monitoring Communication questionnaire: an instrument to measure affect specific to blood glucose monitoring. Diabetes Care 2004;27:2610–2615 [DOI] [PubMed] [Google Scholar]
- 18. Varni JW, Seid M, Rode CA: The PedsQL: measurement model for the Pediatric Quality of Life Inventory. Med Care 1999;37:126–139 [DOI] [PubMed] [Google Scholar]
- 19. Antisdel JE: Diabetes-specific distress among parents of youth with type 1 diabetes (Abstract). Dissertation Abstracts International 2001;61:6123 [Google Scholar]
- 20. Ingersoll GM, Marrero DG: A modified quality-of-life measure for youths: psychometric properties. Diabetes Educ 1991;17:114–118 [DOI] [PubMed] [Google Scholar]
- 21. Jacobson AM, Hauser ST, Lavori P, Wolfsdorf JI, Herskowitz RD, Milley JE, Bliss R, Gelfand E, Wertlieb D, Stein J: Adherence among children and adolescents with insulin-dependent diabetes mellitus over a four-year longitudinal follow-up: I. The influence of patient coping and adjustment. J Pediatr Psychol 1990;15:511–526 [DOI] [PubMed] [Google Scholar]
- 22. Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, Schwartz CE: Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 [DOI] [PubMed] [Google Scholar]
- 23. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 2002;11:1–190 [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text revision. Washington D.C., American Psychiatric Association, 2004. [Google Scholar]
- 25. Fairburn CG, Cooper Z: The Eating Disorder Examination. In Binge Eating: Nature, Assessment, and Treatment. Fairburn CG, Wilson GT. Eds. New York, Guilford Press, 1993, p. 317–360 [Google Scholar]