Abstract
OBJECTIVE
To evaluate the effects of missed insulin boluses for snacks in youth with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Three months of simultaneous continuous subcutaneous insulin infusion and continuous glucose monitoring data from nine subjects were retrospectively evaluated. Glucose excursions between 1330 and 1700 h were defined as relating to snacks with insulin or snacks with no insulin administered. Area under the curve >180 mg/dl (AUC >180), average Δ glucose, and rate of change were analyzed and compared within and between groups.
RESULTS
A total of 94 snacks without insulin and 101 snacks with insulin were analyzed. Snacks without insulin had significantly higher log (AUC >180 + 1) (1.26 vs. 0.44 mg/dl per event; P < 0.001), Δ glucose (114 vs. 52 mg/dl; P < 0.001), and average rate of change (1.3 vs. 1.1 mg/dl per minute; P < 0.001).
CONCLUSIONS
This study shows that afternoon snacks without insulin boluses are common and result in significantly higher glucose excursions than snacks with insulin administration.
Previous studies have demonstrated the deleterious effect of missed insulin doses for meals (1–4). None, however, have examined the effect of missed insulin boluses for snacks. Because youth frequently snack when unsupervised, it is likely that missed insulin boluses are even more common for snacks than for meals. The purpose of this investigation was to use data from continuous glucose monitoring (CGM) and continuous subcutaneous insulin infusion (CSII) together to evaluate the glycemic profiles of missed insulin boluses for afternoon snacks.
RESEARCH DESIGN AND METHODS
This is a retrospective (Institutional Review Board approved) analysis of 810 days of CSII and CGM data from nine youth with diabetes. All subjects used the Minimed Paradigm REAL Time System (Northridge, CA) for insulin delivery and CGM. Reports were downloaded using Medtronic CareLink software. Afternoon snacks were identified on CGM as glucose excursions beginning between 1330 and 1700 h. A glucose excursion was considered resolved when glucose levels remained steady for ≥15 min. Glucose excursions with incomplete CGM data or CSII suspension >15 min were discarded. Glucose excursions were identified as a snack with no insulin (SNI) or a snack with insulin (SWI) as described below, and possible snacks not fitting these criteria were excluded from analysis.
SNI criteria were as follows: 1) no bolus administered within ±30 min of the beginning of the glucose excursion, 2) increase in glucose level ≥50 mg/dl, 3) average rate of change from baseline to peak of excursion ≥0.8 mg/dl per minute, 4) starting glucose level >80 mg/dl (to exclude treatment of hypoglycemia), and 5) determined not to be the dinner meal. Carbohydrate contents for snacks are not known for SNI.
SWI criteria were as follows: 1) bolus administered within ±30 min of the beginning of the glucose excursion, 2) determined not to be the dinner meal, and 3) determined not to be an exclusive correction bolus (confirmed on pump download).
Each glucose excursion was characterized by baseline glucose level, peak glucose level, end glucose level, duration of excursion, time spent >180 mg/dl, area under the curve >180 mg/dl (AUC >180), total amplitude of excursion (Δ glucose), average rate of change, and insulin administered. The study's primary outcome was the comparison between the AUC >180 of the glucose excursions for SNI and SWI. Secondary outcomes included comparing the average rate of change and Δ glucose for SNI and SWI.
All statistical analysis used Bonferroni's adjusted P values for multiple comparisons. Results are expressed as mean ± 1 SD. Exploratory analyses revealed positive skew in the outcome variables (AUC >180, Δ glucose, and rate of change). Because AUC >180 was used, this resulted in a zero inflated distribution; thus, a two-stage model was used. Generalized estimating equations were used to determine the distribution of events with blood glucose levels ≤180 versus >180 (5) between SNI and SWI. Mixed models were applied by regressing log(AUC>180 + 1.0), log(Δ glucose), and log(rate of change) on to SNI/SWI (unbolused or bolused snack) adjusting for age, sex, and repeated measures on subjects. Results are presented as mean or geometric mean and 95% CI. A general linear mixed-model approach suggested by Cnaan et al. (6) was used to model blood glucose curves.
RESULTS
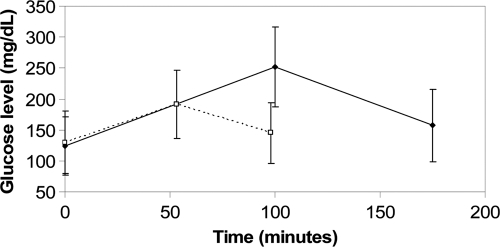
Data from nine subjects (five female) with a mean A1C of 7.6 ± 0.7%, mean duration of diabetes of 8.6 ± 6.3 years, and a mean age of 15.1 ± 8.8 years were analyzed. Of 195 glucose excursions identified, 94 were classified as SNI and 101 as SWI. Baseline glucose values between SNI and SWI were not significantly different (P = 1.0). A total of 76 of 94 (81.7%) SNI resulted in blood glucose levels >180 compared with 51 of 101 (50.5%), for a resulting OR of 4.80 (95% CI 2.46–9.40) (P < 0.0001 after adjusting for age, sex, and repeated measures among subjects). Mean time spent above 180 mg/dl was 105 ± 89 min for SNI and 34 ± 42 min for SWI. The average glucose excursion for SNI began at 124 ± 47 mg/dl, peaked at 252 ± 65 mg/dl after 100 ± 58 min, and resolved after 175 ± 97 min at 157 ± 59 mg/dl (Fig. 1). The average glucose excursion for SWI began at 130 ± 51 mg/dl, peaked at 191 ± 55 mg/dl after 53 ± 27 min, and resolved after 98 ± 48 min at 145 ± 49 mg/dl. Both the main effects of time and SNI versus SWI were significant (P > 0.0001) as well as the second order effects and interactions concluding that the two curves were significantly different (P < 0.0001). A table of the ratio of covariate effects and confidence intervals is included in the online appendix, which is available at http://care.diabetesjournals.org/cgi/content/full/dc09-1840/DC1.
Figure 1.
A comparison of glucose excursions for snacks with insulin (dashed line) and snacks without insulin (solid line).
Glucose excursions from SNI had a mean log (AUC >180 + 1) of 1.26 (95% CI 1.06–1.46) compared with 0.44 mg/dl per event (95% CI 0.31–0.57) for SWI (P < 0.001). Neither age nor sex had a significant effect on AUC >180 (P = 1.0 and P = 0.50, respectively).
The Δ glucose-adjusted mean for SNI (114 mg/dl [95% CI 101–129]) was significantly different (P < 0.001) from SWI (52 mg/dl [47–59]). Age was not found to have an effect (P = 0.08).
The average rate of change was significantly different (P < 0.005) between SNI (1.3 [95% CI 1.2–1.5]) and SWI (1.1 mg/dl/min [1.0–1.2]). Neither age nor sex significantly affected the rate of change (P = 1.0 for both).
CONCLUSIONS
This study shows that when insulin is omitted for afternoon snacks, the area under the curve (>180 mg/dl) is twice that of excursions for bolused snacks. Furthermore, SNI excursions demonstrated a steeper increase in glucose levels and twice the amplitude of SWI excursions. In this study, ∼50% of boluses for snacks (94 of 195) were missed.
Diabetes care providers often put much emphasis on mealtime insulin boluses but fail to focus on snacks. Because snacking involves smaller amounts of food over a longer period of time when compared with meals, the glycemic profiles are different. Future prospective studies should include many more subjects, as well as data relating to insulin reduction and food intake with exercise, to further characterize these excursions. Overall, missed insulin boluses for snacks contribute to significant hyperglycemia. Diabetes care providers need to stress the importance of bolusing for snacks as well as for meals.
Supplementary Material
Acknowledgments
Appreciation is expressed to the Juvenile Diabetes Research Foundation for funding this research.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Chase HP, Rainwater NG: Missed insulin injections: a common syndrome. Pract Diabetol 1989;8:20–23 [Google Scholar]
- 2. Burdick J, Chase HP, Slover R, Knievel K, Scrimgeour L, Maniatis A, Klingensmith G: Missed insulin pump meal boluses cause elevated HbA1c values. Pediatr 2004;113:221–224 [DOI] [PubMed] [Google Scholar]
- 3. Olinder AL, Kernell A, Smide B: Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes 2009;10:142–148 [DOI] [PubMed] [Google Scholar]
- 4. Pańkowska E, Skórka A, Szypowska A, Lipka M: Memory of insulin pumps and their record as a source of information about insulin therapy in children and adolescents with type 1 diabetes. Diabetes Technol Ther 2005;7:308–314 [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care in diabetes 2009. Diabetes Care 2009;32(Suppl. 1):S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cnaan A, Laird NM, Slasor P: Using the general linear model to analyze unbalanced repeated measures and longitudinal data. Stat Med 1997;16:2349–2380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.