Abstract
OBJECTIVE
The high diabetes incidence among Japanese Americans and Native Hawaiians cannot be explained by BMI. Therefore, we examined the influence of three dietary patterns of “fat and meat,” “vegetables,” and “fruit and milk” on diabetes risk in the Hawaii component of the Multiethnic Cohort with 29,759 Caucasians, 35,244 Japanese Americans, and 10,509 Native Hawaiians.
RESEARCH DESIGN AND METHODS
Subjects aged 45–75 years completed a baseline food frequency questionnaire. After 14 years of follow-up, 8,587 subjects with incident diabetes were identified through self-reports or health plan linkages. Risk was assessed using Cox regression stratified by age and adjusted for ethnicity, BMI, physical activity, education, total energy, smoking, alcohol intake, marital status, and hypertension.
RESULTS
Fat and meat was significantly associated with diabetes risk in men (hazard ratio 1.40 [95% CI 1.23–1.60], Ptrend < 0.0001) and women (1.22 [1.06–1.40], Ptrend = 0.004) when extreme quintiles were compared. Except in Hawaiian women, the magnitude of the risk was similar across ethnic groups although not always significant. After stratification by BMI, fat and meat remained a predictor of disease primarily among overweight men and among overweight Japanese women. Vegetables lowered diabetes risk in men (0.86 [0.77–0.95], Ptrend = 0.004) but not in women, whereas fruit and milk seemed to be more beneficial in women (0.85 [0.76–0.96], Ptrend = 0.005) than in men (0.92 [0.83–1.02], Ptrend = 0.04).
CONCLUSIONS
Foods high in meat and fat appear to confer a higher diabetes risk in all ethnic groups, whereas the effects of other dietary patterns vary by sex and ethnicity.
Native Hawaiians have extremely high rates of obesity and diabetes, but despite their relatively low body weight, individuals with Japanese ancestry are also disproportionately affected by diabetes (1). Among the >44,000 Japanese Americans, 14,000 Native Hawaiians, and 35,000 Caucasians in the Hawaii component of the Multiethnic Cohort (MEC), a previous analysis had found diabetes incidence rates of 15.5, 12.5, and 5.8 per 1,000 person-years, respectively, that could not be explained by BMI (2). Dietary patterns have been identified as additional predictors of disease but have only rarely been investigated prospectively among non-Caucasian populations (3–5). The most commonly identified patterns are the so-called “western,” “unhealthy,” or “conservative” pattern (3–11), which is high in meat, high-fat foods, and sweets, and the “prudent” or “healthy” pattern, rich in fruit and vegetables (3–8,10,12,13). With the goal to contribute to the prevention of diabetes, we examined the effect of three dietary patterns, “fat and meat,” “vegetables,” and “fruit and milk,” which had been previously identified in the MEC, on diabetes risk (14).
RESEARCH DESIGN AND METHODS
The MEC study was established from 1993 to 1996 to examine diet and cancer among different ethnic groups in Hawaii and California (15). The Hawaii component of the MEC consists of 103,898 members, primarily Caucasians, Japanese Americans, and Native Hawaiians. Subjects aged 45–75 years entered the cohort by completing a 26-page, self-administered mailed survey that included a food frequency questionnaire and asked about demographics, medical conditions, anthropometric measures, and lifestyle factors (16). Although response rates were highest for Japanese Americans (46% for men and 51% for women) and lowest for Native Hawaiians (28% for men and 35% for women), the MEC yielded a representative population as evidenced by a comparison of educational levels and marital status with census data (15). After exclusion of ineligible subjects (10,028 with prevalent diabetes, 8,797 of other ethnic groups, 6,202 with missing covariates, 2,537 with missing dietary information, 812 subjects with unconfirmed diabetes, and 10 with missing information on follow-up or diabetes at baseline), 36,256 men and 39,256 women were part of this analysis.
Case ascertainment
The detailed identification of case subjects was only available for the Hawaii component of the MEC (2). Subjects with incident diabetes were identified through three sources. A follow-up questionnaire sent to all MEC members in 1999–2003 asked about medical conditions including diabetes and achieved a response rate of 84%. A medication questionnaire administered in 2001–2007 was available for 38% of subjects who had agreed to a blood draw. In 2007, diabetic subjects were identified through a linkage with the two major health plans in Hawaii, Kaiser Permanente and Blue Cross/Blue Shield. After excluding 812 subjects with self-reported diabetes not confirmed by a health plan, 2,251 of the 8,587 subjects with incident diabetes were identified in the follow-up questionnaire, 996 in the medication questionnaire, and 5,340 through the health plans. Annual linkages with state and national death certificate files provided information on vital status.
Dietary patterns
Based on the food frequency questionnaire calibrated within the different ethnic groups (16), nutrients were determined and Food Guide Pyramid servings were computed using an ethnicity-specific food composition database with information from the U.S. Department of Agriculture and additional laboratory analyses performed in Hawaii (15). Subjects who reported energy, fat, protein, or carbohydrate intakes outside the mean ± 3 relative SDs were excluded.
In a previous analysis, exploratory factor analysis with an acceptable goodness of fit was applied to the MEC (14). Three distinct dietary patterns were identified, and factor scores were obtained for each participant (Table 1). The pattern fat and meat was characterized by discretionary fat, meat, eggs, and cheese and explained 30% of variation. The vegetables pattern (20% variation explained) included high amounts of vegetables and also fruits with a relatively low loading, whereas fruit and milk had high loadings on milk, yogurt, cheese, and fruits and explained 14% of variation. The factor analysis was repeated in each ethnic group and produced similar results (14). Therefore, the patterns are expected to be unchanged after the exclusion of the California component with primarily African Americans and Latinos.
Table 1.
Food groups with high factor loadings for the three dietary patterns
| Food groups | Factor loadings | |
|---|---|---|
| Fat and meat | Discretionary fat | 88 |
| Meat and organ meat | 83 | |
| Frankfurters, sausage, and luncheon meat | 72 | |
| White potatoes | 68 | |
| Non–whole grains | 68 | |
| Eggs | 67 | |
| Cheese | 63 | |
| Vegetables | Dark-green vegetables | 87 |
| Other vegetables | 86 | |
| Deep-yellow vegetables | 79 | |
| Other fruits | 44 | |
| Citrus fruits, melons, and berries | 36 | |
| Fruit and milk | Milk and yogurt | 71 |
| Cheese | 35 | |
| Other fruits | 71 | |
| Citrus fruits, melons, and berries | 71 |
Dietary patterns from ref. 14.
Statistical methods
All statistical analyses were performed using SAS statistical software (version 9.2, SAS Institute, Cary, NC). We used Cox proportional hazards regression models with follow-up time as the underlying time metric to estimate hazard ratios (HRs) and 95% CI for sex-specific quintiles of factor scores in relation to diabetes. Ordinal variables representing the median values for each quintile were used to test for linear trends. The final models were stratified by age at cohort entry and adjusted for ethnicity (Japanese Americans and Native Hawaiians versus Caucasians), BMI (continuous), physical activity (quintiles), education (13–15 and >15 vs. ≤12 years), energy intake (log-transformed), alcohol intake (quintiles), marital status, smoking status (past and current versus never), and self-reported hypertension at baseline. The effect of the fat and meat pattern independent of BMI was determined after stratification by BMI. No major violations of the proportional hazards assumption were observed when examined with Kaplan-Meier survival curves and Schoenfeld residuals.
RESULTS
BMI, median factor scores, and intake from major food groups differed significantly by ethnicity and sex (P < 0.001) (Table 2). Caucasians had higher median scores for the fruit and milk pattern and consumed more dairy foods than the other groups. Japanese Americans had a higher proportion of normal-weight subjects, scored higher on the vegetables pattern, and consumed the most rice. Native Hawaiians were more likely to be obese, to have high median scores on the fat and meat pattern, and to report high energy intakes. Women consumed more dairy foods and fruits than men, who had a higher meat intake. All dietary patterns were significantly correlated with BMI (rs = 0.3 for fat and meat and <0.1 for the other patterns).
Table 2.
Baseline characteristics of the Hawaii component of the MEC, 1993–2007
| Caucasian |
Japanese American |
Native Hawaiian |
All | ||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| n | 15,116 | 14,643 | 16,572 | 18,672 | 4,568 | 5,941 | 75,512 |
| Cases (%) | 7 | 5 | 16 | 13 | 18 | 16 | 11 |
| Noncases (%) | 93 | 91 | 84 | 87 | 83 | 84 | 89 |
| Age (%) | |||||||
| 45–54 years | 45 | 47 | 33 | 32 | 51 | 53 | 41 |
| 55–64 years | 28 | 27 | 28 | 31 | 29 | 28 | 28 |
| ≥65 years | 28 | 26 | 39 | 37 | 20 | 19 | 31 |
| Education (%) | |||||||
| ≤12 years | 19 | 23 | 39 | 41 | 48 | 52 | 34 |
| 13–15 years | 29 | 34 | 29 | 28 | 32 | 30 | 30 |
| >15 years | 52 | 43 | 32 | 31 | 21 | 18 | 36 |
| BMI (%) | |||||||
| <25.0 kg/m2 | 47 | 63 | 58 | 74 | 27 | 39 | 57 |
| 25.0–29.9 kg/m2 | 41 | 25 | 37 | 21 | 44 | 33 | 32 |
| ≥30 kg/m2 | 12 | 13 | 6 | 5 | 29 | 28 | 11 |
| Cigarette smoking (%) | |||||||
| Never | 33 | 44 | 30 | 69 | 33 | 45 | 44 |
| Past | 51 | 40 | 54 | 22 | 45 | 31 | 40 |
| Current | 16 | 17 | 16 | 9 | 23 | 24 | 15 |
| Fat and meat | 0.33 | −0.28 | 0.12 | −0.48 | 0.57 | 0.07 | −0.06 |
| Vegetables | −0.22 | −0.01 | 0.32 | 0.47 | 0.18 | 0.29 | 0.18 |
| Fruit and milk | 0.10 | 0.22 | −0.68 | −0.30 | −0.44 | −0.14 | −0.19 |
| Total energy (kcal) | 2,316 ± 891 | 1,824 ± 689 | 2,293 ± 833 | 1,823 ± 674 | 2,800 ± 1,322 | 2,341 ± 1,219 | 2,125 ± 907 |
| Red meat (g/day) | 43 ± 35 | 28 ± 24 | 44 ± 32 | 30 ± 23 | 60 ± 45 | 46 ± 37 | 38 ± 32 |
| Dairy foods (g/day) | 262 ± 211 | 254 ± 207 | 137 ± 137 | 157 ± 154 | 211 ± 211 | 230 ± 231 | 201 ± 192 |
| Vegetables (g/day) | 339 ± 202 | 332 ± 205 | 318 ± 189 | 322 ± 194 | 385 ± 267 | 407 ± 300 | 337 ± 213 |
| Fruits (g/day) | 326 ± 273 | 340 ± 268 | 306 ± 265 | 371 ± 296 | 337 ± 335 | 405 ± 412 | 342 ± 295 |
| Rice (g/day) | 110 ± 134 | 73 ± 92 | 408 ± 245 | 270 ± 180 | 353 ± 261 | 221 ± 193 | 231 ± 223 |
| Physical activity (METs) | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.2 | 1.7 ± 0.4 | 1.6 ± 0.3 | 1.6 ± 0.3 |
Data are %, medians (pattern scores only), or means ± SD. The following subjects were excluded from the 103,898 members of the Hawaii component of the MEC: 10,028 with prevalent diabetes, 8,797 of other ethnicity, 812 with unconfirmed diabetes, 6,202 with missing covariates, 2,537 with missing dietary information, and 10 with lack of follow-up information or missing diabetes information at baseline.
Fat and meat was significantly associated with diabetes risk in men with HR 1.40 ([95% CI 1.23–1.60], Ptrend < 0.0001) when the highest quintile of the factor score was compared with the lowest (Table 3). This trend was consistent across ethnic groups although not statistically significant for Native Hawaiians. High scores on the fat and meat pattern also showed a significant trend with diabetes risk in women overall (HR 1.22, [1.06–1.40], Ptrend = 0.004). The association was significant in Japanese American women (Ptrend = 0.045), the group with a largest sample size, whereas it was similar in magnitude, although not significant, in Caucasian women, and showed no association in Native Hawaiian women (Table 4).
Table 3.
Dietary patterns and diabetes risk in men, Hawaii component of the MEC, 1993–2007
| All men |
Caucasian |
Japanese American |
Native Hawaiian |
|||||
|---|---|---|---|---|---|---|---|---|
| n * | HR (95% CI)† | n * | HR (95% CI)† | n * | HR (95% CI)† | n * | HR (95% CI)† | |
| n | 4,555 | 1,080 | 2,677 | 798 | ||||
| Fat and meat | ||||||||
| Quintile 1 | 773 | 1.00 | 142 | 1.00 | 539 | 1.00 | 92 | 1.00 |
| Quintile 2 | 812 | 1.03 (0.93–1.14) | 166 | 0.99 (0.78–1.24) | 523 | 1.01 (0.89–1.14) | 123 | 1.13 (0.86–1.49) |
| Quintile 3 | 912 | 1.17 (1.06–1.30) | 216 | 1.24 (0.99–1.56) | 572 | 1.14 (1.00–1.30) | 124 | 0.99 (0.74–1.32) |
| Quintile 4 | 958 | 1.23 (1.10–1.37) | 238 | 1.25 (0.98–1.59) | 543 | 1.18 (1.02–1.36) | 177 | 1.12 (0.84–1.50) |
| Quintile 5 | 1,100 | 1.40 (1.23–1.60) | 318 | 1.38 (1.05–1.81) | 500 | 1.38 (1.16–1.64) | 282 | 1.22 (0.88–1.29) |
| Ptrend | <0.0001 | 0.007 | <0.0002 | 0.27 | ||||
| Vegetables | ||||||||
| Quintile 1 | 783 | 1.00 | 362 | 1.00 | 303 | 1.00 | 118 | 1.00 |
| Quintile 2 | 907 | 0.98 (0.89–1.08) | 232 | 0.92 (0.78–1.09) | 527 | 1.02 (0.88–1.18) | 148 | 1.03 (0.81–1.32) |
| Quintile 3 | 982 | 1.03 (0.94–1.14) | 203 | 0.97 (0.81–1.16) | 605 | 1.03 (0.90–1.19) | 174 | 1.21 (0.95–1.54) |
| Quintile 4 | 976 | 0.98 (0.88–1.08) | 183 | 0.99 (0.82–1.19) | 612 | 0.97 (0.83–1.12) | 181 | 1.16 (0.91–1.49) |
| Quintile 5 | 907 | 0.86 (0.77–0.95) | 100 | 0.67 (0.53–0.84) | 630 | 0.86 (0.74–0.99) | 177 | 1.17 (0.90–1.51) |
| Ptrend | 0.004 | 0.01 | 0.007 | 0.17 | ||||
| Fruits and milk | ||||||||
| Quintile 1 | 1,144 | 1.00 | 124 | 1.00 | 819 | 1.00 | 201 | 1.00 |
| Quintile 2 | 1,011 | 0.98 (0.90–1.07) | 168 | 0.72 (0.57–0.91) | 675 | 1.05 (0.95–1.17) | 168 | 0.96 (0.78–1.18) |
| Quintile 3 | 925 | 0.97 (0.89–1.06) | 232 | 0.79 (0.63–0.99) | 520 | 1.02 (0.91–1.14) | 173 | 1.02 (0.83–1.27) |
| Quintile 4 | 770 | 0.89 (0.81–0.98) | 253 | 0.72 (0.57–0.89) | 390 | 0.96 (0.85–1.10) | 127 | 0.84 (0.67–1.07) |
| Quintile 5 | 705 | 0.92 (0.83–1.02) | 303 | 0.71 (0.56–0.89) | 273 | 1.08 (0.93–1.26) | 129 | 0.85 (0.66–1.09) |
| Ptrend | 0.04 | 0.02 | 0.76 | 0.14 | ||||
*Number of case subjects with diabetes.
†HRs (95% CI) were stratified by age at cohort entry and adjusted for ethnicity (Japanese American and Native Hawaiian vs. Caucasian), physical activity (quintiles), education (12–15 and >15 vs. ≤12 years), energy intake (log-transformed), BMI (continuous), alcohol intake (quintiles), smoking status (past and current vs. never), marital status, and self-reported high blood pressure at baseline.
Table 4.
Dietary patterns and diabetes risk in women, Hawaii component of the MEC, 1993–2007
| All women |
Caucasian |
Japanese American |
Native Hawaiian |
|||||
|---|---|---|---|---|---|---|---|---|
| n * | HR (95% CI)† | n * | HR (95% CI)† | n * | HR (95% CI)† | n * | HR (95% CI)† | |
| n * | 4,032 | 715 | 2,374 | 843 | ||||
| Fat and meat | ||||||||
| Quintile 1 | 657 | 1.00 | 83 | 1.00 | 480 | 1.00 | 94 | 1.00 |
| Quintile 2 | 691 | 1.01 (0.91–1.13) | 114 | 1.04 (0.78–1.38) | 465 | 1.00 (0.87–1.14) | 112 | 1.02 (0.76–1.35) |
| Quintile 3 | 784 | 1.09 (0.98–1.22) | 135 | 1.07 (0.80–1.44) | 498 | 1.10 (0.96–1.26) | 151 | 1.04 (0.79–1.36) |
| Quintile 4 | 823 | 1.07 (0.95–1.21) | 161 | 1.08 (0.80–1.45) | 481 | 1.08 (0.92–1.25) | 181 | 0.94 (0.71–1.24) |
| Quintile 5 | 1,077 | 1.22 (1.06–1.40) | 222 | 1.21 (0.87–1.68) | 450 | 1.20 (1.00–1.44) | 405 | 1.10 (0.81–1.48) |
| Ptrend | 0.004 | 0.24 | 0.045 | 0.60 | ||||
| Vegetables | ||||||||
| Quintile 1 | 665 | 1.00 | 207 | 1.00 | 277 | 1.00 | 181 | 1.00 |
| Quintile 2 | 808 | 1.06 (0.95–1.17) | 162 | 1.12 (0.91–1.38) | 473 | 1.17 (1.01–1.36) | 173 | 0.83 (0.67–1.02) |
| Quintile 3 | 816 | 1.03 (0.93–1.15) | 152 | 1.21 (0.97–1.50) | 467 | 1.06 (0.91–1.23) | 197 | 0.93 (0.76–1.15) |
| Quintile 4 | 858 | 1.05 (0.94–1.17) | 113 | 1.02 (0.80–1.30) | 559 | 1.16 (0.99–1.35) | 186 | 0.88 (0.70–1.09) |
| Quintile 5 | 885 | 1.02 (0.91–1.14) | 81 | 1.03 (0.78–1.35) | 598 | 1.11 (0.95–1.30) | 206 | 0.89 (0.71–1.11) |
| Ptrend | 0.93 | 0.78 | 0.41 | 0.48 | ||||
| Fruits and milk | ||||||||
| Quintile 1 | 984 | 1.00 | 96 | 1.00 | 664 | 1.00 | 224 | 1.00 |
| Quintile 2 | 862 | 0.96 (0.88–1.05) | 139 | 1.10 (0.85–1.43) | 546 | 0.95 (0.85–1.07) | 177 | 0.93 (0.76–1.13) |
| Quintile 3 | 816 | 0.95 (0.86–1.04) | 143 | 0.94 (0.72–1.23) | 484 | 0.94 (0.83–1.06) | 189 | 1.06 (0.87–1.30) |
| Quintile 4 | 725 | 0.90 (0.82–1.00) | 150 | 0.81 (0.62–1.06) | 400 | 0.95 (0.83–1.08) | 175 | 0.93 (0.75–1.15) |
| Quintile 5 | 645 | 0.85 (0.76–0.96) | 187 | 0.88 (0.67–1.16) | 280 | 0.86 (0.74–1.01) | 178 | 0.86 (0.68–1.09) |
| Ptrend | 0.005 | 0.09 | 0.09 | 0.29 | ||||
*Number of case subjects with diabetes.
†HRs (95% CI) were stratified by age at cohort entry and adjusted for ethnicity (Japanese American and Native Hawaiian vs. Caucasian), physical activity (quintiles), education (12–15 and >15 vs. ≤12 years), energy intake (log-transformed), BMI (continuous), alcohol intake (quintiles), smoking status (past and current vs. never), marital status, and self-reported high blood pressure at baseline.
The pattern vegetables was inversely associated with diabetes risk in men overall (HR 0.86 [95% CI 0.77–0.95], Ptrend = 0.004) as well as in Caucasian and Japanese American men but not in Native Hawaiian men and not in women. Whereas the fruit and milk pattern was weakly related to diabetes in all men (Ptrend = 0.04), the association was stronger among Caucasians (Ptrend = 0.02) and in all women (0.85 [0.76–0.96], Ptrend = 0.005). Although the risk reduction was similar in all ethnic groups for women, the trend tests failed to reach statistical significance.
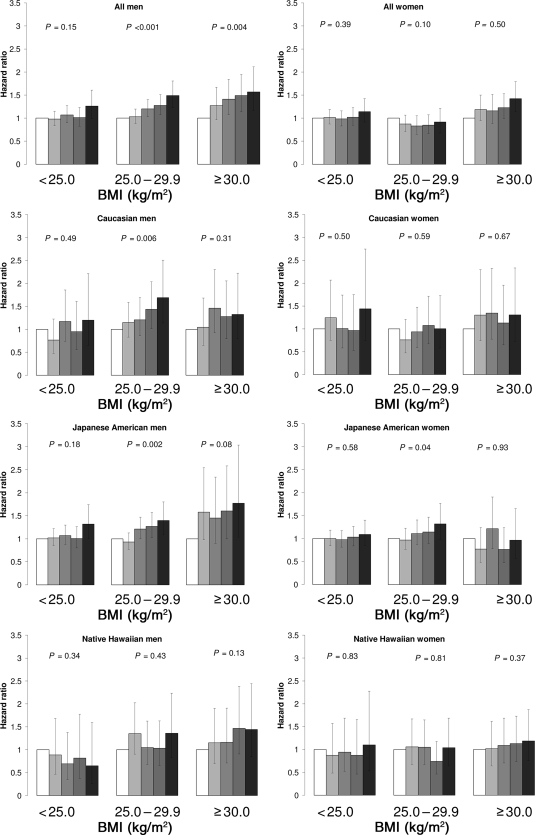
Because of the fairly consistent fat and meat results, we stratified the analysis by BMI (Fig. 1). In all men, the risk for diabetes increased with higher factor scores for fat and meat among overweight (HR 1.49 [95% CI 1.23–1.81], Ptrend < 0.0001) and obese (1.57 [1.16–2.12], Ptrend = 0.004) individuals. By ethnicity, the effect was observed in overweight Caucasian (Ptrend = 0.006) and Japanese American (Ptrend = 0.002) men with borderline associations among obese Japanese American (Ptrend = 0.08) and Native Hawaiian (Ptrend = 0.13) men. In women, no significant trends were observed for the entire population; only the trend for overweight Japanese American women was significant (Ptrend = 0.04).
Figure 1.
Diabetes risk and “fat and meat” dietary pattern by weight status, Hawaii component of the MEC, 1993–2007. The models were stratified by age at cohort entry and adjusted for ethnicity (Japanese American and Native Hawaiian vs. Caucasian), physical activity (quintiles), education (12–15 and >15 vs. ≤12 years), BMI (continuous), energy intake (log transformed), alcohol intake (quintiles), smoking status (past and current versus never), marital status, and self-reported high blood pressure at baseline.
CONCLUSIONS
In this multiethnic population, high scores in the fat and meat pattern were associated with elevated diabetes risk among all ethnic groups in men and to a lesser degree in all and in Japanese American women. After stratification by BMI, the effects were primarily seen in overweight Caucasian and Japanese American men as well as in overweight Japanese American women. The vegetables pattern lowered diabetes risk in Caucasian and Japanese American men but not in women, whereas fruit and milk lowered diabetes risk more in women than in men. These findings indicate that the type of food consumed might contribute to diabetes risk beyond its effects on body weight.
The positive association with the fat and meat pattern is consistent with similar patterns in several other cohort studies (4–11) and agrees with a recent meta-analysis that associated red and processed meat with diabetes (17). However, in a Japanese study, the animal food pattern was not related to diabetes risk (3). Meat may be harmful because of its content of saturated fat, nitrites (processed meat), and iron and may lead to hyperglycemia and hyperinsulinemia (18). Because women had lower loadings on the fat and meat pattern and lower meat intake than men, the use of sex-specific quintiles might explain the weaker associations among women. The more pronounced risk estimates for the fat and meat pattern in overweight than in normal-weight men are consistent with previous studies (6–8), although another investigation did not detect such an interaction (4).
The inverse associations for vegetables in men and fruit and milk in women are consistent with studies that showed a reduced risk of diabetes for subjects adhering to a prudent or healthy diet high in fruits and vegetables (3,4,6,8,10,12,13). However, contradictory results have been reported for vegetables and fruits. Two reports did not detect a protective effect for a prudent pattern (5,7) and one analysis found a protective effect for the high-vegetable pattern but no effect for the high-fruit pattern (10). Whereas fruit but not vegetables were protective in a U.S. cohort (19), vegetable but not fruit intake was protective among Chinese women (20). The protective effects of fruits and vegetables on diabetes have been attributed to antioxidants, fiber, carotenoids, magnesium, and folic acid (21). Some ingredients in fruits, e.g., dietary fiber, may have beneficial effects on glucose metabolism, whereas others, e.g., sugars, may have adverse effects. Dairy products have been associated with diabetes risk due to their high fat content, but low-fat dairy products may have beneficial effects (4,22). Unfortunately, we were not able to differentiate between high- and low-fat products. The inconsistent results for the vegetables and fruit and milk pattern by sex may be due to diverse dietary habits observed in men and women. Women had higher scores on the vegetables and the fruit and milk pattern (14) and relatively higher intakes of fruits and dairy products (Table 2) (15).
Similar to our nonsignificant associations among Native Hawaiians, a report from diverse ethnic groups in Hawaii indicated that ethnicity was a stronger predictor of diabetes risk than dietary patterns (5). It is possible that the high rate of obesity among Native Hawaiians is a stronger determinant of diabetes than nutritional habits (Fig. 1). The smaller sample size of Native Hawaiians, the high intake of total energy, and the low loadings on fruit and milk (Table 2) may have also contributed to the absence of significant associations. The fact that a diet high in animal fat has been associated with intra-abdominal fat deposition and insulin resistance (23) might explain the significant results for the fat and meat pattern among overweight Japanese Americans. Despite their relatively low BMI, individuals of Japanese ancestry seem to be more susceptible to central obesity with a higher proportion of visceral fat than Caucasians (24) that predisposes to insulin resistance (25).
It is necessary to note some weaknesses of this study. Because of the multiple comparisons, some of the findings might be due to chance. We did not have information on the type of diabetes. However, given the median age of 59 years at baseline, >90% of cases of diabetes are probably type 2. The results stratified by BMI should be interpreted with care; residual confounding may be present, and it is unclear whether BMI functions as a confounder or intermediate variable. One limitation of the dietary pattern approach is the difficulty in separating the effects of individual nutrients (8). Because dietary patterns are thought to capture synergistic and antagonistic effects of interrelated nutrients, they may be able to detect the cumulative effect of individual foods whose association with disease risk cannot be detected separately (4). Patterns of diet can also be more easily translated into practical public health advice for diabetes prevention. Other strengths, besides the multiethnic population with a great variation in diabetes risk and BMI, are the large sample size, the long follow-up, and the case ascertainment through health plans (2).
Our findings support previous research that a diet rich in meat and fat predisposes to diabetes independent of its effect on body weight (17), in particular among overweight individuals (6–8). Because our findings were more consistent among Caucasians and Japanese Americans, it seems possible that most of the adverse effect of the fat and meat pattern in Native Hawaiians is mediated through BMI. Our analyses agree with investigations that included individuals with Asian ancestry and reported effects of dietary patterns similar to those in Caucasians (3–5). The results for patterns rich in fruit, vegetables, and dairy products are ambiguous and need to be investigated in other cohorts. A better understanding of dietary factors related to diabetes risk in Japanese Americans and Native Hawaiians will be useful in developing preventive strategies in these high-risk groups. Despite improvements in treatment, ultimately only prevention can reduce the disease burden.
Acknowledgments
The Multiethnic Cohort was supported by National Cancer Institute Grant R37-CA-54281 (principal investigator [PI] Dr. L.N. Kolonel). The recruitment of Native Hawaiians was funded by Grant DAMD 17-94-T-4184 (PI Dr. A. Nomura). The diabetes project is funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R21-DK-073816 (PI Dr. G. Maskarinec).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the World Diabetes Congress, Montreal, Quebec, Canada, 18–22 October 2009.
We thank Mark M. Schmidt and Aileen Uchida at Kaiser Permanente Center for Health Research (Honolulu, HI) and Deborah Taira Juarez and Krista Hodges at HMSA, Blue Cross Blue Shield of Hawaii, for their assistance in linking the cohort with the health plans. We also thank Grace Matsuura for her help with the literature review.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. McNeely MJ, Boyko EJ: Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 2004;27:66–69 [DOI] [PubMed] [Google Scholar]
- 2. Maskarinec G, Erber E, Grandinetti A, Verheus M, Oum R, Hopping BN, Schmidt MM, Uchida A, Juarez DT, Hodges K, Kolonel LN: Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes 2009;58:1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mizoue T, Yamaji T, Tabata S, Yamaguchi K, Ogawa S, Mineshita M, Kono S: Dietary patterns and glucose tolerance abnormalities in Japanese men. J Nutr 2006;136:1352–1358 [DOI] [PubMed] [Google Scholar]
- 4. Nettleton JA, Steffen LM, Ni H, Liu K, Jacobs DR, Jr: Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008;31:1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HS, Park SY, Grandinetti A, Holck PS, Waslien C: Major dietary patterns, ethnicity, and prevalence of type 2 diabetes in rural Hawaii. Nutrition 2008;24:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB: Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 2002;136:201–209 [DOI] [PubMed] [Google Scholar]
- 7. Fung TT, Schulze M, Manson JE, Willett WC, Hu FB: Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 2004;164:2235–2240 [DOI] [PubMed] [Google Scholar]
- 8. Montonen J, Knekt P, Härkänen T, Järvinen R, Heliövaara M, Aromaa A, Reunanen A: Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol 2005;161:219–227 [DOI] [PubMed] [Google Scholar]
- 9. Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB: Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–684; quiz 714–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodge AM, English DR, O'Dea K, Giles GG: Dietary patterns and diabetes incidence in the Melbourne Collaborative Cohort Study. Am J Epidemiol 2007;165:603–610 [DOI] [PubMed] [Google Scholar]
- 11. McNaughton SA, Mishra GD, Brunner EJ: Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care 2008;31:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidemann C, Hoffmann K, Spranger J, Klipstein-Grobusch K, Möhlig M, Pfeiffer AF, Boeing H: A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study cohort. Diabetologia 2005;48:1126–1134 [DOI] [PubMed] [Google Scholar]
- 13. Brunner EJ, Mosdøl A, Witte DR, Martikainen P, Stafford M, Shipley MJ, Marmot MG: Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr 2008;87:1414–1421 [DOI] [PubMed] [Google Scholar]
- 14. Park SY, Murphy SP, Wilkens LR, Yamamoto JF, Sharma S, Hankin JH, Henderson BE, Kolonel LN: Dietary patterns using the Food Guide Pyramid groups are associated with sociodemographic and lifestyle factors: the Multiethnic Cohort Study. J Nutr 2005;135:843–849 [DOI] [PubMed] [Google Scholar]
- 15. Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS: A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AM, Earle ME, Nagamine FS, Kolonel LN: Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000;151:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aune D, Ursin G, Veierød MB: Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–2287 [DOI] [PubMed] [Google Scholar]
- 18. Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB: Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 2001;73:61–67 [DOI] [PubMed] [Google Scholar]
- 19. Bazzano LA, Li TY, Joshipura KJ, Hu FB: Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008;31:1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villegas R, Shu XO, Gao YT, Yang G, Elasy T, Li H, Zheng W: Vegetable but not fruit consumption reduces the risk of type 2 diabetes in Chinese women. J Nutr 2008;138:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H: Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007;167:956–965 [DOI] [PubMed] [Google Scholar]
- 22. Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB: Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med 2005;165:997–1003 [DOI] [PubMed] [Google Scholar]
- 23. Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Kahn SE, Leonetti DL, McNeely MJ, Newell LL, Shofer JB, Tsunehara CH, Wahl PW: Preventing diabetes—applying pathophysiological and epidemiological evidence. Br J Nutr 2000;84(Suppl. 2):S173–S176 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka S, Horimai C, Katsukawa F: Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol 2003;40(Suppl. 1):S302–S304 [DOI] [PubMed] [Google Scholar]
- 25. Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY: Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 2008;57:1269–1275 [DOI] [PubMed] [Google Scholar]