Abstract
OBJECTIVE
The study's goal was to evaluate the performance of A1C and fasting capillary blood glucose (FCG) tests as mass screening tools for diabetes and pre-diabetes, as determined by the standard oral glucose tolerance test (OGTT).
RESEARCH DESIGN AND METHODS
Data from 2,332 individuals aged 35–74 years who participated in a population-based cross-sectional diabetes survey in Qingdao, China, were analyzed. A 2-h 75-g OGTT was used to diagnose diabetes. The performance of A1C and FCG was evaluated against the results of the OGTTs by using receiver operating characteristic curve (ROC) analysis.
RESULTS
The prevalence of newly diagnosed diabetes and pre-diabetes (impaired fasting glucose and/or impaired glucose tolerance) was 11.9 and 29.5%, respectively. For subjects with newly diagnosed diabetes, the area under the ROC curve was 0.67 for A1C and 0.77 for FCG (P < 0.01) in men and 0.67 and 0.75 (P < 0.01) in women, whereas for pre-diabetes, these values were 0.47 and 0.64 (P < 0.001) in men and 0.51 and 0.65 (P < 0.001) in women. At the optimal A1C cutoff point of ≥5.6% for newly diagnosed diabetes, sensitivities (specificities) were 64.4% (61.6%) for men and 62.3% (63.3%) for women.
CONCLUSIONS
As a screening tool for newly diagnosed diabetes and pre-diabetes, the FCG measurement performed better than A1C in this general Chinese population.
Type 2 diabetes has become a serious public health threat worldwide, and it leads to increased premature mortality and morbidity including blindness, renal failure, amputation, and cardiovascular disease. Diabetes and its complications may occur several years before a clinical diagnosis is made. Mass screening for diabetes can lead to an early diagnosis and timely treatment or intervention, which have been shown to reduce diabetes-associated complications and to prevent or delay the onset of diabetes per se. Currently, diabetes and pre-diabetes are classified according to the 2-h 75-g oral glucose tolerance test (OGTT) (1), which has been considered as a standard diagnostic criterion for diabetes for several decades. However, there are several limitations associated with the OGTT. These limitations include the uncertainty of the fasting stage, the poor reproducibility of the 2-h glucose tests (2), and the poor concordance between the fasting plasma glucose (FPG) and the 2-h plasma glucose levels (3).
The capillary blood glucose test is a point-of-care determination and involves only one finger prick. Because it is easy to use and cheap, it is applied as a first-step screening test for mass screening of subjects in either the fasting (4,5) or the random (4) state. In contrast, A1C is the mean of the long-term glucose level and does not require the subject to be in a fasting state with only one blood sample drawn. It is used to monitor glucose levels in diabetic individuals who have received antihyperglycemic treatment. Because the A1C test is less standard and relatively expensive, it is not widely available. Consequently, its use in mass screening has been limited. Data on the performance of the A1C test in mass screening for diabetes are still lacking. In the light of the recent development in standardizing the method of A1C measurement (6), the A1C test has been adopted as a diagnostic criterion for diabetes (7). To evaluate the performance of the A1C as a mass screening tool for diabetes as determined by OGTTs in a general population and to compare its performance with that of the FCG test, data from a population-based study in Qingdao, China, were analyzed.
RESEARCH DESIGN AND METHODS
A population-based cross-sectional diabetes survey was conducted in Qingdao, China, in 2006. A stratified, random cluster sampling method was used to recruit a representative sample of those in the general population who had lived in Qingdao city for at least 5 years. The survey was conducted in three urban and three rural administrative areas. Five resident communities from each area with 200–250 individuals from each community were randomly selected. A total of 6,100 individuals aged 35–74 years were invited to participate in the survey and 5,355 attended, which gave a response rate of 87.8%. Among 5,355 participants, 4,808 individuals who did not have a prior history of diabetes underwent either FPG and/or 2-h plasma glucose tests, and 4,203 (87.4%) underwent A1C and 4,371 (90.9%) FCG tests. For data analysis strict inclusion criteria were applied: 1) undiagnosed diabetes identified based on both FPG and 2-h plasma glucose criteria; 2) data for both A1C and FCG; and 3) no data missing for BMI, waist circumference, and blood pressure. A total of 2,332 (986 men and 1,346 women) individuals met the strict inclusion criteria. By using the strict inclusion criteria, we were able to correctly classify diabetes status and to compare A1C with FCG, but at the cost of reducing the sample size by 51.5% (2,476 of 4,808). To check whether this strategy biased the results, a sensitivity analysis was performed including all individuals with either A1C (n = 4,203) or FCG (n = 4,371) test values regardless of missing data for either FPG or 2-h plasma glucose. Undiagnosed diabetes was, thus, classified according to either FPG or 2-h plasma glucose criteria in the sensitivity analysis. The survey was approved by the Qingdao Municipal Health Bureau and the local ethics committee, and informed consent was obtained from all participants.
Participants were interviewed by trained doctors or nurses in the local community clinics. Height and weight were measured with the participants wearing only light clothes and without shoes. Waist circumference was measured at the middle point between the rib cage and top of the iliac crest to the nearest 0.1 cm. Three consecutive blood pressure readings were recorded at least 30 s apart. They were taken from the upper right arm of seated individuals, and the mean of the three readings was used in the data analysis. BMI was calculated as weight in kilograms divided by the square of height in meters.
After an overnight fast of at least 10 h, survey participants in their respective survey sites were given the FCG test over the 0700–0930 period, using a Bayer Ascensia BRIO blood glucose monitoring system that was calibrated to give capillary plasma/serum glucose equivalent results (Bayer HealthCare Company, Shanghai, China). A standard OGTT was also performed on the same day over the 0700–1130 period, and blood samples for glucose determinations were collected from the antecubital vein into a vacuum tube containing sodium fluoride. All blood samples were analyzed in the central laboratory of Hiser Medical Center. An Olympus AU system (Olympus, Tokyo, Japan) was used for plasma glucose, A1C, serum total cholesterol, triglycerides, and HDL cholesterol levels. Plasma glucose levels were determined using the glucose oxidase method. A1C was measured using an immunoturbidimetry method (Tina-qu.a A1C HIT 917 large; Roche Diagnostics). The A1C concentration was calculated by using the formula provided by Roche Diagnostics: [calculated A1C (%) = 0.81 × A1C (test result) + 2.39] to match the values with those found in a conventional high-performance liquid chromatography method. The calculated A1C was subsequently used in the data analysis. The reference range for the calculated A1C was 4.3–5.8%. Fasting insulin concentration was measured in 1,636 individuals using the chemiluminescence immunoassay method (Abbott AxSym). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the formula [HOMA-IR = fasting insulin (micro-international units per milliliter) × FPG (millimoles per liter)/22.5], and the homeostasis model assessment of β-cell function (HOMA-B) was calculated using the formula {HOMA-B = 20 × fasting insulin [micro-international units per milliliter]/[FPG (millimoles per liter) − 3.5]} (8).
Previously diagnosed diabetes was defined as self-report of diabetes diagnosed by a doctor and/or on information on treatment, and subjects with diagnosed diabetes were excluded from the analysis. According to the World Health Organization definition (1), newly diagnosed diabetes is defined as having either FPG ≥7.0 mmol/l and/or 2-h plasma glucose ≥11.1 mmol/l. IGT is defined as having FPG <7.0 mmol/l and 2-h plasma glucose ≥7.8 mmol/l and 2-h plasma glucose <11.1 mmol/l. Impaired fasting glucose (IFG) is defined as having FPG ≥6.1 mmol/l and FPG <7.0 mmol/l and 2-h plasma glucose <7.8 mmol/l. Normal glucose tolerance (NGT) is defined as FPG <6.1 mmol/l and 2-h plasma glucose <7.8 mmol/l.
Statistical analysis
Statistical analysis was performed using SPSS for Windows 15.0 (SPSS, Chicago, IL) and STATA 9.1 (StataCorp, College Station, TX). Continuous variables are presented as means (95% CI), and categorical data are given as number (percentage). Correlations between variables were assessed by Pearson correlation coefficient. The differences between the means of the groups were tested using a univariate general linear model with adjustments for age, and categorical data were analyzed by using the χ2 test. The sensitivity and specificity of the A1C and FCG cutoff values for detecting newly diagnosed diabetes, pre-diabetes, or newly diagnosed diabetes plus pre-diabetes based on the OGTT response were calculated. The receiver operating characteristic (ROC) curve was obtained by plotting sensitivity against 1 − specificity for each cutoff value. The optimal cutoff point was identified as the coordinate closest to the y intercept (0,1) of the ROC curve, and at this point, the sum of the sensitivity and the specificity is maximal. Diagnostic accuracy was assessed by the area under the curve (AUC) (9).
RESULTS
The respective prevalence of pre-diabetes and newly diagnosed diabetes among the participants was 29.5 and 11.9%. Individuals of both sexes with pre-diabetes and newly diagnosed diabetes were older, more obese, hypertensive, dyslipidemic, and insulin resistant and had poorer β-cell function (estimated by HOMA-B) compared with individuals with NGT (Table 1). The correlation coefficients of the A1C with FPG (0.57) and 2-h plasma glucose (0.61) were higher in diabetic individuals than in nondiabetic individuals (−0.32 and 0.001, respectively).
Table 1.
Characteristics of male and female subjects stratified by glucose tolerance status defined according to both FPG and 2-h plasma glucose level
| All | Normal: FPG <6.1 mmol/l and 2-h PG <7.8 mmol/l | Pre-diabetes: FPG 6.1–6.9 mmol/l and/or 2-h PG 7.8–11.0 mmol/l | Undiagnosed diabetes: FPG ≥7.0 mmol/l and/or 2-h PG ≥11.1 mmol/l | |
|---|---|---|---|---|
| Male subjects | ||||
| n (%) | 986 | 588 (59.6) | 266 (27.0) | 132 (13.4) |
| Age (years) | 49.5 (48.9−50.2) | 48.1 (47.3−48.9) | 51.2 (50.0−52.4)* | 52.6 (50.9−54.3)* |
| Waist circumference (cm) | 87.2 (86.6−87.8) | 85.6 (84.9−86.4) | 89.6 (88.5−90.7)* | 89.1 (87.5−90.7)* |
| BMI (kg/m2) | 25.7 (25.5−25.8) | 25.2 (24.9−25.4) | 26.4 (26.1−26.8)* | 26.3 (25.8−26.8)* |
| Blood pressure (mmHg) | ||||
| Systolic | 134 (133−135) | 132 (130−133) | 136 (134−138)† | 138 (135−141)† |
| Diastolic | 87 (87−88) | 86 (86−87) | 88 (87−90) | 90 (87−92)‡ |
| Plasma glucose (mmol/l) | ||||
| Fasting | 5.7 (5.6−5.8) | 5.1 (5.0−5.2) | 5.9 (5.8−6.1)* | 7.9 (7.7−8.1)* |
| 2-h | 7.1 (6.9−7.3) | 5.7 (5.5−5.9) | 7.5 (7.2−7.8)* | 12.4 (11.9−12.8)* |
| A1C (%) | 5.7 (5.7−5.8) | 5.6 (5.6−5.7) | 5.5 (5.4−5.6) | 6.4 (6.3−6.5)* |
| A1C ≥6.5% | 116 (11.8) | 67 (11.4) | 12 (4.5)† | 37 (28.0)* |
| FCG (mmol/l) | 6.1 (6.0−6.1) | 5.7 (5.6−5.8) | 6.2 (6.0−6.3)* | 7.4 (7.2−7.6)* |
| Cholesterol (mmol/l) | 5.3 (5.2−5.3) | 5.2 (5.1−5.2) | 5.3 (5.2−5.5) | 5.5 (5.4−5.7)* |
| Triglycerides (mmol/l) | 1.2 (1.2−1.2) | 1.1 (1.0−1.1) | 1.4 (1.3−1.5)* | 1.5 (1.4−1.6)* |
| HDL cholesterol (mmol/l) | 1.64 (1.61−1.67) | 1.68 (1.64−1.72) | 1.55 (1.49−1.60)* | 1.61 (1.53−1.69) |
| HOMA-IR§‖ | 1.1 (1.0−1.1) | 0.8 (0.8−0.9) | 1.5 (1.3−1.7)* | 1.7 (1.4−2.0)* |
| HOMA-B§‖ | 55.1 (51.7−58.7) | 60.9 (56.2−65.9) | 53.9 (47.6−61.0) | 34.6 (28.9−41.4)* |
| Female subjects | ||||
| n (%) | 1,346 | 777 (57.7) | 423 (31.4) | 146 (10.8) |
| Age (years) | 49.3 (48.8−49.8) | 47.2 (46.6−47.9) | 51.4 (50.5−52.3)* | 54.4 (52.9−55.9)* |
| Waist circumference (cm) | 81.9 (81.5−82.4) | 80.8 (80.2−81.4) | 83.1 (82.2−83.9)* | 84.6 (83.2−86.1)* |
| BMI (kg/m2) | 25.8 (25.6−26.0) | 25.3 (25.1−25.6) | 26.4 (26.0−26.7)* | 26.8 (26.2−27.4)* |
| Blood pressure (mmHg) | ||||
| Systolic | 132 (131−133) | 128 (127−130) | 137 (135−139)* | 138 (135−141)* |
| Diastolic | 84 (84−85) | 83 (82−83) | 86 (85−88)* | 87 (85−89)* |
| Plasma glucose (mmol/l) | ||||
| Fasting | 5.6 (5.6−5.7) | 5.2 (5.1−5.2) | 5.8 (5.8−5.9)* | 7.4 (7.3−7.6)* |
| 2-h | 7.3 (7.2−7.5) | 6.0 (5.9−6.1) | 7.9 (7.7−8.1)* | 12.8 (12.5−13.2)* |
| A1C (%) | 5.6 (5.6−5.6) | 5.6 (5.5−5.6) | 5.5 (5.4−5.6) | 6.1 (6.0−6.2)* |
| A1C ≥6.5% | 137 (10.2) | 82 (10.6) | 23 (5.4)† | 32 (21.9)* |
| FCG (mmol/l) | 6.1 (6.0−6.1) | 5.7 (5.6−5.8) | 6.2 (6.1−6.3)* | 7.4 (7.2−7.6)* |
| Cholesterol (mmol/l) | 5.2 (5.2−5.3) | 5.2 (5.1−5.2) | 5.3 (5.2−5.3) | 5.5 (5.4−5.7)* |
| Triglycerides (mmol/l)§ | 1.1 (1.0−1.1) | 1.0 (1.0−1.0) | 1.1 (1.0−1.1)‡ | 1.4 (1.3−1.5)* |
| HDL cholesterol (mmol/l) | 1.68 (1.65−1.70) | 1.73 (1.70−1.77) | 1.61 (1.57−1.65)* | 1.57 (1.50−1.65)* |
| HOMA-IR§‖ | 1.4 (1.3−1.5) | 1.2 (1.1−1.3) | 1.7 (1.6−1.8)* | 2.3 (2.0−2.6)* |
| HOMA-B§‖ | 67.9 (64.7−71.3) | 74.4 (69.9−79.2) | 65.0 (59.5−70.9)‡ | 44.7 (38.4−52.1)* |
Data are age-adjusted means (95% CI) or n (%).
*P < 0.001;
†P < 0.01;
‡P < 0.05, for the difference between the indexed and the normal category using a univariate general linear model for continuous data and χ2 test for categorical data.
§Geometric mean (95% CI).
‖With missing data.
As expected, diabetic individuals of both sexes with A1C ≥6.5% had higher mean FPG or 2-h plasma glucose and also poorer β-cell function than diabetic individuals with A1C <6.5%. However, this was not the case in the nondiabetic individuals (Table 2). Among nondiabetic individuals, participants of both sexes with A1C ≥6.5% had a lower prevalence of IGT and/or IFG and lower mean FPG and triglycerides than those with A1C <6.5% (Table 2).
Table 2.
Characteristics of male and female subjects according to A1C level and diabetes status defined by the OGTT
| Undiagnosed diabetes |
Nondiabetes |
|||
|---|---|---|---|---|
| A1C ≥6.5% | A1C <6.5% | A1C ≥6.5% | A1C <6.5% | |
| Male subjects (n = 986) | ||||
| n (%) | 37 (3.8) | 95 (9.6) | 79 (8.0) | 775 (78.6) |
| Age (years) | 53.5 (50.4−56.6) | 52.3 (50.3−54.2) | 47.2 (44.9−49.4) | 49.2 (48.5−50.0) |
| Waist circumference (cm) | 89.3 (86.4−92.3) | 88.5 (86.7−90.4) | 86.8 (84.7−88.9) | 86.9 (86.3−87.6) |
| BMI (kg/m2) | 26.3 (25.2−27.3) | 26.2 (25.5−26.8) | 25.7 (25.0−26.4) | 25.6 (25.3−25.8) |
| Blood pressure (mmHg) | ||||
| Systolic | 138 (132−145) | 140 (136−144) | 135 (131−139) | 133 (131−134) |
| Diastolic | 91 (87−94) | 89 (87−92) | 88 (85−91) | 87 (86−88) |
| Plasma glucose (mmol/l) | ||||
| Fasting | 9.8 (8.8−10.9) | 7.2 (6.5−7.8)* | 4.8 (4.7−5.0) | 5.4 (5.3−5.4)* |
| 2-h | 17.3 (15.6−19.1) | 10.5 (9.5−11.6)* | 6.1 (5.8−6.5) | 6.2 (6.1−6.4) |
| IGT and/or IFG | 12 (15.2) | 254 (32.8)† | ||
| FCG (mmol/l) | 9.0 (8.3−9.7) | 6.8 (6.4−7.2)* | 6.0 (5.7−6.2) | 5.8 (5.8−5.9) |
| Cholesterol (mmol/l) | 5.7 (5.4−6.1) | 5.5 (5.3−5.7) | 5.4 (5.2−5.6) | 5.2 (5.1−5.3) |
| Triglycerides (mmol/l)§ | 1.5 (1.2−1.8) | 1.4 (1.3−1.7) | 0.9 (0.8−1.1) | 1.2 (1.2−1.2)* |
| HDL cholesterol (mmol/l) | 1.68 (1.53−1.84) | 1.59 (1.49−1.68) | 1.75 (1.64−1.85) | 1.63 (1.59−1.66)‡ |
| HOMA-IR§‖ | 1.3 (0.8−1.9) | 1.8 (1.4−2.4) | 0.8 (0.7−1.0) | 1.0 (0.9−1.1) |
| HOMA-B§‖ | 19.7 (13.3−28.9) | 41.7 (32.5−53.4)† | 70.0 (58.3−84.0) | 57.6 (53.8−61.7) |
| Female subjects (n = 1,346) | ||||
| n (%) | 32 (2.4) | 114 (8.5) | 105 (7.8) | 1,095 (81.4) |
| Age (years) | 55.9 (52.6−59.2) | 53.9 (52.2−55.7) | 46.7 (44.9−48.5) | 48.9 (48.3−49.4)‡ |
| Waist circumference (cm) | 88.1 (85.2−91.0) | 85.3 (83.8−86.8) | 84.1 (82.4−85.8) | 81.2 (80.7−81.7)† |
| BMI (kg/m2) | 27.4 (26.3−28.5) | 27.0 (26.4−27.6) | 25.8 (25.2−26.5) | 25.7 (25.4−25.9) |
| Blood pressure (mmHg) | ||||
| Systolic | 138 (130−147) | 143 (139−148) | 133 (129−137) | 131 (129−132) |
| Diastolic | 86 (82−90) | 88 (86−91) | 85 (82−87) | 84 (83−84) |
| Plasma glucose (mmol/l) | ||||
| Fasting | 9.2 (8.6−9.9) | 7.0 (6.6−7.3)* | 4.8 (4.6−4.9) | 5.5 (5.4−5.5)* |
| 2-h | 17.8 (16.3−19.2) | 11.6 (10.8−12.3)* | 6.7 (6.4−7.0) | 6.7 (6.6−6.7) |
| IGT and/or IFG | 23 (21.9) | 400 (36.5)† | ||
| FCG (mmol/l) | 9.8 (9.2−10.5) | 6.8 (6.5−7.2)* | 6.0 (5.8−6.2) | 5.9 (5.8−5.9) |
| Cholesterol (mmol/l) | 6.0 (5.6−6.4) | 5.6 (5.4−5.8) | 5.0 (4.8−5.2) | 5.2 (5.1−5.3)‡ |
| Triglycerides (mmol/l)§ | 1.5 (1.2−1.8) | 1.4 (1.3−1.6) | 0.9 (0.9−1.0) | 1.0 (1.0−1.1)‡ |
| HDL cholesterol (mmol/l) | 1.58 (1.41−1.75) | 1.59 (1.49−1.68) | 1.77 (1.69−1.85) | 1.68 (1.66−1.71) |
| HOMA-IR§‖ | 2.3 (1.7−3.0) | 2.4 (2.0−2.8) | 1.0 (0.9−1.2) | 1.4 (1.3−1.5)* |
| HOMA-B§‖ | 25.6 (19.0−34.5) | 49.5 (42.2−58.1)* | 98.3 (84.6−114.2) | 68.7 (65.2−72.4)* |
Data are age-adjusted means (95% CI) or n (%).
*P < 0.001;
†P < 0.01;
‡P < 0.05, for the difference between the indexed and the A1C ≥6.5% category within the same glucose tolerance class of undiagnosed diabetes or nondiabetes using a univariate general linear model for continuous data and χ2 test for categorical data.
§Geometric mean (95% CI).
‖With missing data.
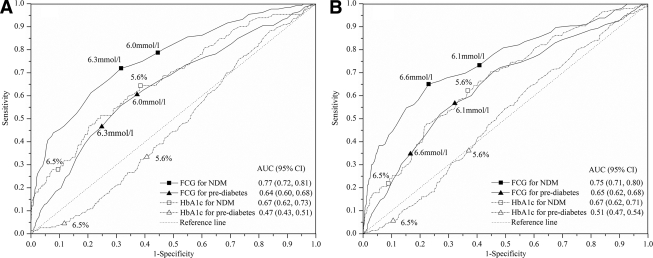
The AUC was lower for A1C than for FCG for detecting newly diagnosed diabetes (0.67 vs. 0.77, P < 0.01, in men; and 0.67 vs. 0.75, P < 0.01, in women) and pre-diabetes (0.47 vs. 0.64, P < 0.001, in men; and 0.51 vs. 0.65, P < 0.001, in women) (Fig. 1). Moreover, for newly diagnosed diabetes plus pre-diabetes, the AUCs for A1C and FCG values were 0.53 vs. 0.69 in men (P < 0.001) and 0.55 vs. 0.68 in women (P < 0.001). The A1C values did not distinguish people with pre-diabetes from those with NGT. The chance-corrected proportion agreement (κ) for classification of diabetes between the criteria of the OGTT and the A1C of ≥6.5% that are recommended by the International Expert Committee (7) was 0.20 in men and 0.14 in women. The optimal A1C cutoff point for newly diagnosed diabetes in this study population at 5.6% was lower than the recommended value of 6.5%. At the optimal A1C cutoff point of 5.6% for newly diagnosed diabetes, the sensitivity was 64.4% in men and 62.3% in women, whereas it was 72.0 and 65.1% at the optimal FCG cutoff point of 6.3 mmol/l for men and 6.6 mmol/l for women (Table 3). Using the recommended diagnostic value of 6.5% for A1C (7), the sensitivity for newly diagnosed diabetes was <30% in both men and women. The optimal FCG cutoff value and the corresponding sensitivity for pre-diabetes and pre-diabetes plus newly diagnosed diabetes are shown in Table 3.
Figure 1.
ROC curve for A1C and FCG to assess the presence of newly diagnosed diabetes (NDM) and pre-diabetes in men (A) and women (B).
Table 3.
Sensitivity and specificity corresponding to the optimal cutoff values of A1C and FCG for detecting newly diagnosed diabetes, pre-diabetes, and newly diagnosed diabetes plus pre-diabetes in male and female subjects
| Optimal cutoff point | NDM |
Pre-diabetes |
NDM + pre-diabetes |
|||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| Male subjects | ||||||
| A1C (%) | ||||||
| ≥5.6 for NDM | 64.4 | 61.6 | 33.5 | 59.4 | 43.7 | 59.4 |
| ≥6.5 (diagnostic criteria)* | 28.0 | 90.5 | 4.5 | 88.3 | 12.3 | 88.3 |
| FCG (mmol/l) | ||||||
| ≥6.3 for NDM | 72.0 | 68.4 | 46.6 | 75.2 | 55.0 | 75.2 |
| ≥6.0 for pre-diabetes | 78.8 | 55.5 | 60.5 | 62.8 | 66.6 | 62.8 |
| ≥6.1 for NDM + pre-diabetes | 75.0 | 61.0 | 55.6 | 68.5 | 62.1 | 68.5 |
| Female subjects | ||||||
| A1C (%) | ||||||
| ≥5.6 for NDM | 62.3 | 63.3 | 36.2 | 62.9 | 42.9 | 62.9 |
| ≥6.5 (diagnostic criteria)* | 21.9 | 91.2 | 5.7 | 89.4 | 9.8 | 89.4 |
| FCG (mmol/l) | ||||||
| ≥6.6 for NDM | 65.1 | 77.0 | 34.8 | 83.4 | 42.5 | 83.4 |
| ≥6.1 for pre-diabetes and NDM + pre-diabetes | 73.3 | 59.2 | 56.7 | 67.8 | 61.0 | 67.8 |
Data are %.
*Adopted as the diagnostic criteria for diabetes (7). NDM, newly diagnosed diabetes.
To check whether the results were biased by excluding half of the survey participants from the data analyses, a sensitivity analysis was made using the maximum number of participants for either A1C or FCG. For undiagnosed diabetes, an AUC of 0.67 (95% CI 0.63–0.72) in men and 0.70 (0.67–0.73) in women for A1C and 0.78 (0.74–0.81) in men and 0.76 (0.73–0.79) in women for FCG was obtained, which did not differ from the results presented in Fig. 1. The group who met the strict inclusion criteria was not different from those who did not with regard to mean age, BMI, and blood pressure, but the former consisted of more men (42.3 vs. 35.6%, P < 0.001) and had a slightly higher prevalence of undiagnosed diabetes (11.9 vs. 10.1%, P = 0.04) than the latter as expected.
CONCLUSIONS
In this population-based study, the FCG test performed better than A1C in ability to identify newly diagnosed diabetes as determined by the standard 2-h 75-g OGTT. The A1C test did not distinguish between pre-diabetic and normal subjects. Less than 30% of the newly diagnosed diabetes could be identified at the A1C cutoff point of ≥6.5%, the diagnostic benchmark recommended by the International Expert Committee (7).
In previous studies, the optimal A1C cutoff points for detecting undiagnosed diabetes ranged from 5.3 to 6.1% in population-based studies among Australian and American populations (10–12). In those studies the sensitivity varied from 63.2 to 86.0%, and the specificity ranged from 82.8 to 97.4% at a given optimal A1C cutoff point. In a study among Hong Kong Chinese who were referred to a hospital for confirmative testing for glucose intolerance, an optimal A1C cutoff point of 6.1% for detecting 2-h plasma glucose ≥11.1 mmol/l was reported, which gave a sensitivity of 77.5% and a specificity of 78.8% (13). This Hong Kong study was, however, performed among a preselected high-risk population of which close to one-fourth of its participants were diagnosed as having diabetes. Moreover, the diagnostic criteria for diabetes in the Hong Kong study were also different from those used in our study. The inconsistency in performance of the A1C test among studies may have been due to the discrepancies in ethnicity, assay methods for A1C, the gold standard for diagnosis of diabetes, study methodology differences (population-based versus clinical based), and the prevalence of other risk factors such as obesity. The extent to which these factors affect the performance of the A1C needs to be further investigated. However, our results are consistent with other studies showing that the A1C test did not detect IGT (11,14,15).
Few studies have evaluated the performance of the FCG test. In a randomly selected population-based study in Brazil, the optimal FCG cutoff point for undiagnosed diabetes was 5.6 mmol/l, which gave a sensitivity of 87.2% and a specificity of 72.4% (16). Another study compared FCG with A1C assays among Caucasians who were referred to a hospital because they were suspected as having diabetes (17). That study also showed that the performance of the FCG test was better than that of the A1C test in detecting either diabetes or diabetes plus pre-diabetes, which was similar to the findings in our present study.
Prospective studies showed that elevated A1C values increase the risk of mortality from all causes (18,19) and cardiovascular disease (19). It was also reported that A1C predicted mortality (20,21) or incident cardiovascular disease (22) depending largely on either the 2-h plasma glucose (20–22) or the FPG (21) levels. In our study, the blood pressure and lipid levels in individuals with A1C <6.5% did not differ from those with A1C ≥6.5%. However, both of these parameters were higher for diabetic and pre-diabetic individuals than they were for normal subjects as defined by the OGTT. The prognosis of those individuals with A1C ≥6.5% needs to be further investigated in prospective studies.
The large number of individuals included in this population-based study provided our study with high statistical power for data analyses. Diabetes and pre-diabetes were defined according to the currently accepted standard criteria based on both FPG and 2-h plasma glucose levels. All individuals included in the analysis provided data on FCG and A1C in addition to the OGTT. These individuals also yielded data on lipid and insulin, which enabled us to study further the clinical phenotypes of individuals of different glycemic categories. Because of the strict inclusion criteria for glucose and other variables, only 55% of the population who had A1C measurements were included in the data analyses. To check whether the results have been biased by the strict inclusion criteria, a sensitivity analysis using all data available for the A1C was made. The results of the sensitivity analysis showed that the study was less likely to be biased by the strict inclusion criteria applied in the study. The high prevalence of diabetes observed in this study reflects the recent increase in the prevalence of diabetes in Qingdao, which has been investigated in detail and reported (23,24). The question as to whether the result can be found in other populations remains to be confirmed. Another limitation of our study is that hemoglobin concentrations were not available, and, thus, a potential influence of conditions such as hemolytic anemia and hemoglobinopathies on A1C assays cannot be excluded. Nevertheless, these conditions are rare in the general population from which our study population was drawn. The prevalence of abnormal hemoglobin in the Shandong province in which Qingdao is located is only 0.04% (25).
In summary, as a mass screening tool, the FCG test performed better than the A1C test in the general population of Chinese. In consideration of its high cost and poor performance, the A1C test is not a suitable test for mass screening, particularly with the purpose of detecting pre-diabetes for early intervention.
Acknowledgments
This study was supported by the Finnish Academy (118492). Bayer HealthCare Company, Shanghai, China, provided unrestricted financial support for the data collection.
No potential conflicts of interest relevant to this article were reported.
We express our sincere thanks to the local research teams from Qingdao Municipal Health Bureau, Qingdao Centers for Disease Control and Prevention, Qingdao, China, for their contribution to the field survey (http://www.qddiabetes.org/Organize-6.asp).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999. (WHO/NCD/NCS/99.2) [Google Scholar]
- 2. Eschwège E, Charles MA, Simon D, et al. : Reproducibility of the diagnosis of diabetes over a 30-month follow-up: the Paris Prospective Study. Diabetes Care 2001;24:1941–1944 [DOI] [PubMed] [Google Scholar]
- 3. Drzewoski J, Czupryniak L: Concordance between fasting and 2-h post-glucose challenge criteria for the diagnosis of diabetes mellitus and glucose intolerance in high risk individuals. Diabet Med 2001;18:29–31 [DOI] [PubMed] [Google Scholar]
- 4. Toscano CM, Duncan BB, Mengue SS, et al. : Initial impact and cost of a nationwide population screening campaign for diabetes in Brazil: a follow up study. BMC Health Serv Res 2008;8:189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spijkerman AM, Adriaanse MC, Dekker JM, et al. : Diabetic patients detected by population-based stepwise screening already have a diabetic cardiovascular risk profile. Diabetes Care 2002;25:1784–1789 [DOI] [PubMed] [Google Scholar]
- 6. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care 2007;30:2399–2400 [DOI] [PubMed] [Google Scholar]
- 7. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews DR, Hosker JP, Rudenski AS, et al. : Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 9. Akobeng AK: Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 2007;96:644–647 [DOI] [PubMed] [Google Scholar]
- 10. Rohlfing CL, Little RR, Wiedmeyer HM, et al. : Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 2000;23:187–191 [DOI] [PubMed] [Google Scholar]
- 11. Colagiuri S, Hussain Z, Zimmet P, et al. : Screening for type 2 diabetes and impaired glucose metabolism: the Australian experience. Diabetes Care 2004;27:367–371 [DOI] [PubMed] [Google Scholar]
- 12. Buell C, Kermah D, Davidson MB: Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007;30:2233–2235 [DOI] [PubMed] [Google Scholar]
- 13. Ko GT, Chan JC, Yeung VT, et al. : Combined use of a fasting plasma glucose concentration and HbA1c or fructosamine predicts the likelihood of having diabetes in high-risk subjects. Diabetes Care 1998;21:1221–1225 [DOI] [PubMed] [Google Scholar]
- 14. Saydah SH, Byrd-Holt D, Harris MI: Projected impact of implementing the results of the Diabetes Prevention Program in the U.S. population. Diabetes Care 2002;25:1940–1945 [DOI] [PubMed] [Google Scholar]
- 15. Mannucci E, Ognibene A, Sposato I, et al. : Fasting plasma glucose and glycated haemoglobin in the screening of diabetes and impaired glucose tolerance. Acta Diabetol 2003;40:181–186 [DOI] [PubMed] [Google Scholar]
- 16. Bortheiry AL, Malerbi DA, Franco LJ: The ROC curve in the evaluation of fasting capillary blood glucose as a screening test for diabetes and IGT. Diabetes Care 1994;17:1269–1272 [DOI] [PubMed] [Google Scholar]
- 17. Herdzik E, Safranow K, Ciechanowski K: Diagnostic value of fasting capillary glucose, fructosamine and glycosylated haemoglobin in detecting diabetes and other glucose tolerance abnormalities compared to oral glucose tolerance test. Acta Diabetol 2002;39:15–22 [DOI] [PubMed] [Google Scholar]
- 18. Khaw KT, Wareham N, Bingham S, et al. : Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004;141:413–420 [DOI] [PubMed] [Google Scholar]
- 19. Nakanishi S, Yamada M, Hattori N, et al. : Relationship between HbA1c and mortality in a Japanese population. Diabetologia 2005;48:230–234 [DOI] [PubMed] [Google Scholar]
- 20. Qiao Q, Dekker JM, de Vegt F, et al. : Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol 2004;57:590–596 [DOI] [PubMed] [Google Scholar]
- 21. Barr EL, Boyko EJ, Zimmet PZ, et al. : Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 22. Meigs JB, Nathan DM, D'Agostino RB, Sr, et al. : Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002;25:1845–1850 [DOI] [PubMed] [Google Scholar]
- 23. Ning F, Pang ZC, Dong YH, et al. : Risk factors associated with the dramatic increase in the prevalence of diabetes in the adult Chinese population in Qingdao, China. Diabet Med 2009;26:855–863 [DOI] [PubMed] [Google Scholar]
- 24. Gao WG, Dong YH, Pang ZC, et al. : Increasing trend in the prevalence of type 2 diabetes and pre-diabetes in the Chinese rural and urban population in Qingdao, China. Diabet Med 2009;26:1220–1229 [DOI] [PubMed] [Google Scholar]
- 25. Zeng YT, Huang SZ: Disorders of haemoglobin in China. J Med Genet 1987;24: 578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]