Abstract
OBJECTIVE
Mortality rates have declined substantially over the past decades in the general population, but the situation among diabetic subjects is less clear. The aim of this study was to analyze mortality trends in diabetic and nondiabetic subjects during 1972–2004.
RESEARCH DESIGN AND METHODS
Since 1972, all patients with diabetes are entered in a diabetes register at Laxå Primary Health Care Center; 776 incident cases were recorded up to 2001. The register has been supplemented with a nondiabetic population of 3,880 subjects and with data from the National Cause of Death Register during 1972 to 2004.
RESULTS
During the 33-year follow-up period, 233 (62.0%) diabetic women and 240 (60.0%) diabetic men and 995 (52.9%) nondiabetic women and 1,082 (54.1%) nondiabetic men died. The age-adjusted hazard ratio (HR) for all-cause mortality among diabetic and nondiabetic subjects was 1.17 (P < 0.0021) for all, 1.22 (P < 0.007) for women, and 1.13 (P = 0.095) for men. The corresponding cardiovascular disease (CVD) mortality HRs were 1.33 (P < 0.0001), 1.41 (P < 0.0003), and 1.27 (P < 0.0093), respectively. The CVD mortality reduction across time was significant in nondiabetic subjects (P < 0.0001) and in men with diabetes (P = 0.014) but not in diabetic women (P = 0.69). The results regarding coronary heart disease (CHD) were similar (P < 0.0001, P < 0.006, and P = 0.17, respectively). The CVD and CHD mortality rate change across time was fairly linear in all groups.
CONCLUSIONS
Diabetic subjects had less mortality rate reduction during follow-up than nondiabetic subjects. However the excess mortality risk for diabetic subjects was smaller than that found in other studies.
The excess mortality, mostly owing to cardiovascular disease (CVD), in diabetic subjects compared with nondiabetic subjects, is well documented (1–4) and has been shown to decline with increasing age at diagnosis (1,3,5). However, studies of the influence of sex on mortality risk for those with and without diabetes show conflicting results (1,6,7).
CVD incidence and mortality rates have declined in most developed countries, including Sweden, over the past decades (8–10). Because CVD is the major cause of death among diabetic and nondiabetic subjects, it would be expected that the general decline in mortality would be reflected in both groups. Data from Framingham reported parallel declines in CVD outcomes for diabetic and nondiabetic women and men between 1950 and 1995 (11). Another U.S. study (12) confirmed a mortality decline during 1971 to 2000 for diabetic men but not women, whereas a Canadian study (13) showed reduced mortality rates for both sexes during 1995 to 2005. A Danish study (14) reported a faster reduction in mortality in diabetic subjects than in a nondiabetic population between 1995 and 2006, and a Norwegian study (15) showed a substantial decrease in mortality from coronary heart disease (CHD) for both diabetic and nondiabetic subjects and for both sexes from the mid-1980s to the mid-1990s. A Swedish study (16) covering the period 1980–2004 showed improved survival rates in diabetic subjects, more so in diabetic women than in men, thereby narrowing the gap to nondiabetic women.
The aim of this study was to analyze mortality trends among diabetic and nondiabetic subjects over the time period 1972–2004 in a defined geographical area in Sweden and to test the hypothesis that changes in all-cause, CVD, and CHD mortality have been parallel among the two cohorts.
RESEARCH DESIGN AND METHODS
Diabetic subjects
The study was undertaken in Laxå, Örebro County, Sweden. From 1 January 1972 and onward all new cases of diabetes among residents in Laxå were entered into a diabetes register at Laxå Primary Health Care Center (PHCC). Patient record files at the local PHCC, at nearby PHCCs, at private practitioners' offices, and at adjacent hospitals and prescription registers at the local pharmacies were scrutinized to find possible missing cases, as described in detail previously (17). During 1972 to 2001, altogether 776 new cases of diabetes were found, 36 type 1 and 740 type 2 diabetes.
Nondiabetic subjects
For each new diabetic subject, five (maximum number available) nondiabetic subjects were sampled in 2007 from the Laxå general population register, matched to the diabetic subjects by sex and age, sampled from the population register version of the diagnosis year, and required to not have a diabetes diagnosis at any time from baseline to end of follow-up. Several diabetes screening rounds during the study period were used to eliminate misclassification. No clinical data were available for the nondiabetic cohort. Altogether 3,880 subjects, 1,880 women and 2,000 men, were sampled.
Death ascertainment
Mortality data until 2004 for the complete study population were obtained from the National Cause of Death Registry. Data obtained included date and place of death, underlying cause of death and contributing causes of death. The causes of death were coded according to the ICD-8, ICD-9, and ICD-10. The Regional Research Ethics Board in Uppsala, Sweden, approved the study.
Statistical considerations
The data were analyzed using SAS (release 9.1, SAS Institute, Cary, NC). Simple differences between groups were tested with Student's t test, variance analysis, or the χ2 test.
Survival analyses were performed with Cox's proportional hazards regression, modeled with the outcome and time as the dependent variables and age, diabetes status, and year of diagnosis and inclusion to the study as independent variables and stratified for sex. In addition, two interaction terms, diabetes status-year of diagnosis and diabetes status-year of diagnosis-sex, were tested. The former was significant for some outcomes, which was used for further modeling.
For illustration purposes some of the results are shown for the three periods 1972–1981, 1982–1991, and 1992–2001, all based on the time of diabetes diagnosis. The follow-up time was right-truncated at 26, 20, and 10 years for the three partial study periods owing to small numbers.
Finally, to allow equal exposure times when mortality rate per year of diagnosis was analyzed, 3-year follow up was used, because those entering the study in 2001 only had 3 years of follow-up. P < 5% was regarded as statistically significant.
RESULTS
Characteristics of the study population
Mean ± SD age at diagnosis for diabetic subjects and fasting blood glucose decreased over the three decades, whereas BMI and the proportion of smokers and former smokers increased, as did the proportion of subjects using diet only as diabetes treatment (Table 1).
Table 1.
Baseline characteristics among diabetic women and men
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Decade 1: 1972–1981 | Decade 2: 1982–1991 | Decade 3: 1992–2001 | Decade 1: 1972–1981 | Decade 2: 1982–1991 | Decade 3: 1992–2001 | |
| n | 131 | 140 | 105 | 133 | 154 | 113 |
| Age at diagnosis (years) | 65.0 ± 15.3 | 66.1 ± 13.4 | 62.1 ± 15.1 | 62.6 ± 14.1 | 61.2 ± 17.1 | 58.4 ± 14.6 |
| FBG (mmol/l) | 9.6 ± 2.8 | 8.3 ± 2.3 | 7.4 ± 2.2* | 9.1 ± 2.6 | 9.1 ± 12.4 | 8.6 ± 3.6 |
| BMI (kg/m2) | 30.2 ± 6.1 | 29.7 ± 6.0 | 31.2 ± 5.6 | 28.8 ± 3.4 | 28.0 ± 4.5 | 30.6 ± 4.8* |
| Smokers (%) | 3.8 | 7.9 | 14.3† | 12.0 | 15.6 | 18.6 |
| Ex-smokers (%) | 3.1 | 5.0 | 7.6 | 8.3 | 20.1 | 31.0* |
| AHD (%) | 51.9 | 63.6 | 53.3‡ | 51.9 | 50.0 | 38.0 |
| Diabetes treatment during the first year | ||||||
| Diet only (%) | 67.9 | 77.9 | 76.2† | 63.2 | 71.4 | 82.3* |
| OHA (%) | 29.8 | 16.4 | 15.2† | 32.3 | 20.1 | 8.8* |
| OHA and insulin (%) | 2.3 | 0.0 | 0.0 | 3.0 | 0.6 | 3.5 |
| Insulin (%) | 0.0 | 5.7 | 8.6 | 1.5 | 7.8 | 5.3 |
Data are means ± SD or %.
*P < 0.001;
†P < 0.01;
‡P < 0.05. AHD, antihypertensive drug; FBG, fasting blood glucose; OHA, oral hypoglycemic agent.
Trends in all-cause mortality
During the 33-year follow up period, 233 (62.0%) diabetic women and 240 (60.0%) diabetic men and 995 (52.9%) nondiabetic women and 1,082 (54.1%) nondiabetic men died (Table 2). Age- and sex-adjusted hazard ratios (HRs) for all-cause mortality during 1972 to 2004 in diabetic and nondiabetic subjects were 1.17 (P = 0.0021) for the total sample (data not shown), 1.22 (P < 0.007) for women, and 1.13 (P = 0.095) for men.
Table 2.
Mortality during follow-up 1972–2004 among diabetic and nondiabetic subjects
| Diabetes |
No diabetes |
Ratio diabetic and nondiabetic women |
Ratio diabetic and nondiabetic men |
|||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | HR (95% CI) | P | HR (95% CI) | P | |
| CVD | 141 (37.6) | 147 (36.7) | 523 (27.8) | 587 (29.3) | 1.41 (1.17–1.70) | 0.0003 | 1.27 (1.06–1.52) | 0.0093 |
| CHD | 80 (21.3) | 103 (25.8) | 274 (14.6) | 385 (19.3) | 1.50 (1.17–1.92) | 0.0015 | 1.35 (1.08–1.68) | 0.0072 |
| Cerebrovascular disease | 33 (8.8) | 28 (7.0) | 130 (6.9) | 97 (4.8) | 1.39 (0.94–2.04) | NS | 1.49 (0.98–2.27) | NS |
| Other CVD | 28 (7.5) | 16 (4.0) | 119 (6.3) | 105 (5.2) | 1.22 (0.81–1.84) | NS | 0.78 (0.46–1.33) | NS |
| Malignant neoplasms | 32 (8.5) | 41 (10.2) | 211 (11.2) | 241 (12.0) | 0.76 (0.53–1.11) | NS | 0.87 (0.63–1.22) | NS |
| Respiratory disease | 14 (3.7) | 11 (2.8) | 62 (3.3) | 65 (3.2) | 1.21 (0.68–2.16) | NS | 0.85 (0.45–1.61) | NS |
| Endocrine disease | 14 (3.7) | 12 (3.0) | 13 (0.7) | 11 (0.5) | 5.53 (2.59–11.81) | <0.0001 | 5.89 (2.59–13.40) | <0.0001 |
| Digestive disease | 11 (2.9) | 9 (2.2) | 42 (2.2) | 40 (2.0) | 1.37 (0.70–2.67) | NS | 1.14 (0.55–2.34) | NS |
| Injury | 6 (1.6) | 5 (1.2) | 33 (1.7) | 53 (2.7) | 0.91 (0.38–2.18) | NS | 0.46 (0.19–1.16) | NS |
| MB disorder | 5 (1.3) | 0 | 23 (1.2) | 13 (0.7) | 1.46 (0.55–3.91) | NS | — | — |
| Infectious disease | 3 (0.8) | 3 (0.8) | 13 (0.7) | 14 (0.7) | 1.24 (0.35–4.36) | NS | 1.05 (0.30–3.64) | NS |
| GU disease | 3 (0.8) | 7 (1.8) | 18 (1.0) | 23 (1.2) | 0.90 (0.26–3.06) | NS | 1.62 (0.69–3.79) | NS |
| Nervous disease | 1 (0.3) | 1 (0.3) | 20 (1.1) | 13 (0.7) | 0.26 (0.03–1.90) | NS | 0.38 (0.05–2.94) | NS |
| All other diseases | 3 (0.8) | 4 (1.0) | 37 (2.0) | 22 (1.1) | 0.44 (0.13–1.41) | NS | 0.93 (0.32–2.70) | NS |
| All causes | 233 (62.0) | 240 (60.0) | 995 (52.9) | 1,082 (54.1) | 1.22 (1.06–1.40) | 0.0071 | 1.13 (0.98–1.30) | NS |
Data are n (%) or HR (95% CI). Ratios between diabetic and nondiabetic subjects were adjusted for the influence of age, sex, and calendar year. GU, genitourinary disease; MB, mental and behavioral disorder.
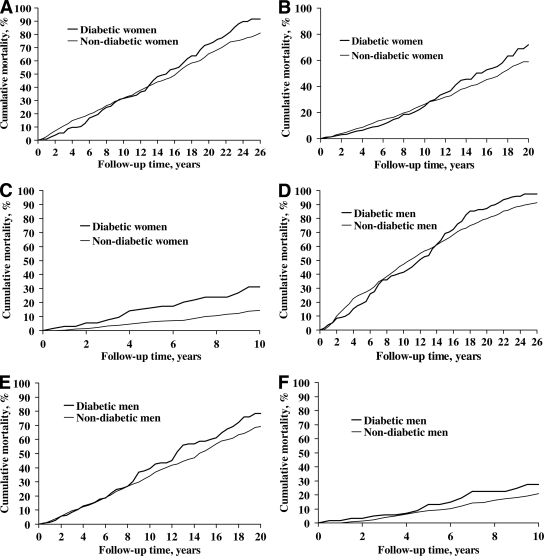
Cumulative mortality rates during the whole follow-up period in the three decades are shown in Fig. 1A–F. Mortality decreased in nondiabetic subjects of both sexes (P < 0.0001). For diabetic men there was a similar tendency (P = 0.045), whereas no such development could be seen for diabetic women (P = 0.72). The mortality trends during the follow-up period in relation to age and year of diagnosis for diabetic and nondiabetic subjects are shown in Table 3. For both cohorts and for both sexes the all-cause mortality risk (HR) increased by age (P < 0.0001). The nondiabetic subjects had a decreased mortality rate over time by almost 30% per 10 years. Diabetic subjects had no significant change over time, although there was a tendency toward a decrease among men with diabetes. No significant interactions between time period and diabetes status for all-cause mortality were found. However, when sex-specific analyses were performed, a statistically significant interaction was found for diabetic women (P < 0.004) but not for diabetic men (P = 0.15).
Figure 1.
Cumulative mortality among diabetic and nondiabetic women and men during three time periods. A: Exposed diabetic (n = 131) and nondiabetic women (n = 655) 1972–1981. B: Exposed diabetic (n = 140) and nondiabetic women (n = 700) 1982–1991. C: Exposed diabetic (n = 105) and nondiabetic women (n = 525) 1992–2001. D: Exposed diabetic (n = 133) and nondiabetic men (n = 665) 1972–1981. E: Exposed diabetic (n = 154) and nondiabetic men (n = 770) 1982–1991. F: Exposed diabetic (n = 113) and nondiabetic men (n = 565) 1992–2001.
Table 3.
Associations between age and diagnosis year on mortality from all causes, CVDs, and malignant diseases among diabetic and nondiabetic subjects
| Diabetes |
No diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Women |
Men |
Women |
Men |
|||||
| HR (95% CI) | χ2 | HR (95% CI) | χ2 | HR (95% CI) | χ2 | HR (95% CI) | χ2 | |
| Mortality, all causes | ||||||||
| Age, 10-year groups | 2.42 (2.07–2.82) | 125.3 | 2.20 (1.93–2.51) | 137.1 | 2.47 (2.31–2.66) | 629.6 | 2.31 (2.18–2.46) | 705.7 |
| Diagnosis year, by 10 years | 1.02 (0.84–1.24) | 0.04 | 0.83 (0.68–1.01) | 3.5 | 0.71 (0.65–0.78) | 48.5 | 0.72 (0.66–0.79) | 51.5 |
| CVD mortality | ||||||||
| Age, 10-year groups | 2.45 (2.00–2.99) | 76.8 | 2.22 (1.87–2.63) | 84.9 | 3.08 (2.78–3.41) | 455.0 | 2.61 (2.39–2.84) | 463.9 |
| Diagnosis year, by 10 years | 0.95 (0.74–1.22) | 0.2 | 0.73 (0.57–0.94) | 6.0 | 0.70 (0.62–0.80) | 26.9 | 0.69 (0.61–0.78) | 37.0 |
| CHD mortality | ||||||||
| Age, 10-year groups | 2.09 (1.63–2.69) | 33.5 | 2.06 (1.69–2.52) | 51.3 | 2.97 (2.58–3.41) | 230.1 | 2.43 (2.19–2.70) | 273.7 |
| Diagnosis year, by 10 years | 0.79 (0.56–1.11) | 1.9 | 0.65 (0.48–0.88) | 7.6 | 0.59 (0.49–0.71) | 31.8 | 0.63 (0.54–0.73) | 38.0 |
| Cerebrovascular disease mortality | ||||||||
| Age, 10-year groups | 3.44 (2.17–5.46) | 27.7 | 2.70 (1.81–4.10) | 23.3 | 3.23 (2.61–3.98) | 118.4 | 3.16 (2.52–3.97) | 99.0 |
| Diagnosis year, by 10 years | 1.24 (0.73–2.09) | 0.6 | 0.98 (0.53–1.78) | 0 | 0.77 (0.58–1.01) | 3.5 | 0.76 (0.56–1.03) | 3.0 |
| Malignant neoplasm mortality | ||||||||
| Age, 10-year groups | 1.43 (1.04–1.97) | 4.8 | 1.93 (1.43–2.60) | 18.5 | 1.61 (1.42–1.83) | 52.2 | 1.85 (1.64–2.10) | 99.3 |
| Diagnosis year, by 10 years | 1.37 (0.83–2.24) | 1.5 | 1.27 (0.80–2.03) | 1.0 | 0.68 (0.56–0.83) | 14.6 | 0.72 (0.59–0.88) | 10.8 |
| Mortality from all other causes | ||||||||
| Age, 10-year groups | 3.59 (2.52–5.11) | 49.9 | 2.39 (1.78–3.19) | 34.3 | 2.47 (2.15–2.84) | 164.7 | 2.22 (1.96–2.52) | 154.8 |
| Diagnosis year, by 10 years | 1.01 (0.67–1.53) | 0.002 | 0.84 (0.54–1.30) | 0.6 | 0.77 (0.63–0.93) | 7.5 | 0.81 (0.67–0.97) | 5.3 |
Data are HRs (95% CI) or χ2 estimate.
Trends in CVD and CHD mortality
The age- and sex-adjusted CVD and CHD mortality rates were significantly higher for diabetic than for nondiabetic subjects: CVD mortality HR 1.33 (P < 0.0001) and CHD mortality HR 1.41 (P < 0.0001) (data not shown). Both diabetic women and men had significantly higher risk than nondiabetic subjects of dying from CVD and within this group of disease from CHD but not from other cardiovascular causes (Table 2). The associations between age and CVD mortality trends were similar to the associations between age and all-cause mortality (Table 3). Diabetic and nondiabetic men had a similar average decrease over calendar time, whereas diabetic women had no such change (nondiabetic subjects P < 0.0001, diabetic men P = 0.014, and diabetic women P = 0.69). For CHD mortality, the associations were similar for both age and diagnosis year to those for CVD mortality (nondiabetic subjects P < 0.0001, diabetic men P < 0.006), and diabetic women P = 0.17). No significant interactions between time period and diabetes status for CVD and CHD mortality were found (P > 0.10).
Trends in non-CVD mortality
As expected, the mortality risk was significantly increased for endocrine disease among diabetic subjects. For all other causes, there were no significant differences (Table 2). A pronounced increase in mortality risk by age was seen for diabetic and nondiabetic subjects for cerebrovascular disease. No significant associations of diagnosis year, either among diabetic subjects or among nondiabetic subjects, were detected. There was a significantly increased HR for malignant neoplasm mortality and mortality from all other causes by age for diabetic and nondiabetic subjects of both sexes, but only nondiabetic subjects had a significant decrease in HR with calendar time (Table 3). There was a significant interaction between time period and diabetes status for malignant neoplasm mortality (P = 0.02) but not for mortality from all other causes (P > 0.10).
Trends across time
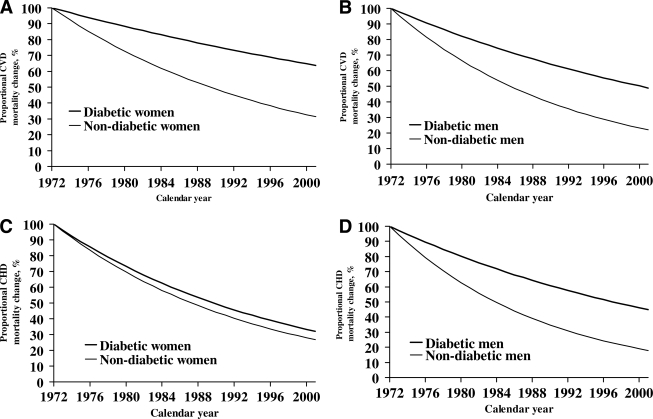
A more detailed analysis of the time sequence in the course of events was made on the basis of year of inclusion of diabetic and nondiabetic subjects and with 3 years of follow-up. The course of events regarding CVD and CHD mortality is shown in Fig. 2A–D. Generally, the mortality rates fell gradually by year of inclusion, but faster among nondiabetic than diabetic subjects.
Figure 2.
Proportion (%) not dead from CVD and CHD across the total study period among diabetic and nondiabetic women and men. A: CVD mortality among diabetic and nondiabetic women 1972–2001. B: CVD mortality among diabetic and nondiabetic men 1972–2001. C: CHD mortality among diabetic and nondiabetic women 1972–2001. D: CHD mortality among diabetic and nondiabetic men 1972–2001.
CONCLUSIONS
The excess mortality risk for diabetic than for nondiabetic subjects was low in our study and especially so for men. Nondiabetic but not diabetic subjects had significantly improved survival during the study period. However, diabetic men had substantially lower CVD mortality, particularly CHD mortality, during the third compared with the previous decades, whereas diabetic women had a slower rate of improvement. The improved survival for nondiabetic subjects was due to reduced mortality from CVD, malignant disease, and all other causes. Diabetic subjects showed no such improvement over the study period. Together with the unchanged CVD mortality, this constituted the basis of the lack of improvement of survival for the diabetic subjects. However, an interesting and promising finding was that diabetic men had as good an improvement in survival as nondiabetic men regarding CVD and CHD mortality. This was not true for diabetic women, although a positive tendency was seen (Table 3).
The reduced CVD and CHD mortality among men but not among women with diabetes, was similar to what others have found (12,18). On the other hand, other studies showed the opposite, a higher mortality rate among men (2,7), and still others found no sex differences (11,15). The lack of improvement among women in our study may have several explanations ranging from sex differences in care provision and/or care adherence, to the diabetes disease itself, which has been claimed to have a greater adverse affect on CVD risk factors in women than in men (19).
Several factors may explain the decrease in mortality among nondiabetic subjects over the last decades. These include successful primary prevention of CVD risk factors such as smoking, improved lipid and blood pressure treatments, and accompanying lifestyle changes in addition to improvements in life-saving technologies for those developing acute CHD over the years.
All of these improvements in CVD mortality in the nondiabetic population may also be seen in the diabetic population, but they occur later and are not of the same order of magnitude and diabetic women lag behind diabetic men. Some possible explanations for this unfavorable development might be that diabetic subjects are smokers and overweight or obese to a larger extent than nondiabetic subjects and do not reach the treatment goals for blood pressure, lipids, and A1C according to the guidelines (20). A further negative circumstance for diabetic compared with nondiabetic subjects and especially so for diabetic women is their worse prognosis after an acute myocardial infarction (21).
Good scientific evidence about the effects of treatment of hyperglycemia, high blood pressure and dyslipidemia came fairly late in our study with the UK Prospective Diabetes Study (UKPDS) (22,23). The UKPDS was followed by several other reports: one meta-analysis showing the advantages of treating hypercholesterolemia (24) and another focusing on multifactorial intervention (25). It will take some time before these findings are implemented in clinical practice widely enough to have an impact on the health and survival of diabetic subjects.
When interpreting the improvements in CVD mortality among diabetic subjects, one should keep in mind the case-finding strategies started in 1983. A possible lead time bias introduced by these strategies might have affected the three diabetes cohorts differently. Subjects with diabetes diagnosed in the last compared with the first decade were younger and had lower fasting blood glucose, indicating a more favorable prognosis. However, taking the influence of these factors into account in the analyses did not affect the result.
The strengths of this study include the fact that all incident diabetic subjects in a defined geographical area were identified using uniform diagnostic criteria and followed over a long time period. All Swedish residents have a unique 12-digit personal identification number, which is an excellent and highly reliable tool for record linkage. Patient fees for hospital outpatient and general practice appointments are heavily subsidized by central and local governments, which means that private financial resources are seldom an obstacle to health care utilization. These factors, together with excellent continuity in the diabetes care given, may be one explanation for the low excess mortality risk seen in our study compared with what others have found (6,7,11,15).
Moreover, the absence of known diabetes in the reference group makes the risk estimates in our study a forceful representation of the true risk. Furthermore, in our study, as opposed to many other studies, all incident diabetic subjects regardless of age were included. The mortality data were based on the official Cause of Death Register and were 100% complete.
The limitations of the study include the fairly small study area with an ethnically homogeneous Caucasian population. As a result, generalizations to ethnically different populations should be made cautiously. Furthermore, nondiabetic subjects sampled for the period 1972–1982 who died before the opportunistic case finding procedures started in 1983 may have included some subjects with undiagnosed diabetes, causing a dilution of the mortality comparisons for this period.
In summary, the novel findings in this study were the low excess mortality risk for diabetic than for nondiabetic subjects. Nondiabetic but not diabetic subjects had a significant all-cause, CVD, and CHD mortality reduction during the 33-year follow-up whereas diabetic men but not women had a significant average reduction of CVD and especially CHD mortality rates.
Acknowledgments
This study was supported by grants from the Primary Health Care Research Unit, Örebro, the Örebro County Council Research Unit, the Family Medicine Research Centre, Örebro University, and Uppsala University.
S.P.O.J. has received honoraria for lecturing engagements from AstraZeneca, GlaxoSmithKline, Novo Nordisk, and sanofi-aventis. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Muggeo M, Verlato G, Bonora E, Bressan F, Girotto S, Corbellini M, Gemma ML, Moghetti P, Zenere M, Cacciatori V: The Verona diabetes study: a population-based survey on known diabetes mellitus prevalence and 5-year all-cause mortality. Diabetologia 1995;38:318–325 [DOI] [PubMed] [Google Scholar]
- 2.de Fine Olivarius N, Andreasen AH: Five-year all-cause mortality of 1323 newly diagnosed middle-aged and elderly diabetic patients. Data from the population-based study, diabetes care in general practice, Denmark. J Diabetes Complications 1997;11:83–89 [DOI] [PubMed] [Google Scholar]
- 3.Morgan CL, Currie CJ, Peters JR: Relationship between diabetes and mortality: a population study using record linkage. Diabetes Care 2000;23:1103–1107 [DOI] [PubMed] [Google Scholar]
- 4.Eberly LE, Cohen JD, Prineas R, Yang L: Intervention Trial Research Group. Impact of incident diabetes and incident nonfatal cardiovascular disease on 18-year mortality: the multiple risk factor intervention trial experience. Diabetes Care 2003;26:848–854 [DOI] [PubMed] [Google Scholar]
- 5.Berger B, Stenström G, Sundkvist G: Incidence, prevalence, and mortality of diabetes in a large population. A report from the Skaraborg Diabetes Registry. Diabetes Care 1999;22:773–778 [DOI] [PubMed] [Google Scholar]
- 6.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA: Mortality in people with type 2 diabetes in the UK. Diabet Med 2006;23:516–521 [DOI] [PubMed] [Google Scholar]
- 7.Gu K, Cowie CC, Harris MI: Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 1998;21:1138–1145 [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Reddy S, Ounpuu S, Anand S: Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104:2746–2753 [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Reddy S, Ounpuu S, Anand S: Global burden of cardiovascular diseases: part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001;104:2855–2864 [DOI] [PubMed] [Google Scholar]
- 10.Rosén M: Chapter 5.1: major public health problems—cardiovascular diseases. Scand J Public Health Suppl 2006;67:51–58 [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino RB, Sr, Wilson PW, Savage PJ: Trends in cardiovascular complications of diabetes. JAMA 2004;292:2495–2499 [DOI] [PubMed] [Google Scholar]
- 12.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC: Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 13.Lipscombe LL, Hux JE: Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: a population-based study. Lancet 2007;369:750–756 [DOI] [PubMed] [Google Scholar]
- 14.Carstensen B, Kristensen JK, Ottosen P, Borch-Johnsen K: Steering Group of the National Diabetes Register. The Danish National Diabetes Register: trends in incidence, prevalence and mortality. Diabetologia 2008;51:2187–2196 [DOI] [PubMed] [Google Scholar]
- 15.Dale AC, Vatten LJ, Nilsen TI, Midthjell K, Wiseth R: Secular decline in mortality from coronary heart disease in adults with diabetes mellitus: cohort study. BMJ 2008;337:a236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliasson M, Talbäck M, Rosén M: Improved survival in both men and women with diabetes between 1980 and 2004—a cohort study in Sweden. Cardiovasc Diabetol 2008;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson SP, Andersson DK, Svärdsudd K: Prevalence and incidence rate of diabetes mellitus in a Swedish community during 30 years of follow-up. Diabetologia 2007;50:703–710 [DOI] [PubMed] [Google Scholar]
- 18.Gu K, Cowie CC, Harris MI: Diabetes and decline in heart disease mortality in US adults. JAMA 1999;281:1291–1297 [DOI] [PubMed] [Google Scholar]
- 19.Howard BV, Cowan LD, Go O, Welty TK, Robbins DC, Lee ET: Adverse effects of diabetes on multiple cardiovascular disease risk factors in women. The Strong Heart Study. Diabetes Care 1998;21:1258–1265 [DOI] [PubMed] [Google Scholar]
- 20.Eliasson B, Cederholm J, Nilsson P, Gudbjörnsdóttir S: Steering Committee of the Swedish National Diabetes Register. The gap between guidelines and reality: type 2 diabetes in a National Diabetes Register 1996–2003. Diabet Med 2005;22:1420–1426 [DOI] [PubMed] [Google Scholar]
- 21.Gitt AK, Schiele R, Wienbergen H, Zeymer U, Schneider S, Gottwik MG, Senges J: Intensive treatment of coronary artery disease in diabetic patients in clinical practice: results of the MITRA study. Acta Diabetol 2003;40(Suppl. 2):S343–S347 [DOI] [PubMed] [Google Scholar]
- 22.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 23.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 24.Cholesterol Treatment Trialists' (CTT) Collaborators. Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C: Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]
- 25.Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]