Abstract
OBJECTIVE
To assess the relative contribution of increased fasting and postload plasma glucose concentrations to the incidence of type 2 diabetes in subjects with a normal 2-h plasma glucose concentration.
RESEARCH DESIGN AND METHODS
A total of 3,450 subjects with 2-h plasma glucose concentration <140 mg/dl at baseline were followed up in the San Antonio Heart Study (SAHS) and the Botnia Study for 7–8 years. The incidence of type 2 diabetes at follow-up was related to the fasting, 1-h, and 2-h plasma glucose concentrations.
RESULTS
In subjects with 2-h plasma glucose <140 mg/dl, the incidence of type 2 diabetes increased with increasing fasting plasma glucose (FPG) and 1-h and 2-h plasma glucose concentrations. In a multivariate logistic analysis, after adjustment for all diabetes risk factors, the FPG concentration was a strong predictor of type 2 diabetes in both the SAHS and the Botnia Study (P < 0.0001). However, when the 1-h plasma glucose, but not 2-h plasma glucose, concentration was added to the model, FPG concentration was no longer a significant predictor of type 2 diabetes in both studies (NS). When subjects were matched for the level of 1-h plasma glucose concentration, the incidence of type 2 diabetes markedly increased with the increase in 1-h plasma glucose, but the increase in FPG was not associated with a significant increase in the incidence of type 2 diabetes.
CONCLUSIONS
An increase in postload glycemia in the normal range is associated with an increase in the incidence of type 2 diabetes. After controlling for 1-h plasma glucose concentration, the increase in FPG concentration is not associated with an increase in the incidence of type 2 diabetes.
Impaired fasting glucose (IFG) was introduced in 1997 by the American Diabetes Association (ADA) (1), and, analogous with impaired glucose tolerance (IGT), it was meant to represent an intermediate stage in the transition from normal glucose tolerance (NGT) to overt type 2 diabetes. Both IFG and IGT indicate an increased risk for future type 2 diabetes (2–4). Previously (5–7), we have shown that the 1-h plasma glucose concentration has better predictive power than either fasting plasma glucose (FPG) or 2-h plasma glucose, suggesting that the 1-h plasma glucose concentration may have greater utility in identifying subjects at increased risk for type 2 diabetes in routine clinical practice.
Previous studies have reported that IFG and IGT represent separate clinical entities, which are characterized by distinct metabolic abnormalities (8–13). Subjects with IGT manifest insulin resistance in skeletal muscle (9–12) and impaired β-cell function (both early and late phases of insulin secretion) (10,14–16), whereas subjects with IFG are characterized by increased hepatic insulin resistance (9,16), impaired early insulin response (12), and decreased non–insulin-dependent glucose clearance (15). Because of the prominent role of progressive β-cell failure in the development of hyperglycemia (17), the impairment in β-cell function in subjects with IGT represents a major pathogenic factor for their increased risk for future type 2 diabetes. Although the increase in fasting plasma glucose is associated with a decrease in first-phase insulin secretion (11–13,18), subjects with IFG have robust second-phase insulin secretion, and, when related to their prevailing level of insulin resistance, they have second-phase insulin secretion comparable with that of subjects with NGT (12,13). Thus, impaired β-cell function cannot fully explain the increased incidence of type 2 diabetes associated with the increase in FPG concentration, e.g., in subjects with isolated IFG.
Previously we have shown a strong correlation between insulin resistance in skeletal muscle and liver (16). Thus, a strong correlation between FPG and postload plasma glucose concentrations is anticipated. Therefore, we hypothesized that the increased type 2 diabetes risk associated with the increase in FPG, at least in part, is due to the increased postprandial plasma glucose concentration associated with the increase in FPG and is not due to the increase in FPG per se. The aim of this study was to test this hypothesis.
RESEARCH DESIGN AND METHODS
Subjects were participants in the San Antonio Heart Study (SAHS) (19–21) and the Botnia Study (22), who were free of diabetes at baseline. The two studies are prospective longitudinal studies in which nondiabetic subjects (Caucasian and Mexican American in the SAHS and Caucasian in the Botnia Study) were recruited and followed for 7–8 years. Detailed descriptions of the Botnia Study and SAHS were published previously (19–22). Only subjects with 2-h plasma glucose concentrations <140 mg/dl were included in this study. Table 1 presents the baseline patient characteristics. All subjects completed a 7- to 8-year follow-up examination and had their diabetes outcome determined with a repeat oral glucose tolerance test (OGTT).
Table 1.
Baseline patient characteristics in the SAHS and Botnia Study
| SAHS | Botnia Study | P | |
|---|---|---|---|
| n | 1,390 | 2,060 | <0.0001 |
| Age (years) | 43 ± 03 | 45 ± 0.3 | <0.0001 |
| Sex (% male) | 56 | 52.9 | <0.0001 |
| BMI | 27.3 ± 0.2 | 25.5 ± 0.1 | <0.0001 |
| Systolic blood pressure (mmHg) | 117 ± 0.4 | 126 ± 0.4 | <0.0001 |
| Diastolic blood pressure (mmHg) | 71 ± 0.3 | 94 ± 0.2 | <0.0001 |
| FPG (mg/dl) | 85 ± 0.3 | 99 ± 0.2 | <0.0001 |
| 1-h plasma glucose (mg/dl) | 125 ± 1 | 133 ± 0.5 | <0.0001 |
| 2-h plasma glucose (mg/dl) | 96 ± 0.6 | 104 ± 0.5 | <0.0001 |
| HDL (mg/dl) | 47 ± 0.4 | 54 ± 0.6 | <0.0001 |
| Triglycerides (mg/dl) | 132 ± 2 | 108 ± 2 | <0.0001 |
| Type 2 diabetes incidence (%) | 6.33 | 3.64 | <0.0001 |
During the baseline studies, data for clinical and anthropometric parameters (age, sex, BMI, and ethnicity) were collected. Blood pressure and lipid profile were measured. In addition, all subjects underwent a 75-g OGTT after a 12-h overnight fast. Plasma glucose and serum insulin concentrations were measured at 0, 30, 60, and 120 min. After 7–8 years of follow-up (mean of 7.6 years in Botnia Study participants and 7.2 years in the SAHS participants), a repeat OGTT was performed, and the diagnosis of diabetes was based on ADA criteria: 2-h plasma glucose 200 mg/dl or FPG 126 mg/dl (1). Plasma glucose was measured by the glucose oxidase method using a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA) in both studies.
Data analysis and statistical methods
To assess the contribution of FPG to the risk of type 2 diabetes, a multivariate logistic model was constructed and included the incidence of type 2 diabetes at follow-up as the dependent variable and FPG, age, sex, BMI, ethnicity, family history for type 2 diabetes, HDL cholesterol, and systolic blood pressure as the independent variables. To assess the impact of postload plasma glucose concentration on type 2 diabetes risk, the 1-h plasma glucose or the 2-h plasma glucose concentration was added to the model.
To further assess the impact of 1-h plasma glucose concentration compared with FPG on the future risk of type 2 diabetes, we pooled the subjects from the two studies, and subjects were divided based on their FPG into two groups: 1) NGT (FPG <100 mg/dl) and 2) IFG (FPG 100–125 mg/dl). Subjects in each group subsequently were divided into subgroups with matched 1-h plasma glucose. The pooling of the subjects from the two studies (rather than making the subdivision in each study separately) was performed to increase the number of subjects in each subgroup. Because subjects received similar tests at baseline and were followed for a similar period of time, pooling the subjects from the two studies should not introduce a bias to the study. To obtain subgroups with comparable postload plasma glucose levels, subjects with normal fasting glucose and IFG subjects were divided into subgroups based on their 1-h plasma glucose concentration (1-h plasma glucose <100, 100–124, 125–149, 150–175, 175–200, and >200 mg/dl), and the incidence of type 2 diabetes in each group was related to the 1-h plasma glucose concentration. Interestingly, none of the subjects with FPG <100 mg/dl had a 1-h plasma glucose >200 mg/dl. Thus, the subgroup with FPG 100–125 mg/dl and 1-h plasma glucose >200 mg/dl does not have a matched group with FPG <100 mg/dl and 1-h plasma glucose >200 mg/dl.
Variables are presented as means ± SD. The significance of the mean differences was tested with ANOVA. Differences among categorical variables were tested with the χ2 test. Statistical significance was considered at the level of P < 0.05. Statistical analysis was performed with SPSS (version 17; SPSS, Chicago, IL).
RESULTS
The crude incidence rate of type 2 diabetes over 7–8 years of follow-up was greater in the SAHS than in the Botnia Study (6.3 vs. 3.6%, respectively, P = 0.0005). In the SAHS, the crude incidence of type 2 diabetes was ethnicity dependent, with Mexican Americans having a greater incidence of type 2 diabetes than Caucasians (7.9 vs. 3.5%, P = 0.002).
In a multivariate logistic model that included, in addition to FPG concentration, age, sex, BMI, family history of type 2 diabetes, ethnicity, HDL cholesterol, and systolic blood pressure as independent variables, only age, BMI, and ethnicity were significant predictors in the SAHS, and age, BMI, and systolic blood pressure were significant predictors in the Botnia Study (Table 2). FPG concentration was a strong predictor of type 2 diabetes in both studies (P < 0.0001). To assess whether the strong predictive power of FPG is related to the increase in FPG per se or to the strong correlation between FPG and postload plasma glucose concentration, we added 1-h plasma glucose and 2-h plasma glucose concentration to the logistic model. When the 1-h plasma glucose was added to the model, only BMI and ethnicity remained significant predictors in the SAHS and age and BMI in the Botnia Study (Table 2). FPG no longer was a significant predictor in either study, whereas the 1-h plasma glucose was the strongest predictor for type 2 diabetes (P < 0.0001). However, when 2-h plasma glucose was added to the model, it did not significantly affect the predictive power of FPG, age, BMI, ethnicity, and systolic blood pressure (Table 2).
Table 2.
Multivariate logistic model for the future risk of type 2 diabetes as the dependent variable and FPG, age, sex, BMI, ethnicity, family history for type 2 diabetes, blood pressure, and HDL cholesterol as the independent variables (model 1)
| SAHS |
Botnia Study |
|||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95% CI) | P | |
| Model 1 | ||||
| Age | 1.03 (1.004–1.05) | 0.01 | 1.04 (1.02–1.06) | 0.001 |
| BMI | 1.1 (1.06–1.14) | <0.0001 | 1.1 (1.04–1.18) | 0.002 |
| Ethnicity | 0.53 (0.3–0.93 | 0.02 | NA | |
| Systolic blood pressure | 1.01 (0.997–1.03) | NS | 1.02 (1.002–1.03) | 0.02 |
| FPG | 1.06 (1.04–1.08) | <0.0001 | 1.05 (1.02–1.08) | <0.0001 |
| Model 2 | ||||
| 1-h plasma glucose | 1.03 (1.02–1.04) | <0.0001 | 1.02 (1.01–1.03) | <0.0001 |
| Age | 1.02 (0.99–1.04) | NS | 1.04 (1.01–1.06) | 0.004 |
| BMI | 1.08 (1.04–1.13) | <0.0001 | 1.07 (1.006–1.14) | 0.03 |
| Ethnicity | 0.46 (0.256–0.83) | <0.01 | NA | |
| Systolic blood pressure | 1.0 (0.98–1.02) | NS | 1.01 (0.99–1.03) | NS |
| FPG | 1.02 (0.99–1.05) | NS | 1.02 (0.99–1.06) | NS |
| Model 3 | ||||
| 2-h plasma glucose | 1.02 (1.004–1.03) | 0.007 | 1.04 (0.8–1.35) | NS |
| Age | 1.02 (1.001–1.05) | 0.04 | 1.04 (1.015–1.06) | 0.001 |
| BMI | 1.09 (1.05–1.13) | <0.0001 | 1.09 (1.02–1.16) | 0.01 |
| Ethnicity | 0.58 (0.316–0.985) | 0.04 | NA | |
| Systolic blood pressure | 1.0 (0.98–1.02) | NS | 1.02 (1.01–1.03) | 0.02 |
| FPG | 1.05 (1.03–1.08) | <0.0001 | 1.05 (1.02–1.08) | 0.001 |
Data are ORs (95% CI) for the variables that were significant predictors of type 2 diabetes risk. In model 2, the 1-h plasma glucose concentration during the OGTT was added to model 1, and in model 3, the 2-h plasma glucose concentration during the OGTT was added to the model.
To further examine the contribution of FPG and 1-h plasma glucose to type 2 diabetes risk, we combined subjects in the SAHS and Botnia Study and divided subjects with IFG and normal fasting glucose into subgroups matched in their 1-h plasma glucose concentration. The 7- to 8-year incidence of type 2 diabetes in the whole population increased progressively with increasing FPG, 1-h plasma glucose, and 2-h plasma glucose concentrations. When subjects were divided into 10 equal deciles, the incidence of type 2 diabetes markedly increased in deciles 9 and 10 compared with deciles 1–8 (8.1 vs. 3.9, 14.5 vs. 2.3, and 8.6 vs. 3.8% for FPG, 1-h plasma glucose, and 2-h plasma glucose, respectively; all P < 0.0001).
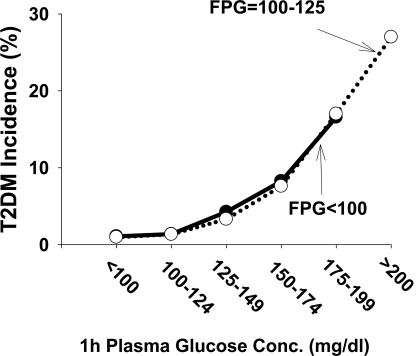
To further assess the impact of 1-h plasma glucose on the predictive power of FPG, we divided the subjects into two groups based on FPG: 1) FPG <100 mg/dl and 2) FPG 100–125 mg/dl. To control for the level of postload hyperglycemia, we divided the subjects in each group into six subgroups with comparable 1-h plasma glucose values. Figure 1 shows that in subjects with IFG and NGT, the incidence of type 2 diabetes rose progressively with the increase in 1-h plasma glucose concentration. However, at any given level of 1-h plasma glucose concentration, the incidence of type 2 diabetes in subjects with IFG was comparable to that in subjects with NGT.
Figure 1.
Seven- to 8-year incidence of type 2 diabetes (T2DM) in subjects with normal fasting glucose (FPG <100 mg/dl) and impaired fasting glucose (FPG 100–125 mg/dl). Subjects were divided into six groups according to fixed intervals of 1-h plasma glucose concentration as follows: <100, 100–125, 125–150, 150–175, 175–200, and >200 mg/dl. The mean 1-h plasma glucose concentration (n) in subjects with FPG <100 mg/dl was 85 (652), 113 (732), 139 (634), 165 (206), and 192 (187) mg/dl and in subjects with FPG >110 mg/dl was 91 (104), 114 (224), 140 (333), 165 (158), 193 (169), and 241 (51) mg/dl, respectively. None of the subjects with FPG <100 mg/dl had a 1-h plasma glucose concentration >200 mg/dl.
Previous studies have reported that the decrease in first-phase insulin secretion begins at an FPG concentration well within the normal range (∼90 mg/dl) (18,23). To examine whether the increased incidence of type 2 diabetes begins at lower FPG concentrations, we divided subjects with NGT into three subgroups (FPG <90, 90–100, and >100 mg/dl) and compared their incidence of type 2 diabetes with that in subjects with IFG. Subjects in these groups were further subdivided into three groups based on their 1-h plasma glucose concentration: <125, 125–150, and >150 mg/dl (Table 3). Again, at any given level of 1-h plasma glucose concentration, the incidence of type 2 diabetes was independent of the FPG concentration and, in each group of FPG values, it rose with increasing 1-h plasma glucose concentration.
Table 3.
Incidence of type 2 diabetes in subjects with NGT divided into three groups based on FPG
| FPG (mg/dl) | 1-h PG (mg/dl) | n (% of SAHS participants) | FPG (mg/dl) | 1-h PG (mg/dl) | 2-h PG (mg/dl) | OR (95% CI) |
|---|---|---|---|---|---|---|
| <90 | <125 | 894 (72) | 81 ± 1 | 95 ± 26 | 87 ± 11 | 1 |
| 125–150 | 286 (73) | 82 ± 6 | 135 ± 10 | 100 ± 22 | 4.7 (2.0–11.1) | |
| >150 | 219 (72) | 83 ± 6 | 175 ± 19 | 109 ± 21 | 7.4 (3.3–17.1) | |
| 90–100 | <125 | 489 (29) | 95 ± 3 | 99 ± 14 | 94 ± 18 | 1.8 (0.6–5.2) |
| 125–150 | 255 (30) | 95 ± 3 | 136 ± 10 | 105 ± 19 | 2.7 (1.1–6.9) | |
| >150 | 265 (34) | 96 ± 3 | 180 ± 18 | 111 ± 20 | 11.3 (5.0–25.8) | |
| >100 | <125 | 329 (7) | 106 ± 5 | 102 ± 12 | 101 ± 17 | 1.7 (0.4–6.7) |
| 125–150 | 277 (9) | 107 ± 5 | 137 ± 10 | 108 ± 19 | 4.0 (1.3–12.5) | |
| >150 | 432 (10) | 109 ± 6 | 188 ± 27 | 114 ± 18 | 17.7 (7.5–41.9) |
Data are means ± SD unless indicated otherwise. Subjects with NGT were divided into three groups based on FPG <90, 90–100, and >100 mg/dl. Subjects in each group were further subdivided based on a 1-h plasma glucose (PG) concentration <125, 125–155, and >155 mg/dl. Age, BMI, ethnicity, and systolic blood pressure were included as covariates in the model.
CONCLUSIONS
The major finding of the present study is that, after controlling for the level of postload hyperglycemia, e.g., 1-h plasma glucose concentration, the increase in FPG concentration in subjects with NGT and IFG is not associated with a significant increase in the incidence of type 2 diabetes (Fig. 1, Table 3). When subjects were matched for their 1-h plasma glucose concentration (Table 1), an increase in FPG concentration from <90 up to 100–125 mg/dl (i.e., impaired fasting glucose) is not associated with a significant increase in the incidence of type 2 diabetes. This observation was consistent in subjects with low postload hyperglycemia (e.g., 1-h plasma glucose <125 mg/dl) and also in subjects with a high level of postload hyperglycemia (e.g., 1-h plasma glucose >150 mg/dl). Conversely, after we controlled for other risk factors, the increase in postload hyperglycemia (measured with 1-h plasma glucose) was associated with a marked increase in the incidence of type 2 diabetes, independent of the level of FPG concentration (Fig. 1, Table 3). Our results indicate that an increase in postload hyperglycemia, in the range considered to represent normal glucose tolerance (2-h plasma glucose <140 mg/dl), is the principal risk factor responsible for the increased incidence of type 2 diabetes. In subjects with 2-h plasma glucose <140 mg/dl, an increase in FPG concentration does not significantly contribute to an incidence of type 2 diabetes. For example, subjects with FPG <90 mg/dl and 1-h plasma glucose >150 mg/dl have an ∼13-fold elevated risk for developing diabetes compared with subjects with FPG 100–125 mg/dl and 1-h plasma glucose <125 mg/dl, despite comparable 2-h plasma glucose concentrations (Table 3).
Previous epidemiological studies have shown that subjects with isolated IFG have a significantly increased incidence of type 2 diabetes compared with that of individuals with NGT (2–4,8). However, in these studies, the level of postload hyperglycemia was not taken into consideration. Indeed, in the present study, the incidence of type 2 diabetes in subjects with IFG was significantly greater than that in subjects with NGT. However, after controlling for the level of postload hyperglycemia, measured with the 1-h plasma glucose concentration, the increase in FPG concentration was not significantly associated with an increased incidence of type 2 diabetes. Thus, our results indicate that the increased incidence of type 2 diabetes in subjects with IFG compared with those with NGT is due to inclusion of subjects with postload hyperglycemia, i.e., subjects with 1-h plasma glucose >150 mg/dl, and is not due to the increase in FPG per se. The results of the multivariate logistic analysis also support this conclusion. Although in a model including all type 2 diabetes risk factors, FPG was a strong predictor of type 2 diabetes risk in both studies, after inclusion of 1-h plasma glucose in the logistic model, FPG no longer was a significant predictor of type 2 diabetes risk. This observation suggests that the future type 2 diabetes risk associated with increased FPG is due primarily to its correlation with the 1-h plasma glucose concentration. These results are consistent with previous studies reporting that the 1-h plasma glucose concentration is a strong predictor of type 2 diabetes risk, independent of FPG and 2-h plasma glucose concentrations, and is a stronger predictor than measures taken during the fasting state, e.g., FPG and A1C (6).
We hypothesize that the strong correlation between insulin resistance in skeletal muscle and liver (16) is responsible, in part, for the association between increased postload hyperglycemia and elevated FPG concentration. Indeed, the 1-h plasma glucose concentration correlated well with the FPG concentration (r = 0.37, P < 0.0001).
Progressive β-cell failure is the principal factor responsible for the development and progression of type 2 diabetes (17). In previous studies, we have shown that β-cell failure develops at 2-h plasma glucose concentrations well within the normal range and that the severity of β-cell failure is inversely related to the 2-h plasma glucose concentration (24). For example, subjects with a 2-h plasma glucose concentration between 120 and 139 mg/dl have an ∼40–50% decrease in β-cell function compared with subjects with a 2-h plasma glucose concentration <100 mg/dl. This range of postload hyperglycemia is comparable to that of subjects studied in the present study. The decrease in β-cell function associated with the increase in postload hyperglycemia in this range would be expected to contribute to the increased diabetes incidence associated with the rise in postload hyperglycemia.
The second important finding of this study is that the rise in postload glycemia in the range considered to be normal (2-h plasma glucose <140 mg/dl) is associated with a significant increase in the incidence of type 2 diabetes (Fig. 1, Table 3). This increased diabetes incidence was observed both in subjects with NGT and IFG. When postload glycemia is evaluated using the 1-h plasma glucose concentration, the increase in incidence of type 2 diabetes becomes evident at a 1-h plasma glucose level of 125 mg/dl and increases markedly as the 1-h plasma glucose concentration exceeds 150 mg/dl, independent of the FPG concentration (Fig. 1). The marked increase in the incidence of type 2 diabetes associated with an increase in 1-h plasma glucose concentration >150 mg/dl is consistent with previous studies in which a 1-h plasma glucose of 155 mg/dl identified subjects at high risk for future type 2 diabetes (6,7).
Previous epidemiological studies have reported that, in absolute numbers, ∼25–40% of subjects who develop type 2 diabetes have a 2-h plasma glucose <140 mg/dl at baseline (2–4,8). Our results are consistent with these observations and extend them by demonstrating that the development of type 2 diabetes in this group considered to have normal glucose tolerance is related to the increase in postload glycemia.
The third important finding of this study is that the 1-h plasma glucose concentration is a better measure for future type 2 diabetes risk than the 2-h plasma glucose concentration. Subjects with a 2-h plasma glucose <140 mg/dl and FPG <100 mg/dl, who according to the ADA criteria (1) have NGT, could be stratified into three risk groups for future development of type 2 diabetes based on their 1-h plasma glucose concentration, and subjects with a 1-h plasma glucose >155 mg/dl had a 13.1-fold increased odds ratio (OR) for type 2 diabetes. Thus, this group of subjects with “normal glucose tolerance” has a risk for future type 2 diabetes comparable to that of subjects with IFG and IGT and should be considered to have “glucose intolerance.” IFG and IGT categories were introduced to represent an intermediate stage in the transition from NGT to type 2 diabetes and identify subjects with increased risk for future type 2 diabetes (1). The results of the present study highlight the need either to revise the current ADA cut points for defining glucose intolerance or to change the criteria for NGT.
Acknowledgments
L.G. has been a consultant for and served on advisory boards for sanofi-aventis, GlaxoSmithKline, Novartis, Merck, Tethys Bioscience, and Xoma and received lecture fees from Lilly and Novartis. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 2. Shaw JE, Zimmet PZ, de Courten M, Dowse GK, Chitson P, Gareeboo H, Hemraj F, Fareed D, Tuomilehto J, Alberti KG: Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care 1999;22:399–402 [DOI] [PubMed] [Google Scholar]
- 3. Unwin N, Shaw J, Zimmet P, Alberti KG: Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 4. Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, Yazdi H, Booker L: Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–312 [DOI] [PubMed] [Google Scholar]
- 5. Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M: What is the best predictor for future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 6. Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L: Fasting versus post load plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 2009;32:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA: One hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contribution of β cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 9. Abdul-Ghani MA, Jenkinson C, Richardson D, DeFronzo RA: Insulin secretion and insulin action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study (VEGAS). Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 10. Weyer C, Bogardus C, Pratley RE: Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999;48:2197–2203 [DOI] [PubMed] [Google Scholar]
- 11. Wasada T, Kuroki H, Katsumori K, Arii H, Sato A, Aoki K: Who are more insulin resistant, people with IFG or people with IGT? Diabetologia 2004;47:758–759 [DOI] [PubMed] [Google Scholar]
- 12. Abdul-Ghani MA, Jenkinson C, Richardson D, DeFronzo RA: Impaired early but not late phase insulin secretion in subjects with impaired fasting glucose. Eur J Clin Invest. In press [DOI] [PubMed] [Google Scholar]
- 13. Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J: Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006;29:1909–1914 [DOI] [PubMed] [Google Scholar]
- 14. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Pettiti M, Natali A, Mari A, DeFronzo RA: Predominant role of reduced β-cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia 2003;46:1211–1219 [DOI] [PubMed] [Google Scholar]
- 15. Jani R, Molina M, Matsuda M, Balas B, Chavez A, DeFronzo RA, Abdul-Ghani M: Decreased non-insulin-dependent glucose clearance contributes to the rise in FPG in the non-diabetic range. Diabetes Care 2008;31:311–315 [DOI] [PubMed] [Google Scholar]
- 16. Abdul-Ghani MA, Matsuda M, DeFronzo RA: Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med 2008;25:1289–1294 [DOI] [PubMed] [Google Scholar]
- 17. Kahn SE: The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 18. Godsland IF, Jeffs JA, Johnston DG: Loss of β cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–1166 [DOI] [PubMed] [Google Scholar]
- 19. Stern MP, Rosenthal M, Haffner SM, Hazuda HP, Franco LJ: Sex difference in the effects of sociocultural status on diabetes and cardiovascular risk factors in Mexican Americans: the San Antonio Heart Study. Am J Epidemiol 1984;120:834–851 [DOI] [PubMed] [Google Scholar]
- 20. Stern MP, Patterson JK, Haffner SM, Hazuda HP, Mitchell BD: Lack of awareness and treatment of hyperlipidemia in type II diabetes in a community survey. JAMA 1989;262:360–364 [PubMed] [Google Scholar]
- 21. Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP: Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med 1999;159:1450–1456 [DOI] [PubMed] [Google Scholar]
- 22. Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissén M, Ehrnström BO, Forsén B, Isomaa B, Snickars B, Taskinen MR: Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 1996;45:1585–1593 [DOI] [PubMed] [Google Scholar]
- 23. Abdul-Ghani MA, Matsuda M, Jenkinson C, Richardson D, Kaku K, DeFronzo RA: The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol 2008;295:E401–E406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004;47:31–39 [DOI] [PubMed] [Google Scholar]