Abstract
OBJECTIVE
We examined prevalences of previously diagnosed diabetes and undiagnosed diabetes and high risk for diabetes using recently suggested A1C criteria in the U.S. during 2003–2006. We compared these prevalences to those in earlier surveys and those using glucose criteria.
RESEARCH DESIGN AND METHODS
In 2003–2006, the National Health and Nutrition Examination Survey included a probability sample of 14,611 individuals aged ≥12 years. Participants were classified on glycemic status by interview for diagnosed diabetes and by A1C, fasting, and 2-h glucose challenge values measured in subsamples.
RESULTS
Using A1C criteria, the crude prevalence of total diabetes in adults aged ≥20 years was 9.6% (20.4 million), of which 19.0% was undiagnosed (7.8% diagnosed, 1.8% undiagnosed using A1C ≥6.5%). Another 3.5% of adults (7.4 million) were at high risk for diabetes (A1C 6.0 to <6.5%). Prevalences were disproportionately high in the elderly. Age-/sex-standardized prevalence was more than two times higher in non-Hispanic blacks and Mexican Americans versus non-Hispanic whites for diagnosed, undiagnosed, and total diabetes (P < 0.003); standardized prevalence at high risk for diabetes was more than two times higher in non-Hispanic blacks versus non-Hispanic whites and Mexican Americans (P < 0.00001). Since 1988–1994, diagnosed diabetes generally increased, while the percent of diabetes that was undiagnosed and the percent at high risk of diabetes generally decreased. Using A1C criteria, prevalences of undiagnosed diabetes and high risk of diabetes were one-third that and one-tenth that, respectively, using glucose criteria.
CONCLUSIONS
Although A1C detects much lower prevalences than glucose criteria, hyperglycemic conditions remain high in the U.S., and elderly and minority groups are disproportionately affected.
The A1C test has recently been recommended for diagnosing diabetes, based on a detailed analysis of its attributes by an international expert committee (1). Laboratory-measured A1C is now as accurate and precise as glucose assays due to improvements in instrumentation and standardization. A1C samples can be obtained at any time, require no patient preparation, and are relatively stable at room temperature after collection. A1C has substantially less biologic variability and is unaffected by acute effects of stress or illness. As a measure of long-term glycemic exposure, A1C has been shown to be better and more consistently correlated with retinopathy in the setting of observational studies and clinical trials in type 1 and type 2 diabetic patients, which have established widely accepted A1C treatment goals for diabetes. A cut point of ≥6.5% for the diagnosis of diabetes was recommended by the committee as optimal for detecting a level of retinopathy thought to be diabetes specific and not due to other conditions (e.g., hypertension). A limitation of A1C for diagnosis is that the committee could not define a specific intermediate threshold at which increased risk for diabetes clearly begins. While there is a continuum of risk even at values into the normal range, the committee suggested the range of ≥6.0 to <6.5% to represent the highest risk for progression to diabetes and one at which preventive measures might be implemented, with additional consideration of prevention efforts at lower levels in the presence of other risk factors. The committee hoped that its report would serve as a stimulus to the scientific community and professional organizations for considering the A1C assay for diagnosis of diabetes.
A change in diagnostic criteria has important public health implications pertaining to the magnitude of the population with diabetes or at high risk of diabetes. This report examines the prevalence of diagnosed and undiagnosed diabetes and high risk of diabetes based on self-report and A1C criteria in the U.S. population during 2003–2006. Prevalences are compared with those using the A1C criteria in 1988–1994 and 1999–2002. Finally, we compare the concordance in prevalence of undiagnosed diabetes using the new A1C criteria to criteria based on fasting plasma glucose and 2-h plasma glucose from an oral glucose tolerance test (OGTT).
RESEARCH DESIGN AND METHODS
The National Health and Nutrition Examination Survey (NHANES) 2003–2006 was conducted by the National Center for Health Statistics (2). NHANES is designed to be representative of the U.S. civilian noninstitutionalized population using a complex, multistage probability sample. Participants are interviewed in their homes and subsequently receive a physical and laboratory examination in a mobile examination center. Among eligible subjects in 2003–2006, 79.7% were interviewed and 76.7% were examined (2).
In 2003–2006, 14,611 individuals aged ≥12 years (77.0% of eligible) completed the household interview. Individuals were classified as having previously diagnosed diabetes if they answered affirmatively to the question of whether, other than during pregnancy, a doctor or health care professional had ever told them that they have diabetes. Of interviewed subjects, 13,970 individuals (95.6%) were subsequently examined after random assignment to either a morning or afternoon/evening session, during which an A1C was obtained. Of examined individuals, 13,094 individuals (93.7%) had valid responses to the diabetes interview question and a valid A1C value. Pregnant women (n = 610) were included, none of whom had undiagnosed diabetes based on A1C.
Beginning in 2005, the OGTT was performed in the NHANES after a decade hiatus. In 2005–2006, there were 2,050 individuals aged ≥20 years without diagnosed diabetes based on interview who were examined in the morning and asked to fast overnight. Fasting plasma glucose (FPG) values were obtained from 1,780 (86.8%) after fasting 8 to <24 h. These individuals then underwent an OGTT (2), consisting of a 75-g glucose-equivalent oral glucose challenge (Trutol) and a blood sample draw 2 h (±15 min) later. Exclusion criteria included known pregnancy, hemophilia, chemotherapy, refusal of phlebotomy, and inability/refusal to drink all of the Trutol. The 2-h glucose value was obtained for 1,508 individuals (91.5% of those eligible for the OGTT).
Procedures for blood collection and processing are described elsewhere (2). A1C was measured using whole blood at a central laboratory by a high-performance liquid chromatographic assay and standardized according to the method of the Diabetes Control and Complications Trial (3), with a coefficient of variation of 1.0–1.7% (2). Plasma glucose was measured using a hexokinase enzymatic method, with a coefficient of variation of 1.3–2.2% (2). Because there were changes to the equipment and laboratory that measured A1C and glucose in 2005–2006 since earlier NHANES surveys, values from 2005–2006 were converted via a linear transformation to make them comparable to values from NHANES III (1988–1994) and NHANES 1999–2004 (2).
A1C criteria recommended by the recent international expert committee report (1) were used to classify people without diagnosed diabetes as to whether they had undiagnosed diabetes (A1C ≥6.5%) or were at high risk for diabetes (A1C ≥6.0 to <6.5%). American Diabetes Association diagnostic criteria for diabetes were also used based on FPG ≥7.0 mmol/l and/or 2-h glucose ≥11.1 mmol/l (4).
Estimates for prevalence of diabetes and high risk for diabetes are compared with those from NHANES 1988–1994 and NHANES 1999–2002. These surveys used similar interview questions and collection and assay methods for blood specimens (2).
Prevalence estimates in Tables 1 and 2 were calculated among examined individuals with known diabetes status based on interview and with valid A1C, using examination sample weights; a similar percent of individuals with and without diagnosed diabetes had valid A1C values. Population counts of diabetes and high risk for diabetes were obtained by applying rates to the 2003–2006 U.S. population estimates (5). The comparison of prevalence of diabetes based on A1C to that based on FPG and 2-h glucose from an OGTT (Fig. 1) was calculated among individuals in the OGTT subsample in 2005–2006, using OGTT sample weights. These weights were calibrated so that the respective subsamples are representative of the full U.S. population (2).
Table 1.
Crude prevalence of diagnosed diabetes, undiagnosed diabetes (A1C ≥6.5%), total diabetes (diagnosed and undiagnosed combined), total diabetes that is undiagnosed, and at high risk for diabetes (A1C ≥6.0 to <6.5%), by age, sex, and race/ethnicity: NHANES 2003–2006 (n = 13,094)
| Diagnosed diabetes | Undiagnosed diabetes | Total diabetes | Total diabetes that is undiagnosed | At high risk for diabetes | |
|---|---|---|---|---|---|
| Combined age-groups (years) | |||||
| ≥12 | 6.8 (6.1–7.5) | 1.6 (1.2–1.9) | 8.4 (7.6–9.2) | 19.0 (15.2–22.7) | 3.1 (2.7–3.4) |
| ≥20 | 7.8 (7.0–8.6) | 1.8 (1.4–2.2) | 9.6 (8.7–10.5) | 19.0 (15.2–22.7) | 3.5 (3.0–3.9) |
| ≥65 | 17.7 (15.6–19.7) | 3.5 (2.6–4.3) | 21.1 (18.7–23.5) | 16.3 (12.9–19.8) | 8.1 (6.6–9.6) |
| Age-specific groups (years) | |||||
| 12–19 | 0.4 (0.2–0.7) | 0.1 (0.0–0.2) | 0.5 (0.2–0.8) | 17.5 (0.0–36.4) | 0.3 (0.1–0.5) |
| 20–39 | 1.9 (1.4–2.4) | 0.6 (0.3–0.9) | 2.5 (1.9–3.1) | 25.6 (16.3–35.0) | 0.7 (0.4–1.0) |
| 40–59 | 8.1 (6.9–9.4) | 2.0 (1.3–2.7) | 10.1 (8.7–11.5) | 19.5 (13.1–25.9) | 3.7 (3.1–4.4) |
| 60–74 | 17.6 (15.7–19.5) | 3.5 (2.1–4.9) | 21.1 (18.7–23.4) | 16.5 (10.7–22.2) | 7.1 (5.5–8.8) |
| ≥75 | 15.2 (12.9–17.6) | 3.5 (2.4–4.6) | 18.7 (16.6–20.9) | 18.8 (12.7–24.9) | 8.7 (7.2–10.2) |
| Sex by age (years) | |||||
| Men | |||||
| ≥12 | 6.5 (5.6–7.5) | 1.8 (1.2–2.4) | 8.3 (7.4–9.3) | 21.8 (15.2–28.4) | 3.1 (2.7–3.6) |
| ≥20 | 7.5 (6.4–8.5) | 2.1 (1.4–2.8) | 9.6 (8.5–10.6) | 21.8 (15.2–28.4) | 3.5 (3.0–4.1) |
| ≥65 | 18.1 (15.3–20.9) | 3.8 (2.2–5.3) | 21.8 (18.5–25.2) | 17.2 (11.2–23.2) | 8.3 (6.6–10.0) |
| Women | |||||
| ≥12 | 7.1 (6.2–7.9) | 1.4 (1.1–1.7) | 8.4 (7.4–9.4) | 16.3 (13.3–19.3) | 3.0 (2.4–3.6) |
| ≥20 | 8.0 (7.1–8.9) | 1.6 (1.2–1.9) | 9.6 (8.4–10.7) | 16.3 (13.3–19.3) | 3.4 (2.7–4.1) |
| ≥65 | 17.4 (14.8–20.0) | 3.2 (2.2–4.3) | 20.6 (17.5–23.7) | 15.7 (11.8–19.5) | 7.9 (6.0–9.9) |
| Race/ethnicity by age (years) | |||||
| Non-Hispanic white | |||||
| ≥12 | 6.2 (5.3–7.1) | 1.3 (0.8–1.8) | 7.5 (6.5–8.5) | 17.5 (12.1–22.9) | 2.7 (2.3–3.2) |
| ≥20 | 6.9 (6.0–7.9) | 1.5 (0.9–2.0) | 8.4 (7.3–9.5) | 17.5 (12.1–23.0) | 3.1 (2.6–3.6) |
| ≥65 | 16.0 (13.8–18.2) | 2.8 (1.9–3.8) | 18.8 (16.2–21.4) | 15.1 (10.9–19.3) | 7.7 (6.1–9.3) |
| Non-Hispanic black | |||||
| ≥12 | 9.3 (8.0–10.5) | 2.1 (1.6–2.5) | 11.4 (10.0–12.7) | 18.3 (14.6–21.9) | 5.7 (4.7–6.6) |
| ≥20 | 11.2 (9.7–12.6) | 2.5 (1.9–3.0) | 13.7 (12.1–15.2) | 18.2 (14.5–21.9) | 6.7 (5.6–7.8) |
| ≥65 | 25.2 (19.7–30.7) | 7.5 (4.9–10.1) | 32.7 (26.8–38.5) | 22.9 (15.4–30.4) | 13.2 (9.8–16.7) |
| Mexican American | |||||
| ≥12 | 6.5 (5.3–7.7) | 2.5 (1.5–3.5) | 9.0 (7.4–10.6) | 27.9 (19.4–36.3) | 2.3 (1.5–3.1) |
| ≥20 | 7.9 (6.5–9.2) | 3.0 (1.8–4.2) | 10.9 (9.0–12.8) | 27.7 (19.2–36.2) | 2.8 (1.8–3.7) |
| ≥65 | 26.6 (22.5–30.7) | 5.2 (2.5–8.0) | 31.8 (28.4–35.2) | 16.4 (7.9–25.0) | 8.3 (3.7–12.8) |
Data are % (95% CI). Diagnosed diabetes determined by self-report on interview. Values by age alone and by sex include those of race/ethnic groups not listed separately.
Table 2.
Standardized prevalence of diagnosed diabetes, undiagnosed diabetes (A1C ≥6.5%), total diabetes (diagnosed and undiagnosed combined), percent of total diabetes that is undiagnosed, and at high risk of diabetes (A1C ≥6.0 to <6.5%), by age, sex, and race/ethnicity: NHANES 1988–1994, 1999–2002, 2003–2006
| 1988–1994 | 1999–2002 | 2003–2006 | |
|---|---|---|---|
| n | 15,891 | 8,973 | 9,025 |
| Diagnosed diabetes | |||
| Combined age groups (years) | |||
| ≥20 | 5.3 (4.8–5.8) | 6.6 (5.9–7.3) | 7.6 (6.9–8.3) |
| ≥65 | 12.5 (11.0–13.9) | 14.9 (13.2–16.6) | 17.7 (15.6–19.8) |
| Age-specific groups (years) | |||
| 20–39 | 1.1 (0.6–1.6) | 1.6 (1.0–2.3) | 1.9 (1.4–2.4) |
| 40–59 | 5.5 (4.6–6.4) | 6.8 (5.7–7.9) | 8.1 (6.9–9.4) |
| 60–74 | 11.9 (10.3–13.5) | 15.5 (13.9–17.2) | 17.7 (15.7–19.6) |
| ≥75 | 13.7 (11.8–15.6) | 13.6 (10.6–16.6) | 15.1 (12.8–17.3) |
| Sex (age ≥20 years) | |||
| Men | 5.3 (4.7–5.8) | 7.2 (6.2–8.2) | 7.6 (6.6–8.6) |
| Women | 5.3 (4.6–6.1) | 6.1 (5.3–6.9) | 7.6 (6.8–8.4) |
| Race/ethnicity (age ≥20 years) | |||
| Non-Hispanic white | 4.8 (4.2–5.5) | 5.3 (4.6–6.0) | 6.3 (5.5–7.0) |
| Non-Hispanic black | 8.4 (7.5–9.2) | 10.9 (9.4–12.3) | 12.2 (10.8–13.6) |
| Mexican American | 9.4 (8.5–10.4) | 10.4 (9.4–11.5) | 12.0 (10.4–13.6) |
| Undiagnosed diabetes | |||
| Combined age groups (years) | |||
| ≥20 | 2.1 (1.8–2.5) | 1.6 (1.3–2.0) | 1.8 (1.4–2.2) |
| ≥65 | 4.4 (3.4–5.4) | 3.2 (2.4–4.0) | 3.5 (2.6–4.4) |
| Age-specific groups (years) | |||
| 20–39 | 0.4 (0.2–0.6) | 0.5 (0.1–0.8) | 0.6 (0.3–0.9) |
| 40–59 | 2.6 (1.9–3.2) | 1.8 (1.3–2.3) | 2.0 (1.3–2.7) |
| 60–74 | 4.3 (3.2–5.5) | 3.7 (2.7–4.7) | 3.5 (2.1–4.9) |
| ≥75 | 5.5 (3.8–7.2) | 3.2 (1.9–4.4) | 3.6 (2.4–4.8) |
| Sex (age ≥20 years) | |||
| Men | 2.6 (2.1–3.1) | 2.0 (1.5–2.5) | 2.1 (1.5–2.7) |
| Women | 1.8 (1.4–2.1) | 1.3 (1.0–1.7) | 1.5 (1.2–1.8) |
| Race/ethnicity (age ≥20 years) | |||
| Non-Hispanic white | 1.6 (1.2–1.9) | 1.2 (0.9–1.5) | 1.3 (0.9–1.8) |
| Non-Hispanic black | 5.0 (4.3–5.6) | 3.1 (2.4–3.8) | 2.7 (2.2–3.3) |
| Mexican American | 4.0 (3.0–4.9) | 2.7 (2.0–3.4) | 3.6 (2.3–4.9) |
| Total diabetes | |||
| Combined age groups (years) | |||
| ≥20 | 7.4 (6.8–8.1) | 8.2 (7.3–9.1) | 9.3 (8.6–10.1) |
| ≥65 | 16.9 (15.2–18.6) | 18.1 (16.2–20.1) | 21.2 (18.8–23.6) |
| Age-specific groups (years) | |||
| 20–39 | 1.5 (1.0–2.0) | 2.1 (1.3–2.9) | 2.5 (1.9–3.1) |
| 40–59 | 8.1 (6.8–9.4) | 8.6 (7.3–10.0) | 10.1 (8.7–11.5) |
| 60–74 | 16.2 (14.3–18.1) | 19.2 (17.1–21.3) | 21.1 (18.8–23.5) |
| ≥75 | 19.2 (16.9–21.6) | 16.8 (13.6–19.9) | 18.6 (16.6–20.7) |
| Sex (age ≥20 years) | |||
| Men | 7.8 (7.1–8.6) | 9.2 (7.9–10.5) | 9.7 (8.8–10.6) |
| Women | 7.1 (6.2–8.0) | 7.4 (6.5–8.3) | 9.1 (8.1–10.1) |
| Race/ethnicity (age ≥20 years) | |||
| Non-Hispanic white | 6.4 (5.6–7.2) | 6.5 (5.7–7.3) | 7.6 (6.7–8.5) |
| Non-Hispanic black | 13.3 (12.3–14.4) | 14.0 (12.6–15.4) | 14.9 (13.4–16.5) |
| Mexican American | 13.4 (12.2–14.5) | 13.2 (12.1–14.2) | 15.6 (14.2–16.9) |
| Percent of total diabetes that is undiagnosed | |||
| Combined age groups (years) | |||
| ≥20 | 28.9 (23.2–34.6) | 20.6 (15.1–26.1) | 21.5 (16.6–26.4) |
| ≥65 | 25.6 (20.5–30.7) | 17.6 (13.8–21.4) | 16.4 (12.9–19.9) |
| Age-specific groups (years) | |||
| 20–39 | 28.1 (15.2–40.9) | 21.8 (9.9–33.7) | 25.6 (16.2–34.9) |
| 40–59 | 31.4 (26.1–36.7) | 20.4 (15.3–25.6) | 19.4 (13.3–25.5) |
| 60–74 | 26.7 (20.8–32.7) | 19.1 (14.6–23.5) | 16.4 (10.8–22.0) |
| ≥75 | 27.6 (21.1–34.2) | 19.1 (11.6–26.6) | 19.2 (12.8–25.7) |
| Sex (age ≥20 years) | |||
| Men | 30.4 (22.7–38.1) | 22.6 (13.1–32.2) | 25.4 (16.4–34.3) |
| Women | 27.8 (20.1–35.5) | 18.7 (11.5–26.0) | 17.8 (12.4–23.1) |
| Race/ethnicity (age ≥20 years) | |||
| Non-Hispanic white | 19.5 (11.7–27.3) | 16.9 (10.1–23.8) | 17.1 (6.5–27.6) |
| Non-Hispanic black | 41.6 (35.2–47.9) | 26.7 (13.5–40.0) | 15.7 (8.7–22.7) |
| Mexican American | 44.3 (38.1–50.6) | 15.2 (11.1–19.3) | 34.6 (23.0–46.1) |
| At high risk for diabetes | |||
| Combined age groups (years) | |||
| ≥20 | 4.9 (4.4–5.5) | 3.2 (2.6–3.7) | 3.4 (3.0–3.8) |
| ≥65 | 10.8 (9.7–11.9) | 6.6 (5.3–7.9) | 8.1 (6.6–9.6) |
| Age-specific groups (years) | |||
| 20–39 | 1.8 (1.2–2.3) | 0.8 (0.5–1.2) | 0.7 (0.4–1.0) |
| 40–59 | 5.2 (4.3–6.0) | 3.6 (2.7–4.6) | 3.7 (3.1–4.4) |
| 60–74 | 9.3 (7.9–10.7) | 6.5 (4.9–8.2) | 7.1 (5.5–8.8) |
| ≥75 | 12.1 (9.7–14.5) | 6.9 (5.4–8.4) | 8.7 (7.2–10.2) |
| Sex (age ≥20 years) | |||
| Men | 5.3 (4.4–6.1) | 3.3 (2.6–3.9) | 3.6 (3.1–4.1) |
| Women | 4.6 (4.0–5.2) | 3.1 (2.4–3.8) | 3.2 (2.6–3.9) |
| Race/ethnicity (age ≥20 years) | |||
| Non-Hispanic white | 3.7 (3.1–4.2) | 2.5 (1.9–3.0) | 2.7 (2.3–3.2) |
| Non-Hispanic black | 12.5 (11.3–13.7) | 5.9 (4.5–7.3) | 7.2 (6.2–8.2) |
| Mexican American | 5.9 (4.7–7.2) | 3.8 (2.9–4.7) | 3.6 (2.5–4.6) |
Data are % (95% CI). Diagnosed diabetes determined by self-report on interview. Values by age alone and by sex include those of race/ethnic groups not listed separately. Estimates for the total population aged ≥20 years and for race/ethnic groups were age- and sex-standardized, estimates for age-specific groups including those aged ≥65 years were sex-standardized, and estimates for sex groups were age-standardized (all using the 2000 U.S. Census population).
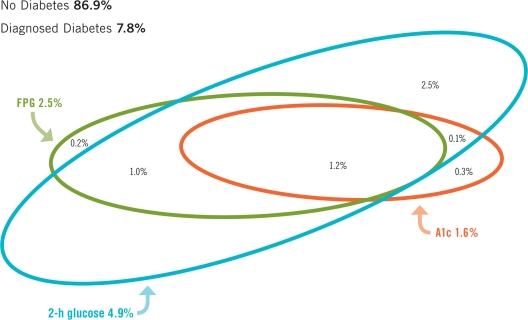
Figure 1.
Undiagnosed diabetes in the U.S. population aged ≥20 years by three diagnostic criteria—NHANES 2005–2006. Comparisons were calculated among individuals in the OGTT subsample in 2005–2006 (n = 2,017); consequently some estimates may differ slightly from those in Table 1. The thresholds of diagnostic criteria for diabetes were A1C ≥6.5%, FPG ≥7.0 mmol/1, and 2-h glucose ≥11.1 mmol/1. Point estimates (%) and 95% CIs for the categories are: A1C alone = 0.3 (0.0–0.7); FPG alone = 0.2 (0.0–0.5); 2-h glucose alone = 2.5 (1.9–3.2); A1C and FPG not 2-h glucose = 0.0; A1C and 2-h glucose not FPG = 0.1 (0.0–0.3); FPG and 2-h glucose not A1C = 1.0 (0.3–1.8); A1C, FPG, and 2-h glucose = 1.2 (0.5–2.0); total A1C = 1.6 (0.7–2.5); total FPG = 2.5 (1.2–3.8); total 2-h glucose = 4.9 (3.4–6.4); diagnosed diabetes = 7.8 (6.7–8.8); nodiabetes = 86.9 (84.6–89.1).
For comparisons of estimates across survey years and across population subgroups (of age, sex, and race/ethnicity) in 2003–2006, we directly standardized estimates to the U.S. 2000 Census population by age and/or sex with age categories of 20–39, 40–59, and ≥60 years. SUDAAN version 10.0.0 (6) was used to calculate standard errors using the Taylor series linearization method (7).
For NHANES 2003–2006, we used one-sample Student's t tests for testing whether differences between subgroups in proportions were significantly different from zero. Two-sample Student's t tests were used to test differences in proportions between 1988–1994 and 2003–2006 and 1999–2002 and 2003–2006. A P value ≤0.05 was considered statistically significant.
RESULTS
Prevalences in 2003–2006
Diagnosed diabetes.
The crude prevalence of diagnosed diabetes was 7.8% in subjects aged ≥20 years, or 16.5 million people; corresponding crude prevalences were 6.8% in individuals aged ≥12 years and 17.7% in those aged ≥65 years (Table 1). Both crude and standardized prevalences (Table 2) increased significantly with age, peaking at age 60–74 years (crude 17.6%), and were similar in men and women. At age ≥20 years, crude prevalence was significantly higher in non-Hispanic blacks (11.2%) than in non-Hispanic whites (6.9%, P < 0.00001) and Mexican Americans (7.9%, P = 0.004). Standardized prevalences at age ≥20 years were nearly twice as high in both non-Hispanic blacks and Mexican Americans as in non-Hispanic whites (both P < 0.00001).
Undiagnosed diabetes.
The crude prevalence of undiagnosed diabetes (A1C ≥6.5%) was 1.8% in subjects aged ≥20 years, or 3.9 million people; crude prevalences were 1.6% in subjects aged ≥12 years and 3.5% in those aged ≥65 years (Table 1). Both crude and standardized prevalences (Table 2) increased significantly with age and peaked at ages 60–74 and ≥75 years (3.5–3.6%). Crude and standardized prevalences did not differ significantly by sex. Both crude and standardized prevalences of undiagnosed diabetes at age ≥20 years were about two times higher in non-Hispanic blacks (standardized, P = 0.0007) and Mexican Americans (standardized, P = 0.0027) than in non-Hispanic whites.
Total diabetes.
The crude prevalence of total diabetes (diagnosed and undiagnosed combined) was 9.6% in subjects aged ≥20 years, or 20.4 million; prevalences were 8.4% in individuals aged ≥12 years and 21.1% in those aged ≥65 years (Table 1). Both crude and standardized prevalences (Table 2) increased significantly with age, peaking at age 60–74 years (21.1%), and were similar in men and women. There were significant differences by race/ethnicity in crude prevalence at all ages, with prevalence always highest in non-Hispanic blacks and lowest in non-Hispanic whites. Standardized prevalence of total diabetes at age ≥20 years was about twice as high in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites (both P < 0.00001).
Percent of total diabetes that is undiagnosed.
Among individuals with diabetes, 19.0% was undiagnosed at both age ≥20 and ≥12 years and 16.3% was undiagnosed at age ≥65 years (Table 1). Both the crude percent of diabetes that was undiagnosed and the standardized percent (Table 2) did not differ significantly by age or sex. At age ≥20 years, the standardized proportion of diabetes that was undiagnosed was about twice as high in Mexican Americans (34.6%) than in non-Hispanic whites (17.1%, P = 0.03) and non-Hispanic blacks (15.7%, P = 0.003).
High risk for diabetes.
The crude prevalence of being at high risk for diabetes was 3.5% among subjects aged ≥20 years, comprising 7.4 million individuals; corresponding crude prevalences were 3.1% in those aged ≥12 years and 8.1% in those aged ≥65 years (Table 1). Both crude and standardized prevalences (Table 2) of individuals at high risk for diabetes increased significantly with age but did not differ by sex. The proportion at high risk for diabetes was two to three times higher in non-Hispanic blacks than in non-Hispanic whites and Mexican Americans (standardized, both P < 0.00001).
Trends in standardized prevalences over time.
Diagnosed diabetes increased significantly from 5.3% in 1988–1993 to 7.6% in 2003–2006 in subjects aged ≥20 years (P < 0.00001, 1988–1994 vs. 2003–2006) (Table 2). Diagnosed diabetes increased significantly in all age-groups except in those aged ≥75 years and increased significantly in both sexes and in all race/ethnic groups. There were nonsignificant decreases in undiagnosed diabetes in almost all groups except non-Hispanic blacks, in whom there was a significant decrease (5.0% in 1988–1994 to 2.7% in 2003–2006, P < 0.00001). Total diabetes (diagnosed and undiagnosed combined) increased significantly from 7.4% in 1988–1994 to 9.3% in 2003–2006 in subjects aged ≥20 years (P = 0.0003, 1988–1994 vs. 2003–2006), as well as in all age-groups (except those aged ≥75 years) and in both sexes. Among racial/ethnic groups, total diabetes increased significantly only among Mexican Americans. Among subjects with diabetes, the percent with undiagnosed diabetes decreased significantly in the combined age-group of ≥65 years (25.6% in 1988–1994 to 16.4% in 2003–2006, P = 0.003), age-specific groups of 40–59 and 60–74 years, women, non-Hispanic blacks, and Mexican Americans. The percent at high risk for diabetes decreased significantly for all ages (e.g., at age ≥20 years, 4.9% in 1988–1994 to 3.4% in 2003–2006, P < 0.00001), except in subjects aged 60–74 years (P = 0.06), and decreased significantly in all sex and racial/ethnic groups.
Comparison of prevalences of undiagnosed diabetes based on A1C, FPG, and 2-h plasma glucose.
Fig. 1 classifies individuals on glycemic status using data from the OGTT subsample who, in addition to the 2-h plasma glucose, had measurement of A1C and FPG. A total of 5.4% of the population aged ≥20 years was classified as having undiagnosed diabetes based on at least one of these criteria; the remaining population was classified as having diagnosed diabetes (7.8%) or being nondiabetic (86.9%). All three criteria simultaneously classified 1.2% of the total population (23% of undiagnosed) as having undiagnosed diabetes. The A1C criterion diagnosed the smallest percent (1.6%) of the total population, or 30% of the undiagnosed diabetic group. In contrast, the 2-h plasma glucose diagnosed 4.9% of the total population, or 90% of those with undiagnosed diabetes; a substantial percent (2.5% of the total population or 47% of undiagnosed diabetes) was detected only by the 2-h plasma glucose but not by the A1C or FPG. In addition, a substantial proportion (1.0% of the total population or 19.0% of undiagnosed diabetes) was diagnosed by both FPG and 2-h plasma glucose but not by A1C. Most people who were diagnosed by A1C were also diagnosed by FPG (1.2%) or 2-h plasma glucose (1.3%).
CONCLUSIONS
Applying A1C criteria to define undiagnosed diabetes (≥6.5%) and high risk of diabetes (6.0 to <6.5%), we find that in the U.S. population aged ≥20 years during 2003–2006, the crude prevalence of total diabetes is 9.6% or 20.4 million, of which 19.0% is undiagnosed (7.8% previously diagnosed and 1.8% undiagnosed). An additional 3.5% of adults, or 7.4 million, are at high risk of diabetes, yielding 13.0% of the U.S. population aged ≥20 years, or 27.8 million, who have a hyperglycemic condition. Prevalence of these conditions is strongly influenced by increasing age. While we find no differences by sex, we continue to find non-Hispanic blacks and Mexican Americans disproportionately affected. Although the prevalence of previously diagnosed diabetes has significantly increased over time in most population groups, the proportion of diabetes that is undiagnosed using A1C criteria has significantly decreased over time in many groups, and the proportion at high risk of diabetes has significantly decreased in almost all groups.
A1C criteria result in substantially lower prevalences of undiagnosed and total diabetes, and being at high risk for diabetes, than prevalences estimated from fasting plasma glucose or 2-h glucose. The prevalences reported here can be compared with our recently reported prevalences of undiagnosed diabetes, impaired fasting glucose (IFG), and impaired glucose tolerance (IGT) in 2005–2006 using glucose criteria (8) (online appendix Table A1, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1524/DC1). Standardized prevalence of undiagnosed diabetes using A1C criteria (1.8%) in this report was one-third that using either FPG or 2-h glucose criteria (5.0% combined, 2.4% by FPG alone, and 4.8% by 2-h alone), resulting in a prevalence of total diabetes using A1C criteria one-quarter less than that of total diabetes using either glucose criteria (9.3 vs. 12.6%). The proportion of total diabetes that is undiagnosed using A1C criteria (21.5%) was 40% less than that using glucose criteria (34.2%). The prevalence of being at high risk of diabetes using A1C criteria (3.4%) was dramatically reduced to about one-tenth the prevalence using FPG/2-h glucose criteria (29.0% combined, 25.2% IFG, and 13.6% IGT).
The substantially lower prevalence of diabetes detected by A1C criteria was illustrated (Fig. 1) by analyzing the concordance of A1C, FPG, and 2-h glucose criteria in 2005–2006. A1C detected only 30% of undiagnosed diabetes defined by any of the three criteria; by contrast, 2-h plasma glucose detected 90% of undiagnosed diabetes. A relatively large proportion (19%) of undiagnosed diabetes was detected by both FPG and 2-h glucose but not by A1C.
The fact that prevalence of being at high risk for diabetes/pre-diabetes detected by A1C is about one-tenth the prevalence of IFG or IGT is striking. The relatively small sample size during 2005–2006 prohibited our assessing discordance of prevalence at high risk for diabetes using A1C versus prevalence of pre-diabetes, undiagnosed diabetes, and normal glucose status using glucose criteria, because of the large number of overlapping categories involved.
Certain relationships by age and race/ethnicity also differed between results based on A1C criteria (herein) and glucose criteria (8) (online appendix Table A1). By age, undiagnosed diabetes increased less dramatically using A1C criteria than it did using glucose criteria, whereas prevalence of being at high risk for diabetes increased relatively more dramatically using A1C criteria. Factors unrelated to glycemic control may explain some of the increase by age in prevalence of high risk for diabetes. A1C increased with age in nondiabetic participants of the Framingham Offspring Study and the NHANES 2001–2004 and in the Framingham Offspring Study participants with normal glucose tolerance, while adjusting for fasting and 2-h glucose levels and other factors (9). Racial/ethnic differences in the prevalence of undiagnosed diabetes and high risk of diabetes were disproportionately greater using A1C criteria compared with glucose criteria, which may also be explained in part by factors unrelated to glucose control. Blacks and Hispanics with IGT in the Diabetes Prevention Program had higher A1C levels than whites, after adjusting for glucose levels and other factors such as age, sex, education, blood pressure, BMI, hematocrit, and insulin resistance (10). This may suggest that hemoglobin glycation or red cell survival differs among racial/ethnic groups.
A limitation of our analyses is that determination of undiagnosed diabetes and high risk for diabetes/pre-diabetes is based on a single measurement of A1C, FPG, and 2-h glucose from subjects who self-reported that they fasted appropriately (for FPG and 2-h glucose), whereas retesting is suggested for diagnosis in a clinical setting. Consequently, some prevalence estimates may be overstated (11). In addition, although there should be limited interference from hemoglobin traits due to the A1C assay methods used in NHANES (2,12), A1C levels may be spurious with conditions that change red cell turnover (e.g., anemia) (12,13) regardless of the assay method used.
Some individuals with self-reported diabetes may not have had diabetes detected if A1C criteria had been used. When we excluded individuals self-reporting diabetes who reported not using glycemic medications and who had A1C <6.5%, prevalence of diagnosed diabetes decreased 0.9%. This was a larger decrease than when we performed the same analysis excluding those with FPG <126 mg/dl (0.3% decrease) or those with 2-h glucose <200 mg/dl (0.2% decrease). Consequently, our estimates of diagnosed and total diabetes may be overstated, and the percent of total diabetes that is undiagnosed may be understated.
The international expert committee emphasized that glucose and A1C levels reflect different aspects of glucose metabolism. The purpose of their report was not to establish identical prevalences in defining new criteria for diabetes and high risk for diabetes but rather to identify individuals at risk for diabetes complications and diabetes so that preventive treatment could follow. It will be important to determine longitudinally whether complication rates differ between individuals detected by glucose criteria or by A1C criteria.
If A1C criteria are adopted by the American Diabetes Association, the identification of a smaller high-risk group may be considered advantageous because of the limited availability of resources. However, a substantial proportion of individuals similar to those in the Diabetes Prevention Program (14) would be missed and not receive preventive intervention to reduce risk. The nearly 1% of the population previously diagnosed with diabetes who no longer have diabetes by A1C criteria may need careful explanation of the alternative diagnostic criteria, the arbitrary cut point for diagnosis along a continuum of risk, and the progressive nature of dysglycemia so that diabetic/pre-diabetic conditions are not thought to be less real or serious. While the change in diagnosis in some from diabetes to high risk of diabetes using A1C criteria might not alter recommended glycemic control and lifestyle management, recommended blood pressure and lipid targets would differ (15), requiring careful consideration of individualized therapeutic goals.
A change to A1C criteria would also impact national surveillance of these conditions. Estimated self-reported diabetes based on surveys and the tracking of temporal trends would likely be difficult to interpret for some time before these criteria are fully assimilated in medical practice. An interim period of measurement both by A1C and glucose may be necessary for international and domestic historical comparisons.
The potential impact of these dramatically lower estimates of diabetes, and particularly of high risk for diabetes, on public perception of the magnitude and seriousness of diabetes is concerning. The fact remains that diabetes and its sequelae are devastating and escalating public health problems that disproportionately affect the elderly, non-Hispanic blacks, and Mexican Americans. A continued commitment to the primary and secondary prevention of diabetes by the public and private sectors is needed to limit the severe toll that diabetes exacts in the U.S.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Centers for Disease Control and Prevention.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey 1988–1994, 1999–2002, 2003–2006 [information online], 2009. Available from http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 1 March 2009
- 3. Little RR, Wiedmeyer HM, England JD, Wilke AL, Rohlfing CL, Wians FH, Jr, Jacobson JM, Zellmer V, Goldstein DE: Interlaboratory standardization of measurements of glycohemoglobins. Clin Chem 1992;38:2472–2478 [PubMed] [Google Scholar]
- 4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32 (Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Census Bureau, Population Division. Table 1: annual estimates of the population by five-year age groups and sex for the United States: April 1, 2000 to July 1, 2006 (NC-EST2006-01) [information online], 2003–2006. Available from www.census.gov/popest/national/asrh/NC-EST2006-sa.html.data. Accessed 17 May 2009
- 6. Research Triangle Institute. SUDAAN User's Manual, Release 9.0.1. Research Triangle Park, NC, Research Triangle Institute, 2005. [Google Scholar]
- 7. LaVange LM, Stearns SC, Lafata JE, Koch GG, Shah BV: Innovative strategies using SUDAAN for analysis of health surveys with complex samples. Stat Methods Med Res 1996;5:311–329 [DOI] [PubMed] [Google Scholar]
- 8. Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS: Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM: Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 2008;31:1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E: Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selvin E, Crainiceanu CM, Brancati FL, Coresh J: Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007;167:1545–1551 [DOI] [PubMed] [Google Scholar]
- 12. National Glycohemoglobin Standardization Program. National Glycohemoglobin Standardization Program [article online]. Available from www.ngsp.org. Accessed 23 October 2009
- 13. Little RR, Sacks DB: HbA1c: how do we measure it and what does it mean? Curr Opin Endocrinol Diabetes Obes 2009;16:113–118 [DOI] [PubMed] [Google Scholar]
- 14. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.