Abstract
OBJECTIVE
To simplify and improve the treatment of patients with type 1 diabetes, we ascertained whether the site of subcutaneous insulin infusion can be used for the measurement of glucose.
RESEARCH DESIGN AND METHODS
Three special indwelling catheters (24-gauge microperfusion [MP] catheters) were inserted into the subcutaneous adipose tissue of subjects with type 1 diabetes (n = 10; all C-peptide negative). One MP catheter was perfused with short-acting insulin (100 units/ml, Aspart) and used for insulin delivery and simultaneous glucose sampling during an overnight fast and after ingestion of a standard glucose load (75 g). As controls, the further two MP catheters were perfused with an insulin-free solution (5% mannitol) and used for glucose sampling only. Plasma glucose was measured frequently at the bedside.
RESULTS
Insulin delivery with the MP catheter was adequate to achieve and maintain normoglycemia during fasting and after glucose ingestion. Tissue glucose concentrations derived with the insulin-perfused catheter agreed well with plasma glucose levels. Median correlation coefficient and median absolute relative difference values were found to be 0.93 (interquartile range 0.91–0.97) and 10.9%, respectively. Error grid analysis indicated that the percentage number of tissue values falling in the clinically acceptable range is 99.6%. Comparable analysis results were obtained for the two mannitol-perfused catheters.
CONCLUSIONS
Our data suggest that estimation of plasma glucose concentrations from the glucose levels directly observed at the site of subcutaneous insulin infusion is feasible and its quality is comparable to that of estimating plasma glucose concentrations from glucose levels measured in insulin-unexposed subcutaneous tissue.
Type 1 diabetes is presently treated by self-administration of insulin, either by a subcutaneous bolus injection using a hypodermic needle (e.g., syringe, insulin pen) or by a continuous subcutaneous infusion using an indwelling catheter connected to an insulin pump (1,2). Type 1 diabetic patients furthermore separately self-monitor glucose levels in blood obtained by finger-pricking to guide the adjustment of insulin dosage, food consumption, and physical activity (1,2). To simplify and improve the glucose management in diabetes, we sought to determine whether the site of subcutaneous insulin administration can also be used for the measurement of glucose. As a first step, we recently assessed the kinetics of insulin action on the tissue glucose concentration at the insulin delivery site in the presence of euglycemic blood plasma levels (3). Using the euglycemic clamp technique together with special indwelling catheters (microperfusion or microdialysis catheters) for coupling insulin delivery with glucose sampling at the same tissue site, we found that within 60 min after exposing adipose tissue of healthy humans to a standard 100 units/ml insulin preparation, insulin's effect on the tissue glucose concentration saturates and a stable ratio between the tissue and plasma glucose concentration is attained. This attainment of steady-state insulin action conditions at the delivery site indicates that glucose sensing and insulin delivery may be carried out simultaneously at the same adipose tissue site via a single tissue catheter. To further validate this single-port treatment approach, the objective of the present study was to ascertain in type 1 diabetic patients whether tissue glucose concentrations observed at the site of subcutaneous insulin delivery can be used to estimate plasma glucose levels. To accomplish this, microperfusion (MP) catheters were inserted in adipose tissue of type 1 diabetic subjects and used to carry out glucose sampling and simultaneous insulin delivery during an overnight fast and after ingestion of a standard glucose load (oral glucose tolerance test [OGTT]).
RESEARCH DESIGN AND METHODS
Ten subjects with type 1 diabetes (two females and eight males; age 39.8 ± 2.9 years, range 27–57; BMI 25.3 ± 1.0 kg/m2, range 21.1–29.5, means ± SE) participated in this study. Their mean duration of diabetes was 22.9 ± 2.6 years (range 7–35) and their percent A1C averaged 7.6 ± 0.3% (range 5.7–8.6%, normal range 4.3–5.9%). Patients were all without residual endogenous insulin secretion, as indicated by undetectable C-peptide levels in blood plasma (i.e., <22 pmol/l). Three patients were treated with continuous subcutaneous insulin infusion and seven with multiple daily injections of insulin. At the time of the study, patients had no evidence of clinically overt diabetes complications and, apart from insulin, were not taking any medication known to influence carbohydrate metabolism and subcutaneous insulin absorption. Written informed consent was obtained after the purpose, nature, and potential risks of the study were explained to the subjects. The studies were approved by the ethics committee of the Medical University of Graz.
Study design
The diabetic subjects were admitted to the clinical research center at ∼2230. Subjects treated with multiple daily injections had been instructed to leave out the injection of long-acting insulin on the evening of the study. On admission to the clinical research center, subjects with continuous subcutaneous insulin infusion treatment were asked to disconnect their own insulin pump. At ∼2300, an intravenous catheter was inserted into an arm vein for blood withdrawal during the night. After catheter insertion, three 24-gauge MP catheters (4,5) were placed into the periumbilical subcutaneous adipose tissue. The distance between adjacent catheters was >35 mm. Subsequently, peristaltic pumps (Minipuls 3; Gilson, Villiers-le-Bel, France) were attached to the inflow and outflow tubing of each catheter (Fig. 1). One catheter was then perfused with a rapid-acting insulin solution (100 units/ml, Aspart; Novo Nordisk, Bagsvaerd, Denmark) to allow insulin delivery and simultaneous sampling of interstitial fluid (ISF) during the experiment. The two further catheters were perfused with an insulin-free solution (5% mannitol) and used for ISF sampling only. After starting catheter perfusion, an equilibration period of 60 min elapsed before the catheter effluent samples were collected in 60-min fractions in vials kept on ice. Outflow rates of all catheters were maintained at a constant value (∼0.45 μl/min) throughout the experiment. The insulin delivery rate of the insulin-perfused catheter was adjusted by simply adjusting the inflow rate of the catheter (dual pump operation mode, Fig. 1A). Adjustments in the inflow rate were done on the basis of frequent plasma glucose measurements (every 10–30 min) to slowly achieve and maintain normoglycemia (∼6 mmol/l) overnight. For comparison purposes, the inflow rates applied in one mannitol-perfused catheter (Fig. 1B) were similar to the periodically adjusted inflow rates of the insulin-perfused catheter (Fig. 1A), whereas the inflow rate applied in the second mannitol-perfused catheter was identical to its outflow rate (Fig. 1C). On the next day, at ∼0700, a hand or forearm vein was cannulated to allow blood withdrawal during the subsequent OGTT phase of the experiment. The forearm with this catheter was then placed in a thermoregulated box to arterialize the venous blood. At 0900, the subject ingested 75 g glucose dissolved in 300 ml of water (Glucoral; Unipack, Wr.-Neustadt, Austria). Twenty minutes before glucose ingestion, an insulin bolus was administered over a period of 15 min via the insulin-perfused MP catheter. Bolus administration periods of comparable length are used in some commercially available insulin pump models (6). The amount of insulin administered as a bolus was determined by using medical records on the subject's insulin sensitivity factor (i.e., subject's insulin-to-carbohydrate ratio). After administration of the insulin bolus, the basal insulin delivery via the insulin-perfused MP catheter was continued and periodically adjusted so as to reestablish normal plasma glucose by ∼5 h after glucose ingestion. During the 8-h OGTT phase and during the 2-h period preceding initiation of the OGTT, the catheter effluent samples were collected in 30-min fractions, and plasma glucose concentrations were determined every 5–30 min. If during experiments the plasma glucose levels decreased below 3.22 mmol/l (58 mg/dl), the subjects were asked to ingest additional glucose.
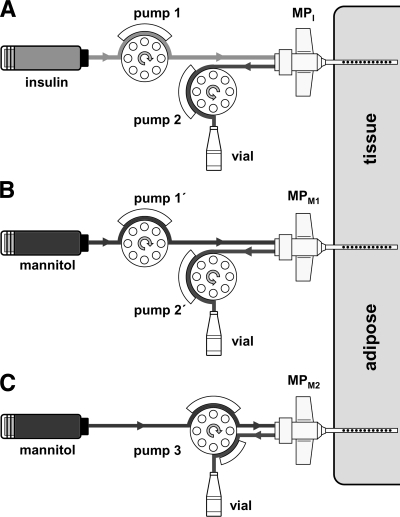
Figure 1.
Schematic of the experimental setup for assessing the feasibility of estimating plasma glucose concentrations from the ISF glucose levels observed at the insulin delivery site. A–C: Three MP catheters were inserted into subcutaneous adipose tissue of diabetic subjects (n = 10). One catheter (MPI) was used for glucose sampling and simultaneous insulin delivery (A), and, as controls, two catheters (MPM1 and MPM2) were used for glucose sampling only (B and C). The catheter for glucose sampling and insulin delivery was perfused with a rapid-acting insulin solution (100 units/ml, Aspart) using two peristaltic pumps (A), with one attached to the inflow tubing and one to the outflow tubing (dual-pump operation mode). Insulin delivery rate was adjusted by adjusting the difference between the inflow and outflow rate of the catheter. The outflow conveying the extracted tissue glucose was collected in vials. The efficiency by which glucose was extracted via diffusion from the ISF of the tissue into the catheter (glucose recovery) was measured by applying the ionic reference technique (4,5). The catheters used for glucose sampling only (MPM1 and MPM2) were perfused with an insulin-free solution (5% mannitol) using either a single-pump operation mode where the inflow equaled the outflow rate (C) or a dual-pump operation mode (B) where the inflow and outflow rates equaled those applied in the insulin-perfused MP catheter (A).
Microperfusion and analytical procedures
MP catheters applied were of concentric design with a cylindrical inner and outer tube (4,5). The outer tube consisted of a conventional intravenous 24-G cannula (shaft length: 19 mm, Neoflon; Becton Dickinson, Helsingborg, Sweden) in which 27 perforations (each 0.3 mm in diameter) were formed in the cannula wall using an Excimer Laser (LZH, Hannover, Germany). Two 750-mm lengths of Tygon tubing (inner diameter: 0.19 mm; Cole-Parmer, Vernon Hills, IL) were used to connect catheter inlet and outlet with perfusate reservoir and sampling vial, respectively.
Plasma glucose concentrations were measured at the bedside using a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA) with a coefficient of variation (CV) of 2%. The glucose concentrations in catheter effluents were determined using an automated CMA600 analyzer (CMA/Microdialysis, Solna, Sweden) with a within-run CV of 2%. The conductivities in the plasma, perfusate, and catheter effluent samples were measured using a contactless conductivity detector (TraceDec; I.S.T., Strasshof, Austria). The electrical conductivity was determined with a within-run CV of <1%. The plasma C-peptide concentrations were determined using a two-site enzyme immunoassay (C-peptide ELISA; Mercodia, Uppsala, Sweden) with a lower limit of quantification of 22 pmol/l. A1C was measured by high-performance liquid chromatography (HA-8160; Menarini Diagnostics, Florence, Italy).
Data analysis
The ISF glucose concentration was calculated (4,5) as the glucose concentration in the catheter effluent sample divided by the glucose recovery of the catheter (R). The recovery (or exchange efficiency) was determined for each sampling period as R = (Cout − Cin)/(Cpl − Cin), where Cin, Cout, and Cpl are the measured electrical conductivity in the perfusate, the effluent sample, and the corresponding plasma sample, respectively. This method (ionic reference technique [4,5]) allowed potential recovery changes caused by changes in the catheter characteristics (e.g., changes in perfusate inflow rates) and/or undesired local variations in the tissue microenvironment (e.g., blood flow changes, accumulation of edematous fluids) to be detected. Application of this technique was possible, because the electrical conductivity in the used catheter perfusates was either negligible (mannitol) or low compared with that in blood plasma (Aspart: ∼22.2% of average Cpl). Because each effluent sample was collected over a specified time interval (i.e., 60-min and 30-min intervals), the concentration in an effluent sample was regarded as an average concentration over the collection period. Thus, the derived ISF glucose values were considered valid at the midpoint of the interval, and an observed change in the ISF glucose concentration was considered as a time-averaged reflection of the changes that occurred in the plasma concentration during a collection interval. Estimates of plasma glucose concentration (termed “tissue glucose concentrations”) were derived from the ISF glucose levels by using a prospective one-point calibration procedure (7) that consisted of dividing the ISF glucose values with the ISF-to-plasma glucose ratio calculated from the ISF glucose concentration and corresponding mean plasma glucose concentration observed during the 60-min sampling period at the beginning of each experiment. Agreement between the tissue glucose concentrations and directly measured plasma glucose concentrations was assessed by applying error grid analysis and the method of residuals (8,9). Agreement index data obtained from the application of the method of residuals and the error grid analysis were examined with Friedman's test and Fisher's exact test, respectively. Implicit in the one-point calibration method is the assumption that there is a proportional relationship (Y = B × X) between the plasma glucose (X) and ISF glucose concentrations (Y). This assumption was tested by performing correlation and linear regression analysis of the full plasma and ISF glucose dataset. Correlation analysis was performed using Pearson's product-moment correlation coefficient, and linear regression analysis was performed by the least squares method. To detect potential effects of fast changes in plasma glucose and insulin concentrations on the relationship between plasma glucose levels and catheter-derived ISF glucose concentrations, linear regression analyses were additionally performed on a fasting dataset containing plasma and ISF glucose values observed during the overnight (euglycemic) phase of the experiment (−8 to 0 h), and on an OGTT dataset containing plasma and ISF glucose values observed during the hyperglycemic/hyperinsulinemic phase of the study (0–8 h). Coefficient and parameter data obtained from correlation and linear regression analysis were examined with Friedman's test or Wilcoxon's signed-rank test. A P value <0.05 was considered to indicate statistical significance. Normality of data were assessed using normal probability plots. Homogenity of variances in the linear regression data were assessed using plots of residuals against fitted values. Data are presented as means ± SE or as median and quartile values. Data analysis was performed using MATLAB (MathWorks, Natick, MA) and SPSS (SPSS, Chicago, IL) software packages.
RESULTS
Plasma and ISF glucose time courses
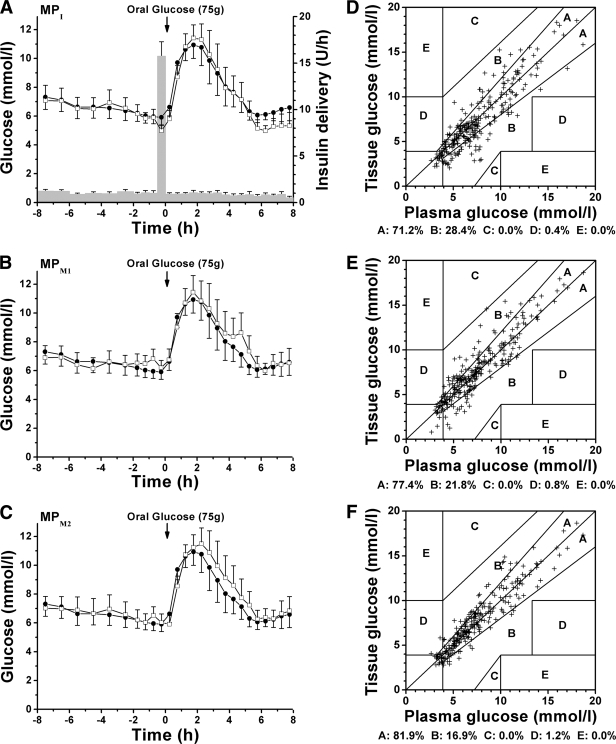
Insulin delivery with the MP catheter was successful both in achieving and maintaining a stable normal plasma glucose during the overnight fasting as well as in reestablishing near-normal plasma glucose by 4–5 h after the ingestion of glucose (Fig. 2A–C and supplementary Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1532/DC1). In addition, glucose concentration time courses observed in the ISF sampled with the three MP catheters paralleled those seen in plasma (supplementary Fig. 1). The basal insulin delivery rates used during the overnight and OGTT period averaged 1.04 ± 0.11 and 0.85 ± 0.16 units/h, respectively. The amount of insulin given as a bolus averaged 7.8 ± 0.8 units (Fig. 2A).
Figure 2.
Comparison of plasma and ISF-derived glucose concentrations observed during an overnight fast and OGTT in diabetic subjects. A: Average time course (n = 10, means ± SE) of plasma glucose concentration (●) and the tissue glucose concentration obtained with the MP catheter used for insulin delivery and simultaneous glucose sampling (MPI, □). A also shows the average time course (n = 10, means ± SE) of the insulin delivery rate (bars) used to control glucose concentration during experiments. B and C: Average time course (n = 10, means ± SE) of plasma glucose (●) and the tissue glucose obtained with the mannitol-perfused MP catheters (MPM1 and MPM2, □). D: Error grid analysis results for the insulin-perfused MP catheter (MPI): One of 268 data points (0.4%) fell outside of the clinically acceptable region A and B. E: Error grid analysis results for the mannitol-perfused catheter MPM1: Two of 266 data points (0.8%) were outside of the clinically acceptable region A and B. F: Error grid analysis results for the mannitol-perfused catheter MPM2: Three of 248 data points (1.2%) fell outside of the clinically acceptable region A and B. In A, the tendency toward lower tissue glucose concentrations than plasma glucose levels at the end of the OGTT is mainly caused by subject 8, whose tissue glucose concentration time course of the insulin-perfused catheter was an apparent outlier, with very low values during the last 4 h of the experiment (supplementary Fig. 1).
Relationship between plasma and ISF glucose concentrations
Results from the correlation analysis of ISF glucose values against plasma glucose values are given in the supplementary Table 2. The correlation coefficient for the insulin-perfused catheters (MPI) was found to be high (median: 0.93; interquartile range: 0.91–0.97) and did not differ from that obtained for the mannitol-perfused catheters (versus MPM1: 0.94 [0.88–0.97] and MPM2: 0.95 [0.91–0.98]; P = 0.67 with Friedman's test). In addition, regression analysis of plasma glucose against ISF glucose values showed that the intercept values obtained for insulin-perfused catheters (MPI: −0.13 mmol/l [−0.71 to 0.10 mmol/l]) and mannitol-perfused catheters (MPM1: 0.31 mmol/l [−0.27 to 1.19 mmol/l]; MPM2: −0.03 mmol/l [−0.36 to 0.18 mmol/l]) are not different from zero (P > 0.19 with Wilcoxon's signed-rank test). Results from an additional regression analysis performed with the straight-line forced through the origin of regression graphs (i.e., intercept was assumed to be zero) are given in supplementary Tables 3 and 4. The standard errors of the slopes as well as the root mean square errors obtained from this regression were similar for the insulin-perfused and mannitol-perfused catheters (P > 0.49 with Friedman's test). These regression results, together with the results from the correlation analysis, indicate that there is a strong proportional relationship between plasma glucose levels and catheter-derived ISF glucose values. Furthermore, the slope of the regression line derived for the insulin-perfused catheters (MPI: 0.726 [0.712–0.774]) was by ∼16% lower than the slopes obtained for the mannitol-perfused catheters (P = 0.002 with Friedman's test). There was no difference between the slopes obtained for the two mannitol-perfused catheters (MPM1: 0.862 [0.801–0.916] vs. MPM2: 0.859 [0.833–0.977]; P = 0.16 with Wilcoxon's signed-rank test). Results from additional regression analyses performed on the fasting and OGTT datasets are given in supplementary Tables 5 and 6. Comparison of the analysis results showed that slopes and standard errors of the slopes obtained for insulin-perfused and mannitol-perfused catheters from the OGTT dataset are similar to those obtained for insulin-perfused and mannitol-perfused catheters from the fasting dataset (MPI: P > 0.48, MPM1: P > 0.41, and MPM2: P > 0.08, all with Wilcoxon's signed-rank test).
Comparison between plasma and tissue glucose concentrations
Tissue glucose time courses that were derived from the ISF glucose time courses using the prospective one-point calibration procedure are shown in supplementary Fig. 1 and Fig. 2A–C. Error grid analysis indicated (Fig. 2D–F) that the percentage number of the tissue values that fall in the clinically acceptable range (zones A and B) is high for the insulin-perfused catheters (99.6%) and comparable to that obtained for the mannitol-perfused catheters (versus 99.2% for MPM1 and 98.8% for MPM2, P > 0.87 with Fisher's exact test). Furthermore, applying the method of residuals, the residual means and 2 SD values obtained for the insulin-perfused catheters were 5.6% (−6.0 to 12.4%) and 31.6% (26.4 to 34.6%), respectively. These values were similar to those calculated for the mannitol-perfused catheters (P > 0.49 with Friedman's test; supplementary Table 1). In addition, the median absolute relative difference calculated for the insulin-perfused catheters (10.9%) was comparable to that of mannitol-perfused catheters (versus 10.5 and 10.1% for MPM1 and MPM2, respectively). Overall, the statistical analysis suggests that the quality of estimation of plasma glucose concentrations from the ISF glucose levels directly observed at the adipose tissue site of insulin delivery is comparable to that of estimating plasma glucose concentrations from the ISF glucose levels measured in insulin-unexposed tissue.
CONCLUSIONS
The present investigation demonstrates that estimation of plasma glucose concentrations from the glucose levels directly observed at the site of subcutaneous insulin infusion is feasible in type 1 diabetic patients. Thus, glucose sensing and insulin delivery may be combined at the same adipose tissue site using a single catheter. Such a single-port treatment approach may offer several important advantages over present-time insulin replacement approaches (1) and recently proposed dual-port treatment strategies (10,11). First, it permits a significant reduction in the number of treatment-related needle-sticks, because the subcutaneously inserted catheter serves the double purpose of delivering insulin and sensing glucose and because the fingerstick testing of blood glucose is only needed to calibrate the ISF-based glucose sensing. Second, it allows a reduction in the size of the treatment system, because both the sensing and delivery components can be fully integrated into one design, and certain device components implemented in dual-port systems can be reduced in number (e.g., power supply units) or can even be eliminated (e.g., receiver-transmitter modules). Third, it increases patient convenience and possibly allows higher treatment compliance, leading to an improved glucose management. Finally, the single-port treatment approach may provide the basis for the future development of an autonomous device able to self-regulate insulin delivery (artificial pancreas).
Estimates of the plasma glucose concentration were derived from the observed ISF glucose concentrations by applying a prospective one-point calibration procedure (7) that implicitly assumes that there is a proportional relationship between plasma and ISF glucose concentrations. This assumption of proportionality may be well satisfied because correlation and linear regression analysis of plasma glucose concentrations and ISF glucose levels from insulin-exposed and insulin-unexposed tissue sites indicated that the derived intercepts are not different from zero and that glucose concentrations in the ISF correlated closely with those in plasma (supplementary Table 2). Furthermore, correlation coefficients, standard errors of the slopes, as well as the root mean square errors obtained from correlation and linear regression analysis of the full ISF and plasma glucose datasets (supplementary Tables 2–4) were similar for insulin-exposed and insulin-unexposed tissue sites. In addition, regression analyses performed individually on fasting and OGTT datasets showed that slopes and standard errors of the slopes obtained for insulin-exposed and insulin-unexposed tissue sites during the fasting were similar to those obtained for insulin-exposed and insulin-unexposed tissue sites during the OGTT, thereby indicating that changes in plasma insulin and glucose concentrations did not affect the relationship between plasma glucose concentrations and ISF glucose levels at these tissue sites (supplementary Tables 5 and 6). Overall, these results suggest that exposure of adipose tissue to standard insulin preparations did not alter the strength of the proportional relationship between plasma glucose concentrations and ISF glucose levels of adipose tissue.
Agreement between the ISF-based estimates of plasma glucose levels and directly measured plasma glucose concentrations was assessed by applying error grid analysis and the method of residuals (8,9). In the present study, values of the agreement indexes obtained for the ISF-based estimation of plasma glucose concentration from insulin-unexposed tissue sites (Fig. 2D–F and supplementary Table 1) were similar to or better than those previously obtained with commercial continuous glucose-sensing devices that also use the ISF of (insulin-unexposed) adipose tissue for the measurement of glucose (12–15). Furthermore, values of the agreement indexes derived in the present study for the insulin-exposed adipose tissue were similar to those derived for the insulin-unexposed adipose tissue (Fig. 2D–F, supplementary Table 1). Thus, our data indicate that the quality of estimation of plasma glucose concentrations from the ISF glucose levels directly observed at the adipose tissue site of insulin delivery is comparable to that of estimating plasma glucose concentrations from the ISF glucose levels measured in insulin-unexposed tissue.
Recently, we assessed the kinetics of insulin action on the ISF glucose concentration at the site of subcutaneous insulin delivery (3). In this earlier study, after the start of insulin delivery, we observed an initial delay of ∼60 min before insulin's effect on the ISF glucose concentration at the delivery site saturated, and the ISF-to-plasma glucose concentration ratio attained steady-state values. These values were ∼20% lower than the ISF-to-plasma glucose ratio values observed during the baseline period before the start of insulin delivery. In the current study, we determined ISF glucose levels under steady-state insulin action conditions. To achieve this, the ISF sampling from the insulin delivery site was begun 60 min after the start of insulin delivery. Thus, comparison of the slopes derived for the insulin-exposed and insulin-unexposed tissue (supplementary Table 2) allowed for estimation of the magnitude of the steady-state effect of insulin on the ISF glucose concentration at the insulin delivery site in diabetic patients. We found that the slopes derived for the insulin-exposed tissue were on average ∼16% lower than the slopes derived for the insulin-unexposed tissue (supplementary Table 2), thereby confirming our recent findings on the magnitude of insulin's effect on the ISF glucose concentration in human adipose tissue (3).
Besides the effect of insulin on the fat cell glucose uptake, an additional mechanism potentially influencing the glucose concentration at the site of insulin delivery may be the local dilution of the ISF by the insulin solvent. To separate and quantitate these potential effects on the glucose concentration at the insulin delivery site, one of the two mannitol catheters (MPM1) was operated using also the dual-pump technique with pump speeds identical to those used in the operation of the insulin catheter (Fig. 1B). We reasoned that if an increase in the perfusate flow fraction directed to the tissue (due to an increase in the difference between the speeds of inflow and outflow pumps) is causing a dilution of the ISF surrounding the catheter, then there will be a decrease in the efficiency of exchange of solutes between the ISF and the perfusates of the two catheters operated with the dual-pump technique (MPI and MPM1). We found that increases in the basal delivery rates did not dilute the ISF at the insulin delivery site. In contrast, bolus administrations apparently diluted the ISF at the insulin delivery site as both exchange efficiency (recovery) and effluent glucose concentration were decreased during and shortly after the bolus administration period (i.e., between −30 and 0 min; supplementary Fig. 2). However, when accounting for this recovery change in the calculation of the tissue glucose concentration from the effluent glucose concentration (by using the ionic reference technique), the obtained tissue glucose concentrations agreed well with the corresponding plasma glucose levels also during bolus administration periods (Fig. 1A and B), thereby indicating that bolus administrations did not disturb steady-state insulin action conditions at the insulin delivery site. Thus, both changes in the basal insulin delivery rates and bolus administrations did not affect the ability of the insulin delivery catheter to predict plasma glucose concentrations.
In summary, here we have shown that glucose concentrations directly measured at the subcutaneous insulin delivery site can be used to reliably estimate blood glucose levels. Thus, glucose sensing and insulin delivery may be simultaneously performed at the same adipose tissue site via a single tissue catheter. Such a single-port treatment approach may provide the basis for the development of an autonomous treatment device able to self-regulate insulin delivery.
Supplementary Material
Acknowledgments
This work was supported in part by funding from Science Park Graz and the Federal Ministry of Economics and Labor of the Republic of Austria and by a research grant from Medingo.
T.R.P. is a cofounder and shareholder of Smart*Med and a consultant for Novo-Nordisk and sanofi-aventis. M.E. is a consultant for Sensile Medical and BBraun and a cofounder and shareholder of Smart*Med. H.K. and L.S. are cofounders and shareholders of Smart*Med. G.K., L.S., M.E., T.R.P., and W.R. have filed patent applications relating to the methodology described in this article. R.K. and O.Y. are employed by Medingo. No other potential conflicts of interest relevant to this article were reported.
We are grateful to A. Wutte, G. Bock, and B. Semlitsch for their expert assistance in conducting the study; M. Suppan for performing the C-peptide assay; A. Groselj-Strele for assistance with the statistical analysis; and all volunteers for participating in the study.
Footnotes
Clinical trial reg. no. NCT00813410, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Daneman D: Type 1 diabetes. Lancet 2006; 367: 847– 858 [DOI] [PubMed] [Google Scholar]
- 2. Shalitin S, Phillip M: The role of new technologies in treating children and adolescents with type 1 diabetes mellitus. Pediatric Diabetes 2007; 8 ( Suppl. 6): 72– 79 [DOI] [PubMed] [Google Scholar]
- 3. Lindpointner S, Korsatko S, Köhler G, Köhler H, Ellmerer M, Schaupp L, Pieber TR, Regittnig W: Glucose concentration at the subcutaneous site of insulin delivery: effect of variable insulin infusion rates. Abstract presented at the 2nd European Diabetes Technology and Transplantation Meeting, 27–29 January 2008, Innsbruck, Austria [Google Scholar]
- 4. Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z, Skrabal F, Pieber TR, Wach P: Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol 1999; 276: E401– E408 [DOI] [PubMed] [Google Scholar]
- 5. Trajanoski Z, Brunner GA, Schaupp L, Ellmerer M, Wach P, Pieber TR, Kotanko P, Skrabal F: Open-flow microperfusion of subcutaneous adipose tissue for on-line continuous ex vivo measurement of glucose concentration. Diabetes Care 1997; 20: 1114– 1121 [DOI] [PubMed] [Google Scholar]
- 6. Diabetes Health: Insulin Pumps Reference Guide [article online], 2008. Available from http://www.diabeteshealth.com/media/pdfs/PRG1208/DH_Insulin-Pumps_08–09.pdf. Accessed 22 October 2009
- 7. Lodwig V, Heinemann L: Glucose Monitoring Study Group. Continuous glucose monitoring with glucose sensors: calibration and assessment criteria. Diabetes Technol Ther 2003; 5: 572– 586 [DOI] [PubMed] [Google Scholar]
- 8. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL: Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987; 5: 622– 628 [DOI] [PubMed] [Google Scholar]
- 9. Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307– 310 [PubMed] [Google Scholar]
- 10. Hovorka R, Chassin LJ, Wilinska ME, Canonico V, Akwi JA, Federici MO, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller HC, Schaupp L, Bodenlenz M, Pieber TR: Closing the loop: the ADICOL experience. Diabetes Technol Ther 2004; 6: 307– 318 [DOI] [PubMed] [Google Scholar]
- 11. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF: Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006; 55: 3344– 3350 [DOI] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration. Summary of safety and effectiveness data: FreeStyle Navigator Continuous Glucose Monitoring System [article online], 2008. Available from http://www.accessdata.fda.gov/cdrh_docs/pdf5/P050020b.pdf. Accessed 20 June 2009
- 13. U.S. Food and Drug Administration. Summary of safety and effectiveness data: DexCom STS Continuous Glucose Monitoring System [article online], 2007. Available from http://www.accessdata.fda.gov/cdrh_docs/pdf5/P050012S001b.pdf. Accessed 20 June 2009
- 14. U.S. Food and Drug Administration. Summary of safety and effectiveness data: Guardian RT [article online], 2006. Available from http://www.accessdata.fda.gov/cdrh_docs/pdf/P980022s011b.pdf. Accessed 20 June 2009
- 15. Maran A, Crepaldi C, Tiengo A, Grassi G, Vitali E, Pagano G, Bistoni S, Calabrese G, Santeusanio F, Leonetti F, Ribaudo M, Di Mario U, Annuzzi G, Genovese S, Riccardi G, Previti M, Cucinotta D, Giorgino F, Bellomo A, Giorgino R, Poscia A, Varalli M: Continuous subcutaneous glucose monitoring in diabetic patients. Diabetes Care 2002; 25: 347– 352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.