Abstract
OBJECTIVE
The objective of this study was to determine the degree to which ramipril and/or rosiglitazone changed β-cell function over time among individuals with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) who participated in the Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) Trial, which evaluated whether ramipril and/or rosiglitazone could prevent or delay type 2 diabetes in high-risk individuals.
RESEARCH DESIGN AND METHODS
The present analysis included subjects (n = 982) from DREAM trial centers in Canada who had oral glucose tolerance tests at baseline, after 2 years, and at the end of the study. β-Cell function was assessed using the fasting proinsulin–to–C-peptide ratio (PI/C) and the insulinogenic index (defined as 30–0 min insulin/30–0 min glucose) divided by homeostasis model assessment of insulin resistance (insulinogenic index [IGI]/insulin resistance [IR]).
RESULTS
Subjects receiving rosiglitazone had a significant increase in IGI/IR between baseline and end of study compared with the placebo group (25.59 vs. 1.94, P < 0.0001) and a significant decrease in PI/C (−0.010 vs. −0.006, P < 0.0001). In contrast, there were no significant changes in IGI/IR or PI/C in subjects receiving ramipril compared with placebo (11.71 vs. 18.15, P = 0.89, and −0.007 vs. −0.008, P = 0.64, respectively). The impact of rosiglitazone on IGI/IR and PI/C was similar within subgroups of isolated IGT and IFG + IGT (all P < 0.001). Effects were more modest in those with isolated IFG (IGI/IR: 8.95 vs. 2.13, P = 0.03; PI/C: −0.003 vs. −0.001, P = 0.07).
CONCLUSIONS
Treatment with rosiglitazone, but not ramipril, resulted in significant improvements in measures of β-cell function over time in pre-diabetic subjects. Although the long-term sustainability of these improvements cannot be determined from the present study, these findings demonstrate that the diabetes preventive effect of rosiglitazone was in part a consequence of improved β-cell function.
Pancreatic β-cell dysfunction plays a central role the pathogenesis of type 2 diabetes (1). It is present in people at high risk for type 2 diabetes, including those with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (2,3), and it predicts the development of type 2 diabetes in prospective studies of people with these disorders (4,5). β-Cell function is also known to decline steadily over the course of type 2 diabetes, highlighting the progressive nature of this disorder (6). It is therefore crucial to understand the factors that erode or preserve β-cell function across the spectrum of glucose tolerance. Relatively little information is available, however, regarding the determinants of β-cell dysfunction in humans (1).
Recent evidence suggests that thiazolidinediones (TZDs) and ACE inhibitors may preserve β-cell function (7,8). Although TZDs have been demonstrated to improve glucose control and β-cell function in type 2 diabetes (9–11), very little is known about the effect of TZDs on β-cell function in people with hyperglycemia in the nondiabetic range, namely those with IGT and/or IFG (12–15). Similarly, while it has been hypothesized that ACE inhibitors may lower glucose via direct effects on the β-cell (16), studies have not been conducted in people with IGT and/or IFG.
The objectives of this study, therefore, were to determine the degree to which ramipril (an ACE inhibitor) and/or rosiglitazone (a TZD) changed β-cell function over time among individuals with IFG and/or IGT who participated in the Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) Trial, which evaluated whether ramipril and/or rosiglitazone could prevent or delay diabetes in high-risk individuals. We also aimed to determine the degree to which changes in indexes of β-cell function over time were modified by baseline glucose tolerance status and whether ramipril and/or rosiglitazone's effect on diabetes incidence was mediated by treatment-induced changes in β-cell function.
RESEARCH DESIGN AND METHODS
The design and principal findings of the DREAM trial have been presented in previous publications (17). Briefly, the DREAM trial was a large, international, multicenter, double-blind, randomized controlled trial designed to determine whether ramipril and/or rosiglitazone could prevent or delay the development of type 2 diabetes in people with IFG or IGT, metabolic states that indicate very high risk for eventual progression to diabetes (17). Eligibility for the DREAM trial included a diagnosis of IFG, IGT, or both IFG and IGT based on a screening 75-g oral glucose tolerance test (OGTT) (17). A total of 5,269 participants with these disorders were recruited and randomized to either ramipril and/or rosiglitazone using a two-by-two factorial design and followed for a median of 3 years after randomization. Participants were assessed at regular intervals to ascertain the occurrence of the primary outcome, which included new-onset diabetes or all-cause mortality. As part of a substudy, 982 DREAM trial participants attending Canadian study centers had OGTTs at baseline, after 2 years, and at the end of the study, with blood samples drawn fasting as well as 30 and 120 min after the glucose challenge.
The primary outcome variable in the present study was change in β-cell function over the course of follow-up. β-Cell function was assessed using two measures: the insulinogenic index (IGI) and proinsulin (PI) concentration, with IGI defined as (30-min insulin − fasting insulin)/(30-min glucose − fasting glucose). Both indexes have previously been validated against gold-standard measures of insulin secretion (18,19) and have been shown to be significant predictors of incident diabetes in large epidemiological studies. To account for the compensatory response of insulin secretion in relation to background insulin resistance, IGI was divided by the homeostasis model assessment of insulin resistance index (HOMA-IR) (defined as fasting glucose × fasting insulin/22.5 [20]) (IGI/IR) for univariate analysis or adjusted for HOMA-IR in multivariate analysis. Similarly, PI concentration was divided by C-peptide concentration (i.e., the PI/C-peptide ratio [PI/C]) for univariate analysis or adjusted for insulin secretion using C-peptide as a covariate in multivariate analysis. Although the PI-to-insulin ratio is often used to identify disproportionate elevations in PI, C-peptide offers advantages over insulin as a denominator for PI because it is cosecreted with insulin in an equimolar ratio, but unlike insulin it is not extracted by the liver and thus it has a constant peripheral clearance.
Glucose concentration was determined using an enzymatic reference method on a Roche Hitachi 917 Instrument and a Roche reagent kit (Roche Diagnostics, Indianapolis, IN). Serum insulin and C-peptide were measured on the Roche Elecsys 2010 immunoassay analyzer using an electrochemiluminescence immunoassay. The insulin assay had a sensitivity of 1.39 pmol/l, an interassay coefficient of variation (CV) of <4.6% at all levels, and <0.05% cross-reactivity with human C-peptide and PI. The C-peptide assay had a sensitivity of 3.0 pmol/l, an interassay CV of <3% at all levels, and <0.005% cross-reactivity with human insulin. PI concentration was measured using a sandwich enzyme-linked immunosorbent assay manufactured by Linco Research (Linco Research, St. Charles, MO). This assay had a sensitivity of 2.0 pmol/l, an interassay CV of <9% at all levels, and no cross-reactivity with human insulin or des (31,32) split PI, although this assay does cross-react with human des (64,65) split PI.
Statistical analysis
Statistical analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant. The distributions of continuous variables were assessed for normality, and transformations of skewed variables were used in the analysis as appropriate. Means and SDs for primary β-cell function measures (IGI/IR and PI/C) were calculated for each time point (baseline, at 2 years, and final visit), according to the marginal treatment group. Change was calculated as baseline minus final visit value. P values for change were based on a t test of the average change being different from zero, while P values for treatment difference were calculated using the Wilcoxon's rank-sum test. Similar analyses were conducted within subgroups of isolated IFG (IIFG), isolated IGT (IIGT), and combined IFG and IGT (IFG+IGT). As there was no significant interaction between ramipril and rosiglitazone's effect on β-cell function, main-effects analyses were conducted according to marginal randomization groups (i.e., rosiglitazone versus placebo, ramipril versus placebo).
Longitudinal analyses of the associations between treatments and changes over time in β-cell function measures were examined using random-effects models in PROC MIXED, which provides appropriate options for handling the covariance structure of the repeated-measures (longitudinal) data. Specifically, we ran four models, which assessed the impact of treatment group on 1) IGI, with age and HOMA-IR as covariates; 2) IGI/HOMA-IR, with age as a covariate; 3) PI, with age and C-peptide as covariates; and 4) PI/C, with age as a covariate.
Finally, using Cox proportional hazards regression, we assessed whether the impact of ramipril and/or rosiglitazone treatment on diabetes incidence was independent of baseline levels and changes over the course of the trial in β-cell function. The outcome variable in these analyses was diabetes status at the final visit, and the primary exposures were the marginal treatment groups (rosiglitazone, ramipril). In separate models, we assessed the impact of treatment on diabetes incidence, adjusting for either baseline β-cell function (including baseline IGI and HOMA-IR or baseline PI and C-peptide as covariates) or change over the course of the trial in β-cell function (including changes in the above-mentioned covariates). Baseline models were also adjusted for age and baseline waist circumference, fasting glucose, triglycerides, and HDL, while change models were also adjusted for age and changes in these covariates.
RESULTS
Baseline characteristics of participants in this DREAM substudy are presented in Table 1. The average age and BMI were 54 years and 31.5 kg/m2, respectively, and 60% of participants were female. The majority (81%) were of European origin, and substantial proportions had a family history of diabetes or a history of gestational diabetes (61 and 16%, respectively), characteristics that were consistent with the recruiting strategy for the DREAM trial (17). There were no significant differences between the marginal randomization groups for any of the baseline characteristics other than family history of diabetes in the ramipril versus placebo marginal group (P = 0.02) (Table 1).
Table 1.
Baseline characteristics of participants with measures of β-cell function overall and by allocation
| Overall | Randomization (marginal groups) |
||||
|---|---|---|---|---|---|
| Rosiglitazone | Placebo | Ramipril | Placebo | ||
| n | 982 | 505 | 477 | 494 | 488 |
| Age (years) | 54.36 ± 10.64 | 54.81 ± 10.49 | 53.9 ± 10.79 | 53.96 ± 10.47 | 54.77 ± 10.81 |
| BMI (kg/m2) | 31.49 ± 5.45 | 31.36 ± 5.33 | 31.63 ± 5.58 | 31.25 ± 5.35 | 31.74 ± 5.54 |
| Waist-to-hip ratio | 0.89 ± 0.09 | 0.89 ± 0.09 | 0.9 ± 0.09 | 0.89 ± 0.09 | 0.9 ± 0.09 |
| Systolic blood pressure (mmHg) | 135.07 ± 16.85 | 135.4 ± 15.92 | 134.71 ± 17.79 | 134.75 ± 16.52 | 135.39 ± 17.18 |
| Diastolic blood pressure (mmHg) | 82.79 ± 9.89 | 82.88 ± 9.5 | 82.69 ± 10.29 | 82.57 ± 10.06 | 83 ± 9.72 |
| Females | 593 (60.39) | 298 (59.01) | 295 (61.84) | 297 (60.12) | 296 (60.66) |
| IIFG | 97 (9.88) | 49 (9.7) | 48 (10.06) | 49 (9.92) | 48 (9.84) |
| IIGT | 609 (62.02) | 312 (61.78) | 297 (62.26) | 310 (62.75) | 299 (61.27) |
| IFG and IGT | 276 (28.11) | 144 (28.51) | 132 (27.67) | 135 (27.33) | 141 (28.89) |
| Gestational diabetes | 93 (15.68) | 52 (17.45) | 41 (13.9) | 51 (17.17) | 42 (14.19) |
| Family history diabetes | 597 (60.79) | 296 (58.61) | 301 (63.1) | 318 (64.37) | 279 (57.17) |
| European | 795 (80.96) | 416 (82.38) | 379 (79.45) | 400 (80.97) | 395 (80.94) |
| Other ethnicity | 187 (19.04) | 89 (17.62) | 98 (20.55) | 94 (19.03) | 93 (19.06) |
| Statin | 56 (15.89) | 77 (15.25) | 79 (16.56) | 73 (14.78) | 83 (17.01) |
| Blood pressure medications | 168 (17.11) | 93 (18.42) | 75 (15.72) | 82 (16.6) | 86 (17.62) |
| Fasting glucose (mmol/l) | 5.76 ± 0.66 | 5.77 ± 0.66 | 5.74 ± 0.67 | 5.75 ± 0.66 | 5.77 ± 0.67 |
| 30-min glucose (mmol/l) | 10.45 ± 1.74 | 10.55 ± 1.74 | 10.35 ± 1.73 | 10.41 ± 1.74 | 10.5 ± 1.74 |
| 2-h glucose (mmol/l) | 8.78 ± 1.35 | 8.8 ± 1.29 | 8.76 ± 1.4 | 8.74 ± 1.36 | 8.82 ± 1.33 |
| Fasting insulin (pmol/l) | 88.92 ± 2.67 | 88.36 ± 2.66 | 89.52 ± 2.67* | 86.5 ± 2.61 | 91.43 ± 2.72* |
| 30-min insulin (pmol/l) | 438.44 ± 3.53 | 435.11 ± 3.44 | 442.0 ± 3.63* | 436.31 ± 3.51 | 440.6 ± 3.55* |
| IGI | 78.38 ± 2.93 | 75.78 ± 2.99 | 81.22 ± 2.86* | 79.8 ± 2.82 | 76.97 ± 3.04* |
| IGI/IR | 30.56 ± 22.78 | 29.43 ± 20.26 | 31.75 ± 25.16 | 31.52 ± 23.18 | 29.59 ± 22.36 |
| Fasting PI (pmol/l) | 13.47 ± 1.92 | 13.56 ± 1.93 | 13.37 ± 1.91* | 13.13 ± 1.9 | 13.82 ± 1.94* |
| Fasting C-peptide (pmol/l) | 1,011.32 ± 454.96 | 1,005.15 ± 450.93 | 1,017.84 ± 459.56 | 999.98 ± 452.87 | 1,022.82 ± 457.24 |
| PI/C | 0.02 ± 2.98 | 0.02 ± 3.01 | 0.02 ± 2.94* | 0.02 ± 2.99 | 0.02 ± 2.97* |
Data are means ± SD or n (%). No significant differences between rosiglitazone versus placebo or ramipril versus placebo other than family history of diabetes in the ramipril versus placebo group (P = 0.02).
*Indicates that statistical testing was performed using geometric means.
Changes in markers of β-cell function in marginal randomization groups are presented in Table 2. Participants receiving rosiglitazone versus placebo had a significant increase in IGI/IR during the study (25.59 vs. 1.94, P < 0.0001) and a significant decrease in PI/C (−0.010 vs. −0.006, P < 0.0001). In contrast, there were no significant changes in IGI/IR or PI/C in participants receiving ramipril versus placebo (11.71 vs. 18.15, P > 0.05, and −0.007 vs. −0.008, P > 0.05, respectively) (Table 2). In the rosiglitazone group, changes in the β-cell function measures were more substantial between the baseline and 2-year visits compared with changes that occurred between the 2-year and final visits (Table 2). The impact of rosiglitazone on IGI/IR and PI/C was similar within subgroups of IIGT and IFG + IGT (IIGT, IGI/IR: 27.74 vs. 2.76, P < 0.0001; PI/C: −0.009 vs. −0.008, P < 0.001; IFG + IGT, IGI/IR: 27.39 vs. −0.70, P < 0.0001; PI/C: −0.014 vs. −0.002, P < 0.0001), although effects were more modest in those with IIFG (IGI/IR: 8.95 vs. 2.13, P = 0.03; PI/C: −0.003 vs. −0.001, P > 0.05) (supplemental Table, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1579/DC1).
Table 2.
Changes in markers of β-cell function
| Study visit | [(Ins30–Ins0)/(Gluc30–Gluc0)]/HOMA-IR |
PI/C |
|||||
|---|---|---|---|---|---|---|---|
| n | P | Means ± SD | n | P | Means ± SD | ||
| A: rosiglitazone marginal group | |||||||
| Placebo | Baseline | 357 | 34.0 ± 22.82 | 449 | 0.022 ± 0.04 | ||
| 2 years | 350 | 41.74 ± 43.94 | 422 | 0.019 ± 0.03 | |||
| Final | 357 | 35.94 ± 38.42 | 449 | 0.016 ± 0.02 | |||
| Change* | 357 | 1.94 ± 36.37 | 449 | −0.006 ± 0.04 | |||
| P † | 0.31 | 0.0044 | |||||
| Rosiglitazone | Baseline | 429 | 29.95 ± 20.44 | 480 | 0.024 ± 0.04 | ||
| 2 years | 417 | 59.82 ± 82.93 | 463 | 0.018 ± 0.03 | |||
| Final | 429 | 55.53 ± 125.14 | 480 | 0.014 ± 0.02 | |||
| Change* | 429 | 25.59 ± 125.22 | 480 | −0.010 ± 0.04 | |||
| P † | <0.0001 | <0.0001 | |||||
| Treatment difference | P ‡ | <0.0001 | <0.0001 | ||||
| B: ramipril marginal group | |||||||
| Placebo | Baseline | 383 | 30.72 ± 21.17 | 461 | 0.023 ± 0.04 | ||
| 2 years | 372 | 50.26 ± 72.91 | 440 | 0.017 ± 0.02 | |||
| Final | 383 | 48.87 ± 128.97 | 461 | 0.014 ± 0.01 | |||
| Change* | 383 | 18.15 ± 128.93 | 461 | −0.008 ± 0.04 | |||
| P † | 0.0062 | <0.0001 | |||||
| Ramipril | Baseline | 403 | 32.80 ± 22.04 | 468 | 0.023 ± 0.04 | ||
| 2 years | 395 | 52.80 ± 64.20 | 445 | 0.020 ± 0.04 | |||
| Final | 403 | 44.51 ± 48.48 | 468 | 0.015 ± 0.03 | |||
| Change* | 403 | 11.71 ± 48.18 | 468 | −0.007 ± 0.05 | |||
| P † | <0.0001 | 0.0009 | |||||
| Treatment difference | P ‡ | 0.89 | 0.64 | ||||
Data are means ± SD and are reported for marginal treatment groups.
*Change was calculated as baseline minus final visit value.
†P value for change were based on a t test of the average change being different from zero.
‡P values for treatment difference were calculated using the Wilcoxon rank-sum test.
We further assessed the impact of treatment on markers of β-cell function by utilizing longitudinal data from multiple study time points in mixed-model analyses. Compared with placebo, rosiglitazone significantly increased IGI after adjustment for age and HOMA-IR (P = 0.015) (Table 3). In contrast, ramipril did not significantly affect adjusted IGI (P > 0.05). Similar findings were seen using PI concentration as a measure of β-cell function. Specifically, rosiglitazone significantly reduced PI concentrations over time after adjustment for age and C-peptide concentration (P = 0.0064) (Table 3). In contrast, ramipril did not significantly change adjusted PI concentrations (P > 0.05).
Table 3.
Longitudinal changes in markers of β-cell function in DREAM trial: analysis of slopes using mixed-model analysis
| Slope | SE | P | Slope difference | |
|---|---|---|---|---|
| Rosiglitazone versus placebo | ||||
| PI | ||||
| PI/C (adjusted for age) | −0.003 | 0.0005 | 0.25 | 0.0008 |
| PI (adjusted for age, fasting C-peptide) | −1.0524 | 0.1344 | 0.0064 | 0.5308 |
| IGI | ||||
| IGI/HOMA-IR (adjusted for age) | 9.0674 | 1.115 | <0.0001 | −7.0191 |
| IGI (adjusted for age and HOMA-IR) | 5.2814 | 1.3232 | 0.015 | −4.7305 |
| Ramipril versus placebo | ||||
| PI | ||||
| PI/C (adjusted for age) | −0.0028 | 0.0005 | 0.57 | 0.0004 |
| PI (adjusted for age, fasting C-peptide) | −0.7796 | 0.14 | 0.87 | 0.0329 |
| IGI | ||||
| IGI/HOMA-IR (adjusted for age) | 5.2084 | 1.1522 | 0.5 | 1.1206 |
| IGI (adjusted for age and HOMA-IR) | 2.7603 | 1.3517 | 0.73 | 0.6681 |
Analysis in table are based on full data; results essentially unchanged when analysis repeated on subjects with information from all visits.
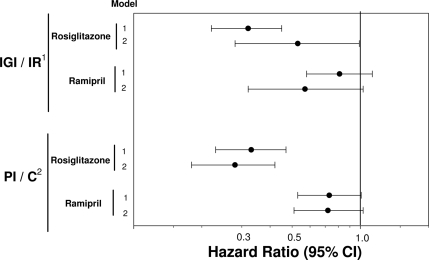
We assessed whether the impact of ramipril and/or rosiglitazone on diabetes incidence was independent of baseline levels or changes in indexes of β-cell function with time (Fig. 1). After accounting for baseline β-cell function as measured by either IGI or PI in models that adjusted for insulin resistance and other covariates, rosiglitazone significantly reduced the risk of developing diabetes (hazard ratio 0.32 [95% CI 0.22–0.45], P < 0.001, and 0.33 [0.23–0.47], P < 0.001, in IGI and PI models, respectively). Adjusting for the change in β-cell function as measured by IGI attenuated the preventive effect of rosiglitazone on incident diabetes (0.53 [0.28–0.99], P = 0.046). Such attenuation was not noted when the change in β-cell was measured using PI (0.28 [0.18–0.42], P < 0.0001) (Fig. 1, model 2). Ramipril showed smaller, nonsignificant reductions in diabetes risk.
Figure 1.
Association of treatment allocation with risk of diabetes: impact of baseline levels and changes in β-cell function. 1For IGI/IR models: model 1 adjusted for age and baseline IGI, HOMA-IR, fasting glucose, waist circumference, triglyceride, and HDL; model 2 adjusted for age and changes in IGI, HOMA-IR, fasting glucose, waist circumference, triglyceride, and HDL. 2For PI/C models: model 1 adjusted for age and baseline PI, C-peptide, fasting glucose, waist circumference, triglyceride, and HDL; model 2 adjusted for age and changes in PI, C-peptide, fasting glucose, waist circumference, triglyceride, and HDL.
CONCLUSIONS
Rosiglitazone, but not ramipril, improved measures of β-cell function over time in people with IFG and/or IGT. Specifically, rosiglitazone increased IGI/IR and reduced PI/C by 86 and 42%, respectively. These findings were consistent across glucose tolerance subgroups (IIFG, IIGT, and IFG+IGT), although there was the suggestion of a more modest effect in the subgroup with IIFG. Finally, rosiglitazone's effect on diabetes prevention persisted after adjustment for baseline β-cell function. The demonstration that an agent that reduces diabetes incidence also improves β-cell function invites the hypothesis that measures of change in β-cell function in response to therapy are indexes of that therapy's ability to reduce the incidence of diabetes. The modest attenuation of rosiglitazone's effect after accounting for the change in β-cell function suggests that some but not all of the effect of rosiglitazone on diabetes prevention/delay is mediated through its effects on β-cell function.
Whereas a number of previous studies (9–11) have documented improvements in β-cell function with TZD treatment in people with diabetes, only four studies (12–15) have investigated this question in people with IFG and/or IGT. The results of these studies have been inconsistent, with two studies (12,15) indicating a significant improvement in measures of β-cell function with TZD treatment and two studies (13,14) reporting no change. These studies all had small sample sizes (n ≤ 30), and the methods used to assess β-cell function varied widely from intensive approaches (insulin responses to glucose infusion [12,14]) to simple fasting-based indexes (HOMA-B [13,15]). Interestingly, the complexity of the method used to assess β-cell function did not appear to explain the differences in findings of these previous studies (12,14). Finally, in a diabetes prevention trial among Hispanic women with previous gestational diabetes, ∼70% of whom had IGT at enrollment, treatment with troglitazone resulted in significant improvements in the frequently sampled intravenous glucose tolerance–determined disposition index after 3 months (21).
Our results suggest a significant improvement in β-cell function with TZD treatment in pre-diabetic subjects. These findings extend the literature in a number of important ways. First, the sample size of our study (n = 982) was much larger that previous investigations. Second, we demonstrated improvements in β-cell function under TZD treatment using two validated, widely used proxy measures of β-cell function, namely IGI and PI. Third, in our analysis we accounted for the compensatory impact on β-cell function of background insulin resistance. Specifically, in analyses of IGI we used HOMA-IR either as a covariate in multivariate analysis or as the denominator in the IGI–to–HOMA-IR ratio. C-peptide was used in a similar fashion in analyses of PI. Accounting for background insulin resistance is crucial to the interpretation of β-cell function measures in this study because TZDs improved insulin sensitivity, thus reducing pancreatic β-cell demand.
The exact mechanisms responsible for the increases in IGI/IR and the reductions in PI/C documented in the present study are not known, although a number of possibilities exist. The reduction in insulin resistance with TZDs would be expected to reduce insulin secretory demand on the β-cells and thus reduce β-cell stress. In addition, TZDs such as rosiglitazone may improve β-cell function indirectly by ameliorating a number of pathogenic processes that have been shown to be detrimental to the β-cells, including lipotoxicity, glucotoxicity, and inflammation (7,8). TZDs lower free fatty acids (22), elevated levels of which result in excess deposition of lipid within β-cells, which in turn leads to increases in ceremide and the stimulation of nitric oxide–mediated β-cell apoptosis. As well, the glucose-lowering effect of TZDs may reduce the impact of reactive oxygen species on the β-cells, which are known to be especially susceptible to oxidative damage (23). Finally, TZDs have been demonstrated to reduce levels of inflammatory cytokines (24), which may induce β-cell apoptosis when chronically elevated. TZDs may also impact β-cell function directly by maintaining β-cell proliferation and/or reducing β-cell apoptosis (25).
In the HOPE study, treatment with ramipril was shown to reduce the incidence of diabetes in middle-aged individuals with vascular disease. In the DREAM trial, although ramipril did not significantly reduce the incidence of diabetes in people with IFG and/or IGT, it did significantly increase regression to normoglycemia. The mechanism by which ramipril might reduce glucose levels and/or prevent/delay diabetes is unknown, although vascular and metabolic effects on the muscle and pancreatic β-cell have been proposed (16). The results of the present study suggest that ramipril does not have significant effects on β-cell function compared with placebo in people at high risk for diabetes, and thus its glucose-lowering effects may operate through other metabolic mechanisms. The improvement in β-cell function in the placebo arm of the ramipril marginal group analysis may be explained by the fact that under the factorial design of the DREAM trial, half of the participants on ramipril placebo were receiving active rosiglitazone.
The major strengths of the present study include the large sample size, the randomized, double-blind design, and the completeness of follow-up (92.6%). Further, participants were thoroughly characterized in terms of glucose tolerance status and were all in the pre-diabetic range (either IIFG, IIGT, or IFG+IGT). The major limitation of the present study is the lack of detailed measures of β-cell function, such as those obtained from the hyperglycemic clamp technique or the frequently sampled intravenous glucose tolerance test. Notwithstanding, we used two proxy measures of β-cell function that have been extensively validated and used in previous studies including the Diabetes Prevention Program and the American Diabetes Association Genetics of Type 2 Diabetes Study (in the case of IGI/IR) and the IRAS study (in the case of PI).
In conclusion, rosiglitazone, but not ramipril, resulted in significant improvements in measures of β-cell function over time. These findings demonstrate that the diabetes preventive effect of rosiglitazone may in part be a consequence of improved β-cell function.
Supplementary Material
Acknowledgments
H.C.G.'s institution has received research grants for the conduct of clinical trials that include rosiglitazone and ramipril from GlaxoSmithKline and sanofi-aventis. He has also received honoraria from these companies for providing advice and speaking. No other potential conflicts of interest relevant to this article were reported.
This substudy was supported by a grant from the Canadian Institutes of Health Research. A.J.H. is supported through a Canadian Diabetes Association scholarship and holds a Tier II Canada Research Chair in Diabetes Epidemiology. B.Z. holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and the University of Toronto.
We thank Kim Hall, Jackie Bosch, and Dr. Janet Raboud for their advice and expert technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Prentki M, Nolan CJ: Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Haeften TW, Pimenta W, Mitrakou A, Korytkowski M, Jenssen T, Yki-Jarvinen H, Gerich JE: Disturbances in β-cell function in impaired fasting glycemia. Diabetes 2002;51(Suppl. 1):S265–S270 [DOI] [PubMed] [Google Scholar]
- 3. Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 4. Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanley AJG, D'Agostino R, Jr, Wagenknecht LE, Saad MF, Savage PJ, Bergman R, Haffner SM: Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the Insulin Resistance Atherosclerosis Study. Diabetes 2002;51:1263–1270 [DOI] [PubMed] [Google Scholar]
- 6. UKPDS 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 7. Decker M, Hofflich H, Elias AN: Thiazolidinediones and the preservation of beta-cell function, cellular proliferation and apoptosis. Diabetes Obes Metab 2008;10:617–625 [DOI] [PubMed] [Google Scholar]
- 8. Bell DS: Beta-cell rejuvenation with thiazolidinediones. Am J Med 2003;115(Suppl. 8A):20S–23S [DOI] [PubMed] [Google Scholar]
- 9. Prigeon RL, Kahn SE, Porte D, Jr: Effect of troglitazone on B cell function, insulin sensitivity, and glycemic control in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 1998;83:819–823 [DOI] [PubMed] [Google Scholar]
- 10. Wallace TM, Levy JC, Matthews DR: An increase in insulin sensitivity and basal beta-cell function in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet Med 2004;21:568–576 [DOI] [PubMed] [Google Scholar]
- 11. Smith SA, Porter LE, Biswas N, Freed MI: Rosiglitazone, but not glyburide, reduces circulating proinsulin and the proinsulin:insulin ratio in type 2 diabetes. J Clin Endocrinol Metab 2004;89:6048–6053 [DOI] [PubMed] [Google Scholar]
- 12. Cavaghan MK, Ehrmann DA, Byrne MM, Polonsky KS: Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest 1997;100:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osei K, Gaillard T, Kaplow J, Bullock M, Schuster D: Effects of rosiglitazone on plasma adiponectin, insulin sensitivity, and insulin secretion in high-risk African Americans with impaired glucose tolerance test and type 2 diabetes. Metabolism 2004;53:1552–1557 [DOI] [PubMed] [Google Scholar]
- 14. Hung YJ, Hsieh CH, Pei D, Kuo SW, Lee JT, Wu LY, He CT, Lee CH, Fan SC, Sheu WH: Rosiglitazone improves insulin sensitivity and glucose tolerance in subjects with impaired glucose tolerance. Clin Endocrinol (Oxf) 2005;62:85–91 [DOI] [PubMed] [Google Scholar]
- 15. Hung YJ, Lin SH, Pei D, Kuo SW, Hsieh CH, He CT, Hsing Lee C, Fan SC, Sheu WH: Rosiglitazone improves insulin sensitivity in nonobese subjects with impaired glucose tolerance: the role of adiponectin and C-reactive protein. Metabolism 2006;55:439–444 [DOI] [PubMed] [Google Scholar]
- 16. Carlsson PO, Berne C, Jansson L: Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 1998;41:127–133 [DOI] [PubMed] [Google Scholar]
- 17. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR: Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 18. Wareham NJ, Phillips DI, Byrne CD, Hales CN: The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 1995;12:931 [DOI] [PubMed] [Google Scholar]
- 19. Mykkanen L, Haffner SM, Hales CN, Ronnemaa T, Laakso M: The relation of proinsulin, insulin, and proinsulin-to-insulin ratio to insulin sensitivity and acute insulin response in normoglycemic subjects. Diabetes 1997;46:1990–1995 [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 21. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP: Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 22. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–E883 [DOI] [PubMed] [Google Scholar]
- 23. Robertson RP, Harmon J, Tran PO, Poitout V: β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004;53(Suppl. 1):S119–S124 [DOI] [PubMed] [Google Scholar]
- 24. Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI: Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 2002;106:679–684 [DOI] [PubMed] [Google Scholar]
- 25. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE: β-Cell mass dynamics in Zucker diabetic fatty rats: rosiglitazone prevents the rise in net cell death. Diabetes 2001;50:1021–1029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.