Abstract
OBJECTIVE
Using the clamp technique, youths with a clinical diagnosis of type 2 diabetes (CDx-type 2 diabetes) and positive pancreatic autoantibodies (Ab+) were shown to have severe impairment in insulin secretion and less insulin resistance than their peers with negative antibodies (Ab−). In this study, we investigated whether oral glucose tolerance test (OGTT)-derived indexes of insulin secretion and sensitivity could distinguish between these two groups.
RESEARCH DESIGN AND METHODS
A total of 25 Ab−, 11 Ab+ CDx-type 2 diabetic, and 21 obese control youths had an OGTT. Fasting and OGTT-derived indexes of insulin sensitivity (including the Matsuda index, homeostasis model assessment [HOMA] of insulin resistance, quantitative insulin sensitivity check index, and glucose-to-insulin ratio) and insulin secretion (HOMA of insulin secretion and 30-min insulogenic and C-peptide indexes) were used. Glucagon and glucagon-like peptide (GLP)-1 responses were assessed.
RESULTS
Fasting C-peptide and C-peptide–to–glucose ratio, and C-peptide area under the curve (AUC) were significantly lower in the Ab+ CDx-type 2 diabetic patients. Other OGTT-derived surrogate indexes of insulin sensitivity and secretion were not different between the Ab+ versus Ab− patients. GLP-1 during the OGTT was highest in the Ab+ youths compared with the other two groups, but this difference disappeared after adjusting for BMI. Ab+ and Ab− CDx-type 2 diabetes had relative hyperglucagonemia compared with control subjects.
CONCLUSIONS
The clinical measures of fasting and OGTT-derived surrogate indexes of insulin sensitivity and secretion, except for fasting C-peptide and C-peptide AUC, are less sensitive tools to distinguish metabolic/pathopysiological differences, detected by the clamp, between Ab+ and Ab− CDx-type 2 diabetic youths. This underscores the importance of using more sensitive methods and the importance of determining antibody status in obese youths with CDx-type 2 diabetes.
Diabetes in youth is generally classified into two major categories: type 1 diabetes characterized by autoimmune destruction of the pancreatic β-cells and absolute insulin deficiency and type 2 diabetes characterized by insulin resistance coupled with a nonimmune-mediated β-cell failure and relative insulin deficiency (1). While type 1 diabetes remains the most common form of childhood diabetes, type 2 diabetes in youth has increased worldwide over the last decade concomitant with the epidemic increase in childhood obesity (2,3). The diagnosis of type 1 versus type 2 diabetes in children relies largely on the clinical presentation with obesity being a major characteristic of children diagnosed with type 2 diabetes (1,2). However, the increasing prevalence of obesity in children, including those newly diagnosed with type 1 diabetes, has made the clinical distinction between the two types of diabetes more difficult (4). Moreover, 10–75% of physician-diagnosed obese youth with type 2 diabetes have islet cell autoantibodies (3,5–7), which is the hallmark of autoimmune type 1 diabetes. In a previous study using the hyperinsulinemic-euglycemic and the hyperglycemic clamp, we demonstrated important distinguishing features in insulin sensitivity and secretion between antibody-positive (Ab+) versus -negative (Ab−) obese youth with a clinical diagnosis of type 2 diabetes (CDx-type 2 diabetes). While insulin sensitivity was severely impaired in Ab− but not Ab+ patients, β-cell function was almost completely abolished in Ab+ and not Ab− type 2 diabetes (8). Moreover, the Ab− CDx-type 2 diabetic patients had features consistent with the metabolic syndrome. These pathophysiological differences have important bearing on the management of diabetes and highlight the importance of making the correct diagnosis. While the clamp technique is considered the gold standard for studying in vivo insulin secretion and sensitivity, its use is limited to the research setting. Therefore, in this study, we investigated whether the oral glucose tolerance test (OGTT), a clinically applicable tool, could be used to distinguish the differences in insulin sensitivity and secretion between the two groups of patients with phenotypic type 2 diabetes versus obese control subjects with normal glucose tolerance.
RESEARCH DESIGN AND METHODS
Thirty-six obese adolescents, all reported previously (8), with CDx-type 2 diabetes diagnosis made by the attending endocrinologist based on the American Diabetes Association diagnostic criteria (1), were recruited from the Diabetes Center at the Children's Hospital of Pittsburgh. Islet cell antibody screening revealed 25 with negative antibodies and 11 with positive antibodies. Islet cell antibodies were tested using the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored harmonization assay. The control group consisted of 21 age-matched obese, otherwise healthy, adolescents recruited from the community. All study participants were pubertal. The treatment modalities at the time of the study and the characteristics of the study population are summarized in Table 1. None of the obese control subjects were on medications that affect blood glucose metabolism. All studies were approved by the University of Pittsburgh Institutional Review Board, and consents and assents were obtained prior to the investigation.
Table 1.
Participants' demographics and fasting laboratory data
| Clinically diagnosed type 2 diabetes |
Obese control subjects | ANOVA P | Post hoc P (Ab+ vs. Ab−)* | ||
|---|---|---|---|---|---|
| Ab− | Ab+ | ||||
| n | 25 | 11 | 21 | ||
| Age (years) | 15.1 ± 0.4 | 14.0 ± 0.7 | 14.5 ± 0.3 | 0.23 | 0.3 |
| Sex (male/female)† | 12/13 | 5/6 | 8/13 | 0.79† | |
| Ethnicity (African American/white)† | 13/12 | 5/6 | 12/9 | 0.82† | |
| Tanner (n)† | 0.31† | ||||
| II–III | 2 | 3 | 3 | ||
| IV–V | 23 | 8 | 18 | ||
| BMI (kg/m2) | 36.4 ± 1.1 | 30.4 ± 1.3 | 36.5 ± 1.1 | 0.005 | 0.008 |
| BMI (%) | 98.8 ± 0.2 | 96.5 ± 1.2 | 98.7 ± 0.4 | 0.008 | 0.01 |
| BMI Z score | 2.39 ± 0.1 | 1.99 ± 0.1 | 2.35 ± 0.1 | 0.005 | 0.005 |
| Waist circumference (cm) | 108.6 ± 2.8 | 92.4 ± 3.6 | 104.2 ± 2.8 | 0.006 | 0.004 |
| Percent body fat (%) | 41.2 ± 1.4 | 39.6 ± 2.5 | 44.3 ± 1.0 | 0.1 | 1.0 |
| Visceral adipose tissue (cm2) | 85.6 ± 8.6 | 52.0 ± 6.9 | 59.2 ± 4.1 | 0.006 | 0.019 |
| Subcutaneous abdominal adipose tissue (cm2) | 532.7 ± 29.5 | 432.8 ± 42.1 | 517.0 ± 38.6 | 0.2 | 0.24 |
| A1C (%) | 6.7 ± 0.2 | 6.3 ± 0.3 | 5.4 ± 0.1 | <0.001 | 0.78 |
| Diabetes duration (months) | 8.2 ± 2.3 | 4.5 ± 1.4 | NA | 0.3 | |
| Treatment modality n (%)† | 0.069† | ||||
| Lifestyle [n (%)] | 7 (28) | 2 (18) | NA | ||
| Insulin | 3 (12) | 1 (9) | NA | ||
| Metformin | 10 (40) | 1 (9) | NA | ||
| Insulin and metformin | 5 (20) | 7 (64) | NA | ||
| Proinsulin-to-insulin ratio | 0.25 ± 0.03 | 0.21 ± 0.08 | 0.28 ± 0.05 | 0.59 | 1.0 |
| Leptin (ng/ml) | 31.6 ± 2.9 | 23.3 ± 4.0 | 42.6 ± 3.8 | 0.004 | 0.37 |
| Adiponectin (μg/ml) | 5.2 ± 0.6 | 7.1 ± 1.6 | 6.3 ± 0.4 | 0.25 | 0.34 |
| Leptin-to-adiponectin ratio | 8.9 ± 1.6 | 4.1 ± 0.9 | 7.9 ± 1.3 | 0.13 | 0.14 |
Data are means ± SE.
*Post hoc Bonferroni correction for Ab− versus Ab+ type 2 diabetic patients.
†χ2 analysis. NA, not applicable.
Autoantibody testing
GAD 65 kDa autoantibody (GAD65 Ab) and insulinoma-associated protein 2 autoantibody (IA2) were measured using a standardized assay protocol and a common serum calibrator developed by the NIDDK-sponsored standardization group. Results are expressed as DKU/ml. Based on analysis of 550 samples, the cutoff values for positivity/negativity are 33 DKU/ml for GAD65 and 5 DKU/ml for IA2. The calculated assay specificity is 97 for GAD65 and 99 for IA2, while the sensitivity is 76 and 64, respectively.
OGTT
Study participants were admitted to the Pediatric Clinical and Translational Research Center at the Children's Hospital of Pittsburgh. After a 10- to 12-h overnight fast, they underwent a 2-h OGTT (1.75 g/kg, maximum 75 g). Blood samples were obtained at −15, 0, 15, 30, 60, 90, and 120 min for determination of glucose, insulin, C-peptide, glucagon, and GLP-1 levels. Metformin was discontinued 36 h prior to the OGTT. Patients did not receive long- or intermediate-acting insulin for 24 h prior to the OGTT. The last dose of short-acting insulin was given 6–8 h prior to the OGTT.
Biochemical measurements
Plasma glucose was measured with a glucose analyzer (Yellow Springs Instrument, Yellow Springs, OH). Insulin, C-peptide, glucagon, leptin, and adiponectin were measured using radioimmunoassay as before (9). One Ab− patient had high levels of nonspecific binding in the insulin radioimmunoassay, and her insulin data were not included in this analysis. A1C was measured by high-performance liquid chromatography (Tosoh Medics) (9). GLP-1 was measured in 22 CDx-type 2 diabetic and 14 control subjects who had plasma samples collected in tubes containing dipeptidyl peptidase-4 inhibitor (Millipore, St. Charles, MO). Serum GLP-1 was measured on the Luminex 200 IS (Luminex, Austin, TX) using a one-plex human endrocrine hormone MILLIplex Kit (catalog no. HENDO-65K-01; Millipore). This assay specifically detects GLP-1 (7–36) amide and GLP-1 (7–37), with no detectable cross-reactivity with GLP-2, GLP-1 (9–36), or glucagon. In our laboratory, the inter- and intra-assay coefficients of variation were 7.7 and 5.1%, respectively. Proinsulin levels were determined at Esoterix (Calabasas Hills, CA).
Body composition
Body composition was determined by dual-energy X-ray absorptiometry. Subcutaneous abdominal adipose tissue and visceral adipose tissue were examined by a single-slice computed tomography (CT) scan at L4–L5 as described (8).
Calculations: OGTT-derived indexes of insulin sensitivity
Composite whole-body insulin sensitivity (WBISIMatsuda) index, the homeostasis model assessment for insulin resistance (HOMA-IR), the quantitative insulin sensitivity check index (QUICKI), the Cederholm (ISICederholm), the Stumvoll (ISIStumvoll), the Gutt (ISIGutt), and the Avignon (ISIAvignon) insulin sensitivity indexes were calculated as per published equations (10,11).
OGTT-derived indexes of insulin secretion
The insulogenic index (ΔI30/ΔG30), using fasting and 30-min OGTT insulin or C-peptide (ΔC-pep30/ΔG30), and the HOMA of insulin secretion (HOMA-%β) were calculated as before (12,13). The area under the curve (AUC) for glucose and insulin were calculated using the trapezoidal rule.
Statistical analysis
Comparison of continuous variables between the three groups (Ab+, Ab−, and control subjects) was performed using univariate ANCOVA with post hoc Bonferroni correction. A three-way repeated-measures analysis was used to compare glucose, insulin, C-peptide, glucagon, and GLP-1 responses during the OGTT among the three groups with time as a factor. ANCOVA and repeated-measures analyses were adjusted for BMI. Categorical variables were compared using the χ2 analysis. A P value of ≤0.05 was considered statistically significant. All values are reported as means ± SE.
RESULTS
Study participants
The clinical and physical characteristics of the study participants are summarized in Table 1. There were no significant differences in age, sex, Tanner stage, and ethnicity among the groups. The Ab+ patients had significantly lower BMI, BMI percentile, Z score, waist circumference, and visceral adipose tissue compared with their Ab− peers and the obese control subjects. A1C at the time of the study was comparable between the Ab+ and the Ab− CDx-type 2 diabetic and higher than obese control subjects. Diabetes duration was similar between the two groups of CDx-type 2 diabetic patients. More Ab− patients tended to be on metformin and lifestyle intervention than Ab+ patients. All subjects in the obese control group had normal glucose tolerance as defined by World Health Organization and American Diabetes Association criteria (1).
Fasting and OGTT glucose, insulin, and C-peptide
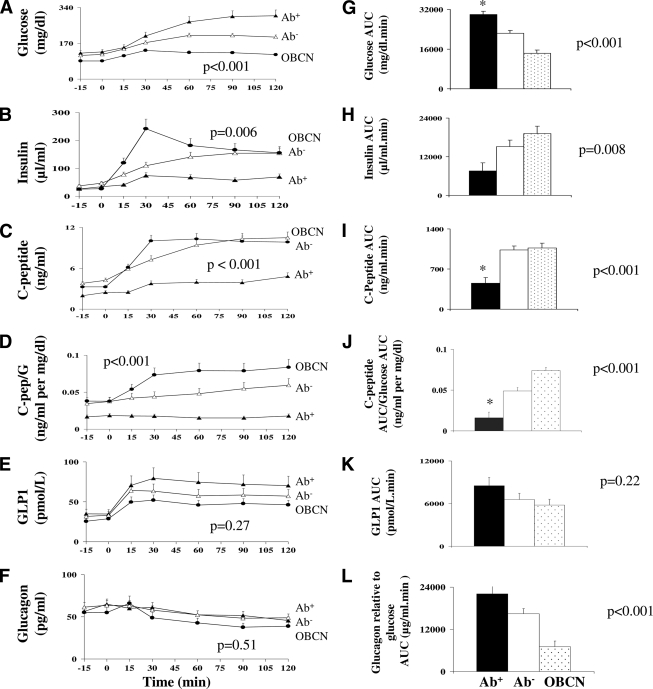
Fasting glucose and insulin were not significantly different between Ab− and Ab+ patients (Table 2) (Fig. 1). However, fasting C-peptide was lower in Ab+ versus Ab− patients (Table 2). Glucose, insulin, and C-peptide responses during the OGTT after adjusting for BMI in the three groups are summarized in Fig. 1A–C. In a three-way repeated-measures analysis with time as a factor and BMI as covariate, glucose levels during the OGTT were significantly different among the three groups (P < 0.001) and were highest in Ab+ versus Ab− (P = 0.003) and lowest in control subects (P < 0.001). Insulin levels were significantly different among the three groups (P = 0.006) and was lowest in Ab+ and highest in control subjects with no significant difference between Ab− and Ab+ patients. C-peptide levels were significantly different among the three groups (P < 0.001) and was highest in Ab− versus Ab+ (P < 0.001), with no significant difference between the Ab− patients and the obese control subjects. Because glucose levels differed during the OGTT among the three groups, C-peptide levels relative to glucose levels are depicted as the C-peptide–to–glucose ratio Fig. 1D. C-peptide–to–glucose ratio differed significantly among the three groups and was highest in control subjects and lowest in Ab+ patients, with significant differences between the Ab− and Ab+ patients (P = 0.001). The glucose AUC was highest in the Ab+ patients (P < 0.001) and significantly higher than Ab− patients (P < 0.001) (Fig. 1G). C-peptide AUC was significantly lower in the Ab+ compared with the Ab− patients and obese control subjects, with no difference between the latter two. C-peptide AUC relative to glucose AUC (Fig. 1J) was significantly different among the three groups (0.016 ± 0.007, 0.049 ± 0.004, and 0.074 ± 0.004 ng/ml per mg/dl), with significant differences between Ab+ and Ab− patients (P < 0.001).
Table 2.
Fasting and OGTT-derived surrogate indexes of insulin sensitivity and secretion in Ab− vs. Ab+ clinically diagnosed type 2 diabetic patients and obese control subjects
| Clinically diagnosed type 2 diabetes |
Obese control subjects | ANCOVA P | Post hoc P (Ab+ vs. Ab−)* | ||
|---|---|---|---|---|---|
| Ab− | Ab+ | ||||
| n | 25 | 11 | 21 | ||
| GF (mg/dl) | 117.9 ± 4.7 | 130.2 ± 7.6 | 85.9 ± 5.1 | <0.001 | 0.55 |
| IF (μU/ml) | 47.1 ± 4.6 | 34.3 ± 7.6 | 25.5 ± 4.9 | 0.008 | 0.5 |
| C-PepF (ng/ml) | 4.2 ± 0.3 | 2.5 ± 0.4 | 3.2 ± 0.3 | 0.001 | 0.002 |
| Fasting and OGTT-derived indexes of insulin sensitivity | |||||
| GF/IF (12) | 3.2 ± 0.4 | 4.5 ± 0.7 | 4.8 ± 0.5 | 0.05 | 0.4 |
| HOMA-IR (10) | 14.9 ± 2.2 | 11.9 ± 3.6 | 5.0 ± 2.3 | 0.01 | 1.0 |
| QUICKI (10) | 0.274 ± 0.004 | 0.282 ± 0.006 | 0.307 ± 0.004 | <0.001 | 0.95 |
| WBISIMatsuda (10) | 1.23 ± 0.2 | 1.74 ± 0.3 | 1.99 ± 0.2 | 0.012 | 0.43 |
| ISICederholm (10) | 23.6 ± 1.6 | 16.7 ± 2.6 | 40.3 ± 1.8 | <0.001 | 0.1 |
| ISIGutt (10) | 2.9 ± 0.1 | 3.1 ± 0.2 | 4.0 ± 0.1 | <0.001 | 1.0 |
| ISIStumvoll (10) | 0.015 ± 0.01 | 0.069 ± 0.02 | 0.016 ± 0.01 | 0.07 | 0.1 |
| ISIAvignon (11) | 0.33 ± 0.06 | 0.39 ± 0.09 | 0.67 ± 0.06 | 0.001 | 1.0 |
| Fasting and OGTT-derived indexes of insulin secretion | |||||
| C-PepF/ GF | 0.036 ± 0.002 | 0.020 ± 0.004 | 0.037 ± 0.003 | 0.001 | 0.002 |
| HOMA-%β (12) | 329.1 ± 42.9 | 254.5 ± 70.9 | 400.9 ± 45.7 | 0.21 | 1.0 |
| ΔI30/ΔG30 | 1.13 ± 0.5 | 0.79 ± 0.9 | 5.0 ± 0.6 | <0.001 | 1.0 |
| ΔC-Pep30/Δ G30 | 0.06 ± 0.01 | 0.02 ± 0.02 | 0.16 ± 0.01 | <0.001 | 0.44 |
Data are means adjusted for BMI ± SE.
*Post hoc Bonferroni correction for Ab− versus Ab+ type 2 diabetic patients.
Figure 1.
A–F: Glucose, insulin, C-peptide, C-peptide–to–glucose ratio, GLP-1, and glucagon, respectively, during the OGTT in Ab− CDx-type 2 diabetes (▵) versus Ab+ CDx-type 2 diabetic patients (▴) versus obese control subjects (●). G–L: Area under the curve (AUC) for glucose, insulin, C-peptide, C-peptide–to–glucose ratio, GLP-1, and glucagon relative to glucose, respectively, during the OGTT in Ab− CDx-type 2 diabetes (□) versus Ab+ CDx-type 2 diabetes (■) versus obese control subjects ( ). Data adjusted for BMI. *P ≤ 0.001 for Ab+ vs. Ab− post hoc Bonferroni correction.
). Data adjusted for BMI. *P ≤ 0.001 for Ab+ vs. Ab− post hoc Bonferroni correction.
Fasting and OGTT-derived indexes of insulin sensitivity
Fasting glucose–to–insulin ratio, HOMA-IR, WBISIMatsuda, QUICKI, ISICederholm, ISIGutt, and ISIAvignon were significantly different among the three groups, while ISIStumvoll was not. None of these indexes, however, were significantly different between Ab− and Ab+ CDx-type 2 diabetes (Table 2).
Fasting and OGTT-derived indexes of insulin secretion
Fasting C-peptide–to–glucose ratio, 30-min insulinogenic index, ΔI30/ΔG30, andΔC-Pep30/ΔG30 were significantly different among the three groups, but HOMA-%β was not. Fasting C-peptide–to–glucose ratio was significantly lower in the Ab+ versus Ab− patients with and without adjustment for BMI, with no significant differences in HOMA-%β, ΔI30/ΔG30, and ΔC-Pep30/ΔG30 (Table 2).
GLP-1 and glucagon responses during the OGTT
A subset of participants (13 Ab−, 9 Ab+, and 14 obese control subjects) had GLP-1 data. GLP-1 response and AUC during the OGTT were significantly different among the three groups and highest in Ab+ patients (Fig. 1E and K). However, this difference disappeared after adjusting for BMI (Fig. 1E).
The glucagon levels (Fig. 1F) and AUC (7,233 ± 587 vs. 6,150 ± 590 and 5,366 ± 713 pg · ml−1 · min−1 in Ab−, Ab+, and obese control subjects, respectively; P = 0.1) during the OGTT were not different among the three groups. However, when corrected for prevailing glucose levels during the OGTT (glucagon AUC × glucose AUC) the glucagon levels were lowest in the control subjects compared with the two groups of diabetic subjects despite adjustment for BMI (Fig. 1L).
Adiponectin, leptin, and proinsulin
Leptin was significantly different among the three groups and lowest in Ab+ patients versus obese control subjects before (P = 0.004) and after (P = 0.03) adjusting for BMI. Adiponectin, leptin-to-adiponectin ratio, and proinsulin-to-insulin ratio were not different among the three groups (Table 1).
CONCLUSIONS
The childhood obesity epidemic has resulted in increasing numbers of youth presenting with type 2 diabetes, but, in addition, type 1 diabetic children have become overweight/obese (1,4). This has made the proper clinical distinction between obese type 1 versus type 2 diabetes challenging, especially when a significant number of physician diagnosed obese youth with type 2 diabetes have circulating β-cell autoantibodies (3,5–7). In the present study, even though the Ab+ youth were statistically significantly less obese than the Ab− ones, from a clinical significance perspective they were still very obese. In a previous publication using the state-of-the-art methodology of the clamp, we reported severe impairment in insulin secretion in Ab+ subjects with CDx-type 2 diabetes in contrast to severe impairment in insulin action in the Ab− patients (8). The current study investigated whether the OGTT, a clinically applicable tool, could reveal differences in insulin secretion and sensitivity in Ab+ versus Ab− patients to assist in the diagnosis of the type of diabetes. Our data show that fasting C-peptide, fasting C-peptide–to–glucose ratio, and C-peptide AUC during the OGTT are significantly lower in Ab+ versus Ab− CDx-type 2 diabetic youths, while none of the other parameters are different. Particularly, none of the sensitivity indexes could distinguish between Ab+ and Ab− CDx-type 2 diabetes.
In our previous study, using the hyperinsulinemic-euglycemic clamp, we demonstrated that insulin sensitivity was significantly lower, ∼45% in Ab− versus Ab+ patients (8). In the current 25 Ab− and 11 Ab+ patients, with the new DK islet cell antibody harmonization assay, the data are consistent with prior publication. In vivo insulin sensitivity in Ab− patients is lower than Ab+ patients (1.5 ± 0.2 vs. 3.1 ± 0.4 mg · kg−1 · min−1 per μU/ml; P = 0.001). However, none of the eight different fasting and OGTT-derived surrogate indexes of insulin sensitivity (Table 2) revealed statistically significant differences between the two groups of type 2 diabetic youths. This may not be surprising since during the clamp, glucose is maintained at euglycemia in all patients, unlike the OGTT where glucose levels are highly variable (Fig. 1A) and dependent on endogenous insulin secretion. The same is true for insulin levels, which are highly variable during the OGTT (Fig. 1B) but controlled with a constant rate infusion of insulin during the hyperinsulinemic clamp, resulting in similar steady-state clamp insulin levels among all patients. Similar to our current findings, a study in adults using the HOMA index reported similar insulin sensitivity between patients with latent autoimmune diabetes of adults (LADA) and patients with GAD-negative type 2 diabetes of short duration (14). In contrast, data from the A Diabetes Outcomes Progression Trial (ADOPT) showed significantly lower HOMA-IR in adult patients with GAD-positive type 2 diabetes compared with GAD negative (15). This finding, though consistent with our previous observation of better insulin sensitivity in Ab+ youths, differs from our current findings. This is most likely due to the much larger cohort in the ADOPT, which increases statistical power despite using a less sensitive method than the clamp. In addition, however, GAD-positive patients had a lower BMI and may therefore have been more insulin sensitive (15). Another small study, using the insulin-modified frequently sampled intravenous glucose tolerance test, showed similar insulin sensitivity between eight GAD-negative and eight GAD-positive adult patients with type 2 diabetes, matched for BMI and glycemic control (16), consistent with our current findings.
With regards to insulin secretion, using the hyperglycemic clamp previously we demonstrated that first- and second-phase insulin secretion was significantly lower (∼50%) in Ab+ versus Ab− patients (8). In the present 25 Ab− and 11 Ab+ patients, first-phase insulin and C-peptide were significantly different (77.1 ± 10.2 vs. 38.4 ± 4.2 μu/ml, P = 0.001; 5.7 ± 0.4 vs. 2.4 ± 0.3 ng/ml, respectively; P < 0.001). However, neither the ΔI30/ΔG30 nor ΔC-Pep30/ΔG30 were significantly different between the two groups of type 2 diabetic youths (Table 2). This, again, may be due to the fact that during the hyperglycemic clamp, the insulin response is standardized to the same glucose stimulus in all patients (∼225 mg/dl), while this is not the case during the OGTT, where glucose levels are highly variable. There are no prior studies comparing insulin secretion during the OGTT in Ab− versus Ab+ young CDx-type 2 diabetic patients. Studies from adults with islet cell antibody–positive LADA showed that fasting and stimulated C-peptide levels were reduced compared with islet cell antibody–negative type 2 diabetic patients (17), consistent with our findings. Similarly HOMA-%β was not different between Ab− and Ab+ patients. In normal children, the reported correlations of HOMA-%β with clamp-derived first-phase insulin secretion range between 0.54 and 0.82 depending on the pubertal stage and glucose tolerance status (13,18). Furthermore, HOMA-%β, fasting insulin, and fasting insulin/glucose are reported not to perform well in detecting differences in insulin secretion in adolescents with impaired glucose tolerance (13). Therefore, it is not surprising that OGTT-derived indexes of insulin secretion were not sensitive enough to detect differences in insulin secretion between Ab+ and Ab− Cx-type 2 diabetes in the current study.
Studies comparing Ab− versus Ab+ young patients with phenotypic type 2 diabetes mostly explored the differences in clinical characteristics at presentation (3,5–7), and none evaluated the differences in metabolic responses during the OGTT between the two groups. In the current study, glucose AUC was significantly higher and C-Peptide AUC, per se, or relative to prevailing glucose levels was significantly lower in Ab+ versus Ab− diabetic youth. This is consistent with a preliminary report from the multicenter study Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) that found a tendency for lower fasting C-peptide levels in 48 Ab+ patients compared with 357 Ab− patients (7). In contrast, another study from Europe reported no differences in C-peptide or glucose levels at manifestation between a group of 82 Ab− and a group of 46 Ab+ youth with CDx-type 2 diabetes (6). Another study from the U.S. did not find significant differences in fasting C-peptide levels between 24 GAD-negative young type 2 diabetic patients compared with 24 obese control subjects (5). The observation that C-Peptide AUC/glucose AUC during the OGTT is significantly lower in Ab+ versus Ab− patients may be a helpful metabolic marker, besides the fasting C-peptide, to distinguish Ab+ and Ab− youths with type 2 diabetes. Despite the lack of statistical significance in the differences in treatment modalities between the Ab+ and Ab− patients in our current study; treatment with metformin versus insulin may have affected the interpretation of the fasting and OGTT-derived indexes of insulin secretion and sensitivity.
Relative hyperglucagonemia is shown in adults with type 2 diabetes and with LADA who had higher glucagon concentrations and less suppression of glucagon with hyperglycemia compared with control subjects (14). In the present study, there was evidence of hyperglucagonemia relative to the prevailing glycemia in CDx-type 2 diabetic patients compared with control subjects consistent with data from adult patients (14).
GLP-1 response during the OGTT was not different between Ab− CDx-type 2 diabetic patients and obese control subjects. Plasma levels of GLP-1 are reported to be unaltered (19), reduced (20,21), or elevated (22) in adults with type 2 diabetes. Furthermore, the duration and the severity of diabetes control and the degree of obesity were shown to be independently associated with GLP-1 responses (23), potentially accounting for the literature-reported divergent findings. No significant differences in GLP-1 responses were detected in adults with type 2 diabetes of short duration (3.2 ± 2.8 years) and with relatively good glycemic control (A1C 6.8 ± 0.9%) compared with subjects with impaired glucose tolerance and control subjects (19). Our similar findings of no significant differences in GLP-1 levels between Ab− and obese control subjects are most likely related to the short duration of diabetes (7.3 ± 1.5 months) and well-controlled glycemia (Table 1). In adults with type 1 diabetes, GLP-1 responses are reported to be normal (20) or reduced compared with control subjects (21). Furthermore, BMI has been shown to correlate inversely with GLP-1 (19,23). Among the subset of patients who had GLP-1 evaluation, the Ab+ group had significantly lower BMI compared with the other two groups. This could explain the observed higher GLP-1 levels in the former group, which disappeared after adjusting for BMI differences.
Last but not least, the observation of significantly lower leptin levels in Ab+ youths compared with control subjects is likely due to the severe insulin deficiency (24). We have shown that in newly diagnosed patients with type 1 diabetes insulin deficiency is associated with low leptin levels and insulin therapy within days corrects this deficiency before any changes in weight are observed (24).
In conclusion, fasting and OGTT-derived surrogate measures of insulin sensitivity and secretion, with the exception of fasting C-peptide and OGTT C-peptide AUC/glucose AUC, are not sufficiently sensitive to detect the significant differences in insulin sensitivity and β-cell function demonstrated by the clamp technique between the two groups of CDx-type 2 diabetic patients. This underscores the importance of using sensitive methods for assessment of β-cell function and insulin sensitivity in pathophysiologic studies. Although relatively small sample sizes may be a limitation of our study, this resonates with the clinical relevance of our findings in that the clinician cannot use fasting or OGTT-derived parameters reliably in distinguishing the individual Ab+ from the Ab− patient. As the presence of autoimmunity implies progressive decline in β-cell function (25), our findings also highlight the importance of determining antibody status in obese pediatric patients with phenotypic type 2 diabetes.
Acknowledgments
This work was supported by U.S. Public Health Service Grant RO1 HD27503 (to S.A.), K24 HD01357 (to S.A.), U01 DK61254 (to S.A.), the Richard L. Day Endowed Chair (to S.A.), the Thrasher Research Fund (to F.B. and N.G.), the Pittsburgh Foundation (to N.G.), MO1 RR00084 (general clinical research center), and UL1 RR024153 (Clinical and Translational Science Award).
No potential conflicts of interest relevant to this article were reported.
These studies would not have been possible without the nursing staff of the Pediatric Clinical and Translational Research Center, the devotion of the research team (Kristin Porter, RN; Sally Foster, RN, CDE; Lori Bednarz, RN, CDE; Nancy Guerra, CRNP; and Sabrina Kadri) and the laboratory expertise of Resa Stauffer and Katie McDowell but most importantly, the commitment of the study participants and their parents. We are thankful to So Jung Lee, PhD, for the interpretation of the abdominal CT data in some of the participants.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 2. Gungor N, Hannon T, Libman I, Bacha F, Arslanian S: Type 2 diabetes mellitus in youth: the complete picture to date. Pediatr Clin North Am 2005;52:1579–609 [DOI] [PubMed] [Google Scholar]
- 3. The Writing Group for the SEARCH for Diabetes in Youth Study Group. Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B: Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 4. Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ: Evidence for heterogeneous pathogenesis of insulin-treated diabetes in black and white children. Diabetes Care 2003;26:2876–2882 [DOI] [PubMed] [Google Scholar]
- 5. Umpaichitra V, Banerji MA, Castells S: Autoantibodies in children with type 2 diabetes mellitus. J Pediatr Endocrinol Metab 2002;15(Suppl. 1):525–530 [PubMed] [Google Scholar]
- 6. Reinehr T, Schober E, Wiegand S, Thon A, Holl R: the DPV-Wiss Study Group. Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child 2006;91:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klingensmith GJ, Coombs LP, Arslanian S, Copeland K, Cuttler L, Haffner S, Kaufman F, Laffel L, Linder B, Marcovina SM, Tollefsen SE, Weinstock RS: the TODAY Study Group. Autoantibody positivity in subjects screened for participation in a treatment trial for type 2 diabetes in youth (Abstract). Diabetes 2006;55(Suppl. 1):A67 [Google Scholar]
- 8. Tfayli H, Bacha F, Gungor N, Arslanian A: Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet-cell antibody negative vs. antibody positive patients. Diabetes 2009;58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gungor N, Bacha F, Saad R, Janosky J, Arslanian S: Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A: Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab 2003;88:1019–1023 [DOI] [PubMed] [Google Scholar]
- 11. Avignon A, Boegner C, Mariano-Goulart D, Colette C, Monnier L: Assessment of insulin sensitivity from plasma insulin and glucose in the fasting or post oral glucose-load state. Int J Obes Relat Metab Disord 1999;23:512–517 [DOI] [PubMed] [Google Scholar]
- 12. Bacha F, Gungor N, Arslanian SA: Measures of beta-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr 2008;152:618–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gungor N, Saad R, Janosky J, Arslanian S: Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr 2004;144:47–55 [DOI] [PubMed] [Google Scholar]
- 14. Carlsson A, Sundkvist G, Groop L, Tuomi T: Insulin and glucagon secretion in patients with slowly progressing autoimmune diabetes (LADA). J Clin Endocrinol Metab 2000;85:76–80 [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Kahn SE, Haffner SM, O'Neill MC, Heise MA, Freed MI: the ADOPT Study Group. Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 2004;53:3193–3200 [DOI] [PubMed] [Google Scholar]
- 16. O'Gorman DJ, Yousif O, Dixon G, McQuaid S, Murphy E, Rahman Y, Gasparro D, Pacini G, Newsholme P, Nolan JJ: In vivo and in vitro studies of GAD-antibody positive subjects with type 2 diabetes: a distinct sub-phenotype. Diabetes Res Clin Pract 2008;80:365–370 [DOI] [PubMed] [Google Scholar]
- 17. Stenström G, Gottsäter A, Bakhtadze E, Berger B, Sundkvist G: Latent autoimmune diabetes in adults: definition, prevalence, β-cell function, and treatment. Diabetes 2005;54(Suppl. 2):S68–S72 [DOI] [PubMed] [Google Scholar]
- 18. Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA: Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care 2002;25:2081–2087 [DOI] [PubMed] [Google Scholar]
- 19. Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ: Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]
- 20. Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, Holst JJ: Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metal 2003;88:2706–2713 [DOI] [PubMed] [Google Scholar]
- 21. Lugari R, Dell'Anna C, Ugolotti D, Dei Cas A, Barilli AL, Zandomeneghi R, Marani B, Iotti M, Orlandini A, Gnudi A: Effect of nutrient ingestion on glucagon-like peptide 1 (7–36 amide) secretion in human type 1 and type 2 diabetes. Horm Metab Res 2000;32:424–428 [DOI] [PubMed] [Google Scholar]
- 22. Orskov C, Jeppesen J, Madsbad S, Holst JJ: Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 1991;87:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muscelli E, Mari A, asolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E: Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008;57:1340–1348 [DOI] [PubMed] [Google Scholar]
- 24. Hanaki K, Becker DJ, Arslanian S: Leptin before and after insulin therapy in children with new onset type 1 diabetes. J Clin Endocrinol Metab 1999;84:1524–1526 [DOI] [PubMed] [Google Scholar]
- 25. Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, Shattock M, Bottazzo GF, Holman R: UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes: UK Prospective Diabetes Study Group. Lancet 1997;350:1288–1293 [DOI] [PubMed] [Google Scholar]