Abstract
OBJECTIVE
Tight glycemic control (TGC) in critically ill patients is associated with an increased risk of hypoglycemia. Whether those short episodes of hypoglycemia are associated with adverse morbidity and mortality is a matter of discussion. Using a case-control study design, we investigated whether hypoglycemia under TGC causes permanent neurocognitive dysfunction in patients surviving critical illness.
RESEARCH DESIGN AND METHODS
From our patient data management system, we identified adult survivors treated for >72 h in our surgical intensive care unit (ICU) between 2004 and 2007 (n = 4,635) without a history of neurocognitive dysfunction or structural brain abnormalities who experienced at least one episode of hypoglycemia during treatment (hypo group) (n = 37). For each hypo group patient, one patient stringently matched for demographic- and disease-related data were identified as a control subject. We performed a battery of neuropsychological tests investigating five areas of cognitive functioning in both groups at least 1 year after ICU discharge. Test results were compared with data from healthy control subjects and between groups.
RESULTS
Critical illness caused neurocognitive dysfunction in all tested domains in both groups. The dysfunction was aggravated in hypo group patients in one domain, namely that of visuospatial skills (P < 0.01). Besides hypoglycemia, both hyperglycemia (r = −0.322; P = 0.005) and fluctuations of blood glucose (r = −0.309; P = 0.008) were associated with worse test results in this domain.
CONCLUSIONS
Hypoglycemia was found to aggravate critical illness–induced neurocognitive dysfunction to a limited, but significant, extent; however, an impact of hyperglycemia and fluctuations of blood glucose on neurocognitive function cannot be excluded.
Since the concept of tight glycemic control (TGC) was introduced in critical care medicine in 2001 (1), its implementation in daily clinical practice has been the subject of a vivid discussion. Several single-center trials in different patient populations largely confirmed the clinical benefits, at least when patients were treated for a few days or longer in an intensive care unit (ICU) (2). Numerous studies have suggested plausible mechanisms behind the clinical benefits (3). However, a recent multicenter trial failed to confirm the strict blood glucose targets (4), and two multicenter trials have been preliminarily stopped because of a high incidence of hypoglycemic episodes (5).
Indeed, hypoglycemia appears as the major side effect of any effort to regulate blood glucose levels with insulin, whatever the blood glucose levels aimed for (2). Although numerous algorithms are available to minimize this risk (6), the fear of hypoglycemia-induced mortality and permanent disability largely impedes the implementation of TGC in daily routine. Scientific evidence supporting the common notion that hypoglycemia is responsible for an increased mortality and profound permanent neurocognitive dysfunction rather than it being just a marker of severity of illness is poor and controversial, however. Efforts to substantiate any evidence are based on post hoc analyses, since confirmation from prospective randomized, controlled trials is precluded for obvious ethical reasons. Some studies imply that any mortality benefits of TGC might be outweighed when the incidence of hypoglycemia is very high (7); however, other analyses revealed conflicting results in this respect (8). Besides direct effects on mortality, neuroglycopenia might cause neuronal damage and at least subtle permanent neurocognitive impairment that potentially affects life quality after discharge. From diabetes, it is known that neuroglycopenia might have a permanent effect on neurocognitive function, at least when it occurs repetitively. Since diabetes and critical illness–induced dysregulations of glucose homeostasis represent substantially different entities, it is inappropriate to extrapolate these data to the ICU population. Cognitive impairment is a relevant problem of patients surviving critical illness in general (9). Currently, there are no data available on the specific impact of hypoglycemic events during treatment in ICU on long-term neurocognitive function. Using a case-control design, we investigated whether hypoglycemic episodes under TGC induce or aggravate permanent neurocognitive deficits in patients surviving critical illness.
RESEARCH DESIGN AND METHODS
The work was approved by the local ethics committee, and written informed consent was obtained from all patients prior to neurocognitive testing.
All patients in the surgical ICU of our university hospital are treated according to our institutional TGC protocol (analog to [1]), aiming for blood glucose between 80 and 110 mg/dl using insulin infusions as necessary. Blood glucose was measured in full blood drawn from an arterial line with an ABL blood gas analyzer (glucose oxidase method with amperometric reading, range 7–540 mg/dl, coefficient of variance <10% for lower detection limit; Radiometer, Copenhagen, Denmark). Quality checks of the device were performed according to the instruction manual.
We identified all patients who suffered from at least one episode of hypoglycemia (blood glucose ≤40 mg/dl) (labeled the hypo group) between 1 January 2004 and 31 December 2007 from our patient data management system. Patients were selected to undergo a battery of validated neuropsychological tests that were designed to assess a full range of cognitive functions (Table 1) at least 1 year after discharge from the unit. To diagnose patients with manifest neurological deficits, a short neurological examination was performed (sensory and motor responses, reflexes including Babinski's sign, and examination of posture and movements). We included all patients, aged between 18 and 80 years, upon admission who were treated for at least 72 h in the ICU. We excluded patients who did not survive until the scheduled time point of testing or had a medical history or medical condition potentially biasing neurocognitive testing, such as neurocognitive, neurodegenerative (Alzheimer's or Parkinson's disease), psychiatric disorders (drug abuse, depression, and schizophrenia and the use of respective medication), severe liver disease (ammonia three times the upper limit of normal or Child C liver insufficiency), or end-stage kidney failure. Patients after neurotrauma, intracranial hemorrhage, stroke, intracranial surgery, and other structural brain lesions were also excluded.
Table 1.
Cognitive domains and tests: results of neurocognitive testing
| Hypo group |
Control group |
||||||
|---|---|---|---|---|---|---|---|
| Score (percentile) | Evaluation | Z scores | Score (percentile) | Evaluation | Z scores | P | |
| Dementia screening | 0.006 | −0.003 | 0.969 | ||||
| Mini-mental state examination | 28.4 | Close below average | 28.8 | Close below average | 0.909 | ||
| Boston Naming Test | 13.8 | Normal | 13.9 | Normal | 0.871 | ||
| Attention and working memory | −0.039 | −0.045 | 0.774 | ||||
| Nuernberg Gerontopsychological Inventory | |||||||
| Digit symbol substitution | 30.0 (56.7) | Normal | 31.1 (60.7) | Normal | 0.770 | ||
| Color word interference task (reading) | 39.8 (10.2) | Far below average | 40.0 (12.5) | Far below average | 0.861 | ||
| Color word interference task (color naming) | 53.3 (28.4) | Close below average | 52.8 (26.6) | Close below average | 0.608 | ||
| Wechsler Memory Scale (revised) | |||||||
| Digit span forward | 11.6 (51.7) | Normal | 12.6 (54.4) | Normal | 0.156 | ||
| Digit span backward | 10.7 (40.6) | Close below average | 11.6 (42.0) | Close below average | 0.892 | ||
| Trail-making test (A) | 60.1 (13.9) | Far below average | 59.6 (13.0) | Far below average | 0.270 | ||
| Executive function | −0.001 | −0.007 | 0.991 | ||||
| Color word interference task (interference condition) | 17.5 (47.9) | Normal | 19.5 (51.3) | Normal | 0.421 | ||
| Regensburg Word Fluency Test (letter fluency) (S) | 14.2 (28.4) | Close below average | 14.2 (28.4) | Close below average | 1.000 | ||
| Trail-making test (B) | 117.0 (27.8) | Close below average | 110.8 (25.6) | Close below average | 0.792 | ||
| Visuospatial skills | −2.084 | −0.145 | 0.001 | ||||
| Rey Osterrieth Complex Figure Test | |||||||
| Copy | 20.4 | 24.7 | 0.007 | ||||
| Delayed recall | 9.4 (22.8) | Close below average | 14.5 (29.9) | Close below average | 0.002 | ||
| Difference copy (delayed) | −54.3% | −41.9% (4.2) | 0.043 | ||||
| Verbal learning and memory | −0.027 | −0.064 | 0.807 | ||||
| Auditory verbal learning test (German) | |||||||
| Recall trial 1 | 4.9 (30.2) | Close below average | 5.5 (38.4) | Close below average | 0.503 | ||
| Recall trial 5 | 10.7 (31.1) | Close below average | 10.5 (28.8) | Close below average | 0.543 | ||
| Total trials 1–5 | 38.0 (30.4) | Close below average | 38.7 (32.1) | Close below average | 0.527 | ||
| Delayed recall | 8.5 (13.8) | Far below average | 9.0 (15.0) | Far below average | 0.240 | ||
| Recognition (true positives, false positives) | 10.9 (30.5) | Close below average | 10.9 (30.5) | Close below average | 1.000 | ||
For each hypo group patient, a matching partner (control group) without any hypoglycemic event meeting the same inclusion and exclusion criteria was identified from the database according to strict demographic- and illness-related matching criteria (Table 2).
Table 2.
Matching criteria
| Demography | |
| Sex | Male/female |
| Age (classified in groups) | <40; 41–60; 61–75; >75 years |
| Simplified acute physiology score (maximum simplified acute physiology score, classified in groups) | <7; 8–14; >14 |
| Year of ICU treatment | |
| Disease-related criteria | |
| Type of surgery | Elective surgery/emergency surgery |
| Cardiopulmonary resuscitation | Yes/no |
| Type 1 or type 2 diabetes | Yes/no |
| Length of stay in ICU* | |
| Mean morning blood glucose* | |
| Duration of sedation (classified in groups) | <3 days; 3–7 days; 1–2 weeks; >2 weeks |
| Respiratory failure (classified by Horrowitz Index in groups)† | >300; 200–300; <200 |
| Cardiovascular failure† | Catecholamine therapy: yes/no; mechanical assist device: yes/no |
| Renal failure† | Hemodialysis of any kind: yes/no; classified by RIFLE criteria |
| Hepatic failure (classified by laboratory liver testing, classified in four groups) | |
| All values <2.5 ULN, one value 2.5–5 ULN, one value >5 ULN, all values >5 ULN | |
| Medication | Steroids: yes/no; immunosuppressants: yes/no |
*Smallest possible difference.
†At time of hypoglycemia ±3 days. ULN, upper limit of normal. RIFLE, Risk, Injury, Failure, Loss, and End-stage classification for acute renal dysfunction.
We recorded and calculated duration (time from last blood glucose above hypoglycemia threshold before, to first blood glucose >40 mg/dl after a hypoglycemic reading), number and severity of hypoglycemia (minimum blood glucose during treatment), mean blood glucose over the whole ICU stay, mean morning blood glucose, maximum blood glucose, Δblood glucose (difference between the minimum blood glucose and maximum blood glucose within 6 h following hypoglycemia), and the difference between minimum and maximum blood glucose during ICU treatment.
Neuropsychological assessment
One investigator, who was unaware of the allocation of the patients, conducted the neuropsychological tests. Test results were primarily analyzed by the same investigator and supervised by an experienced clinical neuropsychologist. Performances in five major areas of cognitive functioning were evaluated. Cognitive domains and their particular tests are listed in Table 1. Concerning the Rey Osterrieth Complex Figure Test, we also calculated the relative difference between both test results since results of delayed recall performance can be influenced by an impairment of initial copying. Additionally, test results from patients were compared with published normative data for age, sex, and educational level. A detailed description of each test can be found in the book by Lezak (10).
Statistical analyses
Data were tested for normal distribution with the Shapiro-Wilk Test. To determine meaningful composite scores of cognitive domains, we performed a principal component analysis using the single test results, followed by an oblique (Oblimin with Kaiser normalization) rotation. The same test was not included in more than one composite score. The resulting five factors of the principal component analysis were Z transformed (mean score of 0 and an SD of 1). For timed tests, the sign of the Z score was reversed so that improved performance resulted in a higher score in all tests.
Primary analysis assessed differences in neurocognitive test results between groups with either paired t test or Mann-Whitney U test as appropriate. Secondary analyses were carried out to test the relation of hypoglycemia severity, length of hypoglycemic episode, and the number of hypoglycemic events to neurocognitive scores and whether maximum glucose values, Δblood glucose, or the difference between minimum and maximum blood glucose were associated with worse test results by means of Pearson's correlation.
Test results of the ICU patients were compared with published normative data for age, sex, and educational level. Differences were expressed semi-quantitatively as normal, close below average, or far below average, respectively. Test results are given as means ± SD. A two-tailed P value <0.05 was considered significant. All data were analyzed using SPSS Statistics version 15.0.
RESULTS
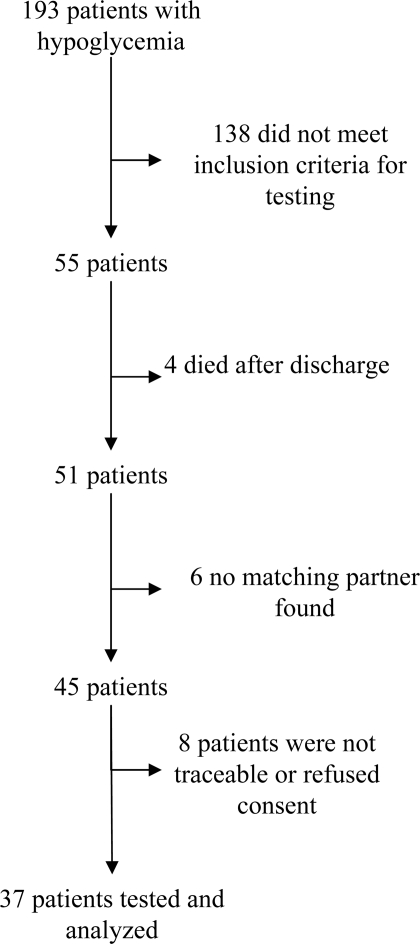
A total of 4,635 patients were treated in our ICU in the study period for >72 h, 193 of whom experienced at least one episode of hypoglycemia (4.2%). Thirty-seven hypo group patients met inclusion criteria, fulfilled no exclusion criteria, and one matching control partner could be identified for each (Fig. 1). Demographic data were as follows (means ± SE): 44 male and 30 female subjects, age 66.3 ± 1.3 years, simplified acute physiology score 39 ± 2.3, length of stay on ICU 15.2 ± 1.6 days, and 32% had diabetes. Admission blood glucose (167.8 ± 7.8 vs. 167.0 ± 8.3 mg/dl; P = 0.941), mean morning blood glucose (131.7 ± 3.0 vs. 126.5 ± 2.6 mg/dl; P = 0.196), and mean blood glucose (139.0 ± 3.0 vs. 137.1 ± 2.5; P = 0.644) did not differ between groups. Mean maximum blood glucose was significantly higher in the hypo group than in the control group (297.8 ± 14.9 vs. 249.8 ± 10.7 mg/dl; P = 0.017). Demographic data did not differ between groups, as patients were matched accordingly.
Figure 1.
Flow chart of patient inclusion in the hypo group.
None of the patients revealed manifest neurological deficits in the neurological examination. Neurocognitive tests in both patient groups showed impaired neurocognitive function in several domains compared with age-matched healthy control subjects (Table 1). Analyses of differences between both patient groups in the five neurocognitive domains revealed solely a significant impairment of visuospatial skills in the hypo compared with the control group (P = 0.001). Within the single subtests, results of both copy (P = 0.007) and delayed (P = 0.002) recall of the Rey Osterrieth Complex Figure Test were lower in the hypo than in the control group. The relative difference between copy and delayed recall also significantly differed between both groups (hypo group < control group; P = 0.043). Results of all other tests did not differ between groups (Table 1).
Solely within the hypoglycemic group, the maximum blood glucose and the difference between minimum and maximum blood glucose serving as rough surrogates for the quality of glycemic control during ICU treatment were negatively correlated with visual-spatial processing parameters. Neither the number nor the duration of hypoglycemic episodes showed a significant correlation. Severity of hypoglycemia was also not significantly associated with visuospatial performance but did show a negative trend (Table 3). In the control group, no correlations between parameters of glycemic control and the performance in the neurocognitive tests were found.
Table 3.
Correlation of the parameters of glycemic control with Rey Osterrieth Complex Figure Test results in the hypo group
| r | P | |
|---|---|---|
| Mean morning blood glucose | −0.055 | 0.747 |
| Mean blood glucose | 0.116 | 0.494 |
| Number of hypoglycemic episodes | −0.097 | 0.414 |
| Duration of hypoglycemic episode | −0.293 | 0.154 |
| Maximum blood glucose during treatment | −0.322 | 0.005 |
| Minimum blood glucose during treatment | −0.299 | 0.072 |
| Difference maximum/minimum blood glucose | −0.309 | 0.001 |
| ΔBlood glucose | 0.052 | 0.765 |
CONCLUSIONS
In the current case-control study, we found that patients who experienced one or more hypoglycemic events during ICU treatment showed an aggravation of critical illness–induced neurocognitive dysfunction compared with patients who did not experience hypoglycemia. Both groups showed significant neurocognitive dysfunctions in all domains compared with healthy control subjects, but hypo group patients had an additional deficit in visuospatial skills. Since tests were done at least 1 year after ICU discharge, these impairments must be considered long term if not permanent.
Former studies investigating the consequences of hypoglycemia under TGC in the critically ill have revealed conflicting results (7,8); however, they have been primarily focused on mortality and gross somatic morbidity. Data on the positive effects of TGC on mortality from prior trials could not be confirmed by the recent multicenter trial Normoglycaemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation (NICE-SUGAR) (4), which investigated the impact of a strict versus a more liberal TGC protocol. NICE-SUGAR revealed a higher mortality in the strict TGC group. The high incidence of hypoglycemia in the strict TGC group might be considered one possible explanation for this controversy. Similarly, from mathematical modeling, Krinsley concluded that negative effects of hypoglycemia might outweigh any benefits on mortality and gross morbidity when they occur in a critical incidence (7). Our study focuses on ICU survivors and is thus the first to explore the long-term effects of hypoglycemia under TGC during ICU treatment on subtle neuropsychological function. With the utilized test battery, we largely confirm and complement prior studies (9) demonstrating neurocognitive impairment in the tested domains in both critically ill patient groups compared with age-, sex-, and educational level–matched healthy control subjects. Furthermore, we could show that in patients surviving the ICU without primary brain damage and preexisting neurocognitive deficits, critical illness–induced deficits of complex neurocognitive functions, in particular visuoconstructive performance as well as figural and spatial aspects of nonverbal memory, might be aggravated by even a single episode of hypoglycemia. Although the aggravation appears minor at first view and is restricted to one single domain, the impairment of visual-spatial processing might have a relevant impact on overall daily functioning (11). It could be associated with the evolution of further cognitive decline over time (12) and, thus, have a significant impact on patients' quality of life.
Recent studies have indicated that an impairment of visuoconstructive skills and both figural and spatial aspects of nonverbal memory are associated with temporal and hippocampal dysfunction (13). Neuroimaging has demonstrated that not all neurons and brain regions are equally sensitive to hypoglycemic injury but that there appears to be a selective vulnerability of especially those hippocampal and/or temporal neurons, followed by neurons in the basal ganglia (14,15). Although the reported abnormalities could be transient and reversible by glucose infusion, several studies in both animals and humans have consistently demonstrated hypoglycemia-induced permanent neuronal damage in regions of the hippocampus, especially in the dentate gyrus (16,17). Although most biochemical studies have focused on cell death, more recent studies indicate that mild, recurrent hypoglycemia can cause synaptic dysfunction even in the absence of neuronal death, particularly in hippocampal neurons (18). Repeated episodes of even moderate hypoglycemia in diabetic patients have been reported as being associated with a decline of intelligence quotient, persistent cognitive impairment, and other long-term effects such as mood changes and affected general well-being (19,20); however, since conflicting results have been published, assigning hypoglycemia as the sole cause of these findings is debatable. Some of the divergent results may be due to methodical issues with regards to the determination of cognitive function; other negative studies may not have been sufficiently long to detect a significant effect. On the other hand, the associations between intellectual disadvantage and episodes of hypoglycemia might exist simply because patients manage their insulin treatment less accurately. It is thus difficult to differentiate between effects of hypoglycemia and modest glycemic control comprising hyperglycemia, hypoglycemia, and blood glucose fluctuations. To conclude from clinical trials that persistent neurocognitive impairment in diabetic subjects is exclusively a consequence of (repeated) episodes of hypoglycemia is plausible but not imperative. Moreover, the underlying pathogenetic mechanisms of the long-term cognitive deficits remain largely unclear; some findings indicate that dopaminergic functional disturbance in the hippocampus (21) and changes in brain glucose transporters or astrocyte-neuron interactions may play a major role (14). The agreement between neurocognitive test results, their probable functional and structural neuroanatomic correlates, and the specific vulnerability of (para-) hippocampal neuron populations to hypoglycemic damage is striking, however.
Current data suggest that a great portion of ICU survivors in general develop persistent cognitive impairment (9,22); we also found neurocognitive impairment in various domains in both our patient groups compared with published normative data. Since critically ill patients, per se, seem to be at risk for neural damage, one might speculate that critical illness can induce a specific vulnerability of neurons to glucose deprivation. Our data show that hypoglycemic events under TGC aggravate these critical illness–induced neurocognitive deficits but that this is limited to one neurocognitive domain. Notwithstanding stringent matching criteria for demographic and severity of illness data including mean blood glucose, we cannot completely exclude confounders. Our groups differ in mean maximum and minimum glucose, suggesting that the hypo group experienced a worse quality of blood glucose control with more variability. Solely within the hypo group, we found a significant association of hyperglycemia and the difference between lowest and highest blood glucose with declined visuospatial skills, whereas for quantity and duration of hypoglycemic episodes, no such correlation was found. No correlations at all were found in our control group. Indeed, previous work showed that hyperglycemia in diabetes, too, is associated with adverse effects on the brain (23), neurocognitive impairment, and affected general well-being (19). Not only hypoglycemia but also hyperglycemia, glucose fluctuations, and their treatments thus might have an impact on cognitive function of ICU survivors. Moreover, neural death is aggravated when glucose concentrations rise rapidly and hyperglycemia occurs after hypoglycemia (“glucose reperfusion injury” [24]). Notably, critically ill patients reveal increased insulin levels, and insulin has also been reported to accelerate neural cell death in the hippocampus during low glucose levels, suggesting that insulin might have a double-edged effect on neuron death dependent on glucose concentration (25). Our findings are in accordance with these data. Since exclusively in the hypo group a correlation of hyperglycemia and a surrogate parameter of the quality of glycemic control with neurocognitive dysfunction was found, we cannot rule out those parameters as relevant confounders of our findings. However, our hypothesis and design only allow to draw a causal link between hypoglycemia and neurocognitive impairment. It is undue to conclude causality between maximum blood glucose or glucose fluctuations from our data; we solely can allude to an association.
To unequivocally prove a causal relation between hypoglycemia and neurocognitive dysfunction, a prospective, randomized controlled trial would be required, but self-evident, ethical considerations preclude this approach. We thus have to rely on the available data from post hoc analysis with its limitations. Another limitation is the absence of brain imaging in all patients. Significant structural brain lesions are unlikely, however, since none of the patients revealed manifest neurological deficits during the study period. However, subtle structural cerebral lesions cannot completely be excluded.
In conclusion, neurocognitive dysfunction is common in patients surviving critical illness. Patients who experienced a hypoglycemic event during ICU treatment show a significant additional impairment in the visuospatial domain compared with patients who did not. In those patients, hyperglycemia and fluctuations of blood glucose levels were also associated with long-term visuospatial dysfunction and might thus confound this conclusion. Every effort should be put in implementing effective blood glucose control algorithms, largely avoiding hypoglycemia and hyperglycemia as well as large fluctuations of blood glucose.
Acknowledgments
The work was supported by a grant from the program “Innovative Medizinische Forschung,” Faculty of Medicine, University of Muenster, Muenster, Germany.
No potential conflicts of interest relevant to this article were reported.
Part of the results of this study was presented at the yearly congress of the European Society of Anaesthesiology, Euroanesthesia ESA, Milan, Italy, 6–8 June 2009, and the Annual Congress of the German Society of Anesthesia and Intensive Care Medicine DGAI, DAC Nürnberg, Germany, 9–12 May 2009.
Footnotes
Clinical trial reg. no. NCT00662922, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in the critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 2. Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D: Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009;180:821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanhorebeek I, Langouche L, Van den Berghe G: Tight blood glucose control with insulin in the ICU: facts and controversies. Chest 2007;132:268–278 [DOI] [PubMed] [Google Scholar]
- 4. NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ: Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 5. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K: the German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–139 [DOI] [PubMed] [Google Scholar]
- 6. Vogelzang M, Loef BG, Regtien JG, van der Horst IC, van Assen H, Zijlstra F, Nijsten MW: Computer-assisted glucose control in critically ill patients. Intensive Care Med 2008;34:1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krinsley JS, Grover A: Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007;35:2262–2267 [DOI] [PubMed] [Google Scholar]
- 8. Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB, Schultz MJ, Rosendaal FR, Hoekstra JB: Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med 2006;34:2714–2718 [DOI] [PubMed] [Google Scholar]
- 9. Hopkins RO, Ely EW, Jackson JC: The role of future longitudinal studies in ICU survivors: understanding determinants and pathophysiology of brain dysfunction. Curr Opin Crit Care 2007;13:497–502 [DOI] [PubMed] [Google Scholar]
- 10. Lezak MD: Neuropsychological Assessment. 4th ed. Oxford, U.K., Oxford University Press, 2004. [Google Scholar]
- 11. Fukui T, Lee E: Visuospatial function is a significant contributor to functional status in patients with Alzheimer's disease. Am J Alzheimers Dis Other Demen 2009;24:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, Masliah E, Thal LJ: Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology 2008;22:729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boxer AL, Kramer JH, Du AT, Schuff N, Weiner MW, Miller BL, Rosen HJ: Focal right inferotemporal atrophy in AD with disproportionate visual constructive impairment. Neurology 2003;61:1485–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suh SW, Hamby AM, Swanson RA: Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 2007;55:1280–1286 [DOI] [PubMed] [Google Scholar]
- 15. Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y: Specific changes in human brain after hypoglycemic injury. Stroke 1997;28:584–587 [DOI] [PubMed] [Google Scholar]
- 16. Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ: Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab 2009;297:E194–E201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Auer RN: Hypoglycemic brain damage. Metab Brain Dis 2004;19:169–175 [DOI] [PubMed] [Google Scholar]
- 18. McNay EC, Williamson A, McCrimmon RJ, Sherwin RS: Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes 2006;55:1088–1095 [DOI] [PubMed] [Google Scholar]
- 19. Warren RE, Frier BM: Hypoglycaemia and cognitive function. Diabetes Obes Metab 2005;7:493–503 [DOI] [PubMed] [Google Scholar]
- 20. Lincoln NB, Faleiro RM, Kelly C, Kirk BA, Jeffcoate WJ: Effect of long-term glycemic control on cognitive function. Diabetes Care 1996;19:656–658 [DOI] [PubMed] [Google Scholar]
- 21. Robinson R, Krishnakumar A, Paulose CS: Enhanced dopamine D1 and D2 receptor gene expression in the hippocampus of hypoglycaemic and diabetic rats. Cell Mol Neurobiol 2009;29:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gunther ML, Jackson JC, Ely EW: The cognitive consequences of critical illness: practical recommendations for screening and assessment. Crit Care Clin 2007;23:491–506 [DOI] [PubMed] [Google Scholar]
- 23. Malone JI, Hanna S, Saporta S, Mervis RF, Park CR, Chong L, Diamond DM: Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr Diabetes 2008;9:531–539 [DOI] [PubMed] [Google Scholar]
- 24. Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA: Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 2007;117:910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka Y, Takata T, Satomi T, Sakurai T, Yokono K: The double-edged effect of insulin on the neuronal cell death associated with hypoglycemia on the hippocampal slice culture. Kobe J Med Sci 2008;54:E97–E107 [PubMed] [Google Scholar]